Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates
Abstract
1. Introduction
2. Materials and Methods
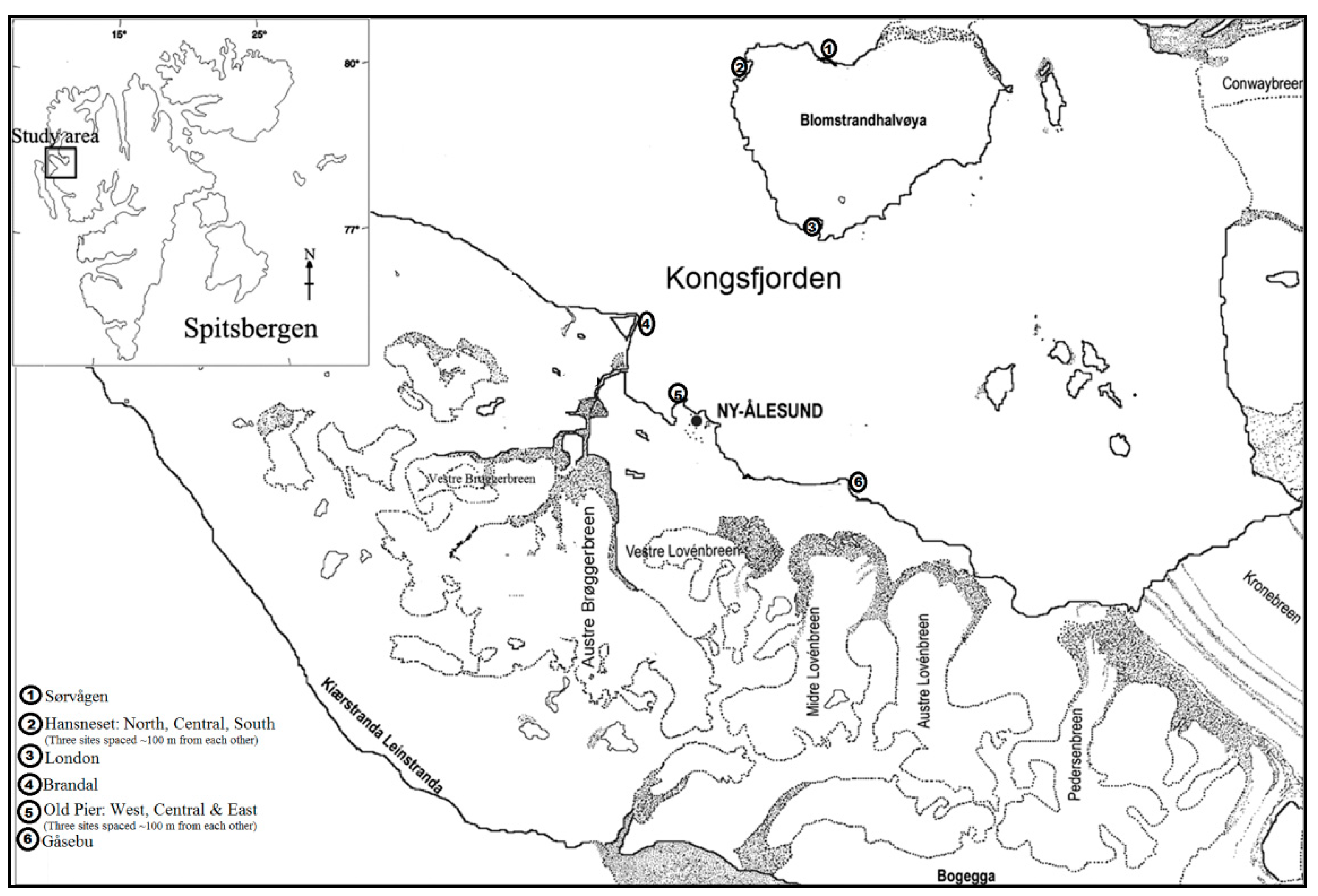
2.1. Study Area and Sampling
2.2. Analytical Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.M.; Kumar, A.; Sharma, P.; Ravindra, M.; Upadhya, A.K.; Ravindra, R. Elemental variations in glacier cryoconites of Indian Himalaya and Spitsbergen, Arctic. Geosci. Front. 2017, 8, 1339–1347. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Lehrbuch Der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Ward, N.T. Trace elements. In Environmental Analytical Chemistry. Blackie Academic and Professional; Fifield, F.W., Haines, P.J., Eds.; Chapman & Hall: London, UK, 1995. [Google Scholar]
- IPCC. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, S., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kamaruzzaman, C.Y.; Rina, Z.; John, B.A.A.; Jalal, K.C.A. Heavy metal accumulation in commercially importantfishes of South West Malaysian coast. Res. J. Environ. Sci. 2011, 5, 595–602. [Google Scholar] [CrossRef]
- Mansour, S.A.; Sidky, M.M. Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem. 2002, 78, 15–22. [Google Scholar] [CrossRef]
- Ni, I.H.; Wang, W.X.; Tam, Y.K. Transfer of Cd, Cr and Zn from zooplankton prey to mudskipper Periophthalmus cantonensis and glassy Ambassisurotaenia fishes. Mar. Ecol. Prog. Ser. 2000, 194, 203–210. [Google Scholar] [CrossRef]
- Roméo, M.; Siau, Y.; Sidoumou, Z.; Gnassia-Barelli, M. Heavy metal distribution indifferent fish species from the Mauritania coast. Sci. Total Environ. 1999, 232, 169–175. [Google Scholar] [CrossRef]
- Yilmaz, B. Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugilcephalus and Trachurus mediterraneusfrom Iskenderun Bay, Turkey. Environ. Res. 2003, 92, 277–281. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Sudaryanto, A.; Monirith, I.; Kan-Atireklap, S.; Iwata, H.; Ismail, A.; Sanguansin, J.; Muchtar, M.; Tana, T.S.; et al. Exposure assessment for trace elements from consumption of marine fish in Southeast Asia. Environ. Pollut. 2007, 145, 766–777. [Google Scholar] [CrossRef]
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, E.; Nieboer, D.G.; Varakina, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966e973. [Google Scholar] [CrossRef]
- Darmody, R.G.; Thorn, C.E. Elevation, age, soil development, and chemical weathering at Storbreen, Jotunheimen, Norway. Geogr. Ann. Ser. A Phys Geogr. 1997, 79, 215–222. [Google Scholar] [CrossRef]
- Pacyna, M.; Vitols, V.; Hanssen, J.E. Size distributed composition of the Arctic aerosol at Ny-Alesund, Spitsbergen. Atmos. Environ. 1984, 18, 2447–2459. [Google Scholar] [CrossRef]
- Maenhaut, W.; Cornille, P.; Pacyna, J.M.; Vitols, V. Trace element composition and origin of the atmospheric aerosol in the Norwegian Arctic. Atmos. Environ. 1989, 23, 2551–2569. [Google Scholar] [CrossRef]
- Wadham, J.L.; Hallam, K.R.; Hawkins, J.; O’Connor, A. Enhancement of snowpack inorganic nitrogen by aerosol debris. Tellus 2006, 59, 229–241. [Google Scholar] [CrossRef]
- Eleftheriadis, K.; Vratolis, S.; Nyeki, S. Aerosol black carbon in the European Arctic: Measurements at Zeppelin station, Ny-Ålesund, Svalbard from 1998–2007. Geophys. Res. Lett. 2009, 36, L02809. [Google Scholar] [CrossRef]
- Boyle, J.F.; Rose, N.L.; Appleby, P.G.; Birks, H.J.B. Recent environmental change and human impact on Svalbard: The lake-sediment geochemical record. J. Paleolimnol. 2004, 31, 515–530. [Google Scholar] [CrossRef]
- Rose, N.L.; Rose, C.L.; Boyle, J.F.; Appleby, P.G. Lake-Sediment Evidence for Local and Remote Sources of Atmospherically Deposited Pollutants on Svalbard. J. Paleolimnol. 2004, 31, 499–513. [Google Scholar] [CrossRef]
- Snyder-Conn, E.; Garbarino, J.R.; Hoffman, G.L.; Oelkers, A. Soluble trace elements and total mercury in Arctic Alaskan snow. Arctic 1997, 50, 201–215. [Google Scholar] [CrossRef]
- Borgå, K.; Campbell, L.; Gabrielsen, G.W.; Norstrom, R.J.; Muir, D.C.G.; Fisk, A.T. Regional and species specific bioaccumulation of major and trace elements in Arctic seabirds. Environ. Toxicol Chem. 2006, 25, 2927–2936. [Google Scholar] [CrossRef]
- Barrie, L.A. Arctic air pollution: An overview of current knowledge. Atmos. Environ. 1986, 20, 643–663. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Ottar, B.; Tomza, U.; Maenhaut, W. Long-range transport of trace elements to NyÅlesund, Spitsbergen. Atmos. Environ. 1985, 19, 857–865. [Google Scholar] [CrossRef]
- Aldahan, A.; Possnert, G.; Scherer, R.; Shi, N.; Backman, J.; Boström, K. Trace-element and major-element stratigraphy in quaternary sediments from the Arctic Ocean and implications for glacial termination. J. Sediment Res. 2000, 70, 1095–1106. [Google Scholar] [CrossRef]
- Hicks, S.; Isaksson, E. Assessing source areas of pollutants from studies of fly ash, charcoal, and pollen from Svalbard snow and ice. J. Geophys. Res. 2006, 111, D02113. [Google Scholar] [CrossRef]
- Lu, Z.; Cai, M.; Wang, J.; Yin, Z.; Yang, H. Levels and distribution of trace metals in surface sediments from Kongsfjorden, Svalbard, and Norwegian Arctic. Environ. Geochem. Health 2013, 35, 257–269. [Google Scholar] [CrossRef]
- Singh, S.M.; Naik, S.; Mulik, R.U.; Sharma, J.; Upadhyay, A.K. Elemental composition and bacterial occurrence in sediment samples on two sides of Brøggerhalvøya, Svalbard. Polar Rec. 2015, 51, 680–691. [Google Scholar] [CrossRef]
- Singh, S.M.; Sharma, J.; Gawas-Sakhalkar, P.; Upadhyay, A.K.; Mulik, R.U.; Naik, S.; Bohare, P.; Ravindra, R. Elemental composition and bacterial incidence in firn-cores at Midre Lovénbreen glacier, Svalbard, Arctic. Polar Rec. 2015, 51, 39–48. [Google Scholar] [CrossRef]
- Singh, S.M.; Sharma, J.; Gawas-Sakhalkar, P.; Upadhyay, A.K.; Naik, S.; Bande, D.; Ravindra, R. Chemical and bacteriological analysis of soil from the middle and late Weichselian from Western Spitsbergen, Arctic. Quatern. Int. 2012, 271, 98–105. [Google Scholar] [CrossRef]
- Singh, S.M.; Sharma, J.; Gawas-Sakhalkar, P.; Upadhyay, A.K.; Naik, S.; Pedneker, S.; Ravindra, R. Atmospheric deposition studies of heavy metals in arctic by comparative analysis of lichens and cryoconite. Environ. Monit. Assess. 2012, 185, 1367–1376. [Google Scholar] [CrossRef]
- Łqokas, E.; Zaborska, A.; Kolicka, M.; Ro_zycki, M.; Zawierucha, K. Accumulation of atmospheric radionuclides and heavy metals in cryoconite holes on an Arctic glacier. Chemosphere 2016, 160, 162–172. [Google Scholar] [CrossRef]
- Brand, M.; Fischer, P. Species composition and abundance of the shallow water fish community of Kongsfjorden, Svalbard. Polar Biol. 2016, 39, 2155–2167. [Google Scholar] [CrossRef]
- Voronkov, A.; Hop, H.; Gulliksen, B. Diversity of hard-bottom fauna relative to environmental gradients in Kongsfjorden. Svalbard. Polar Res. 2013, 32, 11208. [Google Scholar] [CrossRef][Green Version]
- Lippert, H.; Iken, K.; Rachor, E.; Wiencke, C. Macro fauna associated with macroalgae in the Kongsfjord (Spitsbergen). Polar Biol 2001, 24, 512–522. [Google Scholar]
- Werner, E.E. Species packing and niche complementarity in three sunfishes. Am. Nat. 1977, 111, 553–578. [Google Scholar] [CrossRef]
- Keast, A. Thepiscivore feeding guild of fishes in small freshwater ecosystems. Environ. Biol. Fishes. 1985, 12, 119–129. [Google Scholar] [CrossRef]
- Knight, C.A.; Hallett, J.; Devries, A.L. Solute effects on ice recrystallisation: An assessment technique. Cryobiology 1988, 25, 55–60. [Google Scholar] [CrossRef]
- Jia, Z.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- Duman, J.G.; Olsen, T.M. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology 1993, 30, 322–328. [Google Scholar] [CrossRef]
- DeVries, A.L.; Komatsu, S.K.; Feeney, R.E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J. Biol. Chem. 1970, 245, 2901–2908. [Google Scholar] [CrossRef]
- Fletcher, G.L.; Hew, C.L.; Davies, P.L. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 2001, 63, 359–390. [Google Scholar] [CrossRef]
- Ewart, K.V.; Hew, C.L. Fish Antifreeze Proteins; World Scientific: Singapore, 2002. [Google Scholar]
- Able, K.W. A revision of Arctic snailfishes of the genus Liparis (Scorpaeniformes: Cyclopteridae). Copeia 1990, 1990, 476–492. [Google Scholar] [CrossRef]
- Węsławski, J.M.; Linkowski, T.B.; Herra, T. Fishes. In Atlas of the Marine Fauna of Southern Spitsbergen; Klekowski, R.Z., Węsławski, J.M., Eds.; Vertebrate University of Gdańsk, Institute of Oceanology: Gdańsk, Poland, 1990; pp. 67–195. [Google Scholar]
- Muus, B.J.; Nielsen, J.G. Sea Fish; Scandinavian Fishing Year Book: Hedehusene, Denmark, 1999; pp. 2551–2569. [Google Scholar]
- Hayward, P.J.; Ryland, J.S. Handbook of the Marine Fauna of North-West Europe; Oxford University Press Inc.: Oxford, UK, 2005. [Google Scholar]
- Oribhabor, B.J.; Ogbeibu, A.E. The ecological impact of anthropogenic activities on the predatory fish assemblage of a tidal creek in the Niger Delta, Nigeria. Res. J. Environ. Sci. 2010, 4, 271–279. [Google Scholar] [CrossRef][Green Version]
- Emara, H.I.; El-Deek, M.S.; Ahmed, N.S. A comparative study on the levels of trace metals in some Mediterranean and Red Sea fishes. Chem. Ecol. 1993, 8, 119–127. [Google Scholar] [CrossRef]
- Hanna, R.G.M. Levels of heavy metals in some Red Sea fish before Hot Brine pools’ mining. Mar. Pollut. Bull. 1989, 20, 631–635. [Google Scholar] [CrossRef]
- El-Moselhy, K.M.; Othman, A.I.; Abd El-Azem, H.; El-Metwally, M.E.A. Bioaccumulation o heavy metals in some tissues of fish in the Red Sea. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Schenone, N.F.; Avigliano, E.; Goessler, W.; Cirelli, A.F. Toxic metals, trace and major elements determined by, I.C.PMS in tissues of Parapimelodusvalenciennis and Prochiloduslineatus from Chascomus Lake, Argentina. Microchem. J. 2014, 112, 127–131. [Google Scholar] [CrossRef]
- Marx, S.K.; Kamber, B.S.; McGowan, H.A. Provenance of long-travelled dust determined with ultra-trace-element composition: A pilot study with samples from New Zealand glaciers. Earth Surf. Process. Landf. 2005, 30, 699–716. [Google Scholar] [CrossRef]
- DiGiovanni, F.; Fellin, P. Transboundary Air Pollution. In Encyclopedia of Life Support Systems (EOLSS); Inyang, H.I., Daniels, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; p. 339. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- SanPiN 2.3.2.1078-01 Hygienic Demands of the Safeness and Nutritional Values of Food. Prescript N_ 18, 31.05 [CaнПиH 2.3.2.1078-01 «Gигиеничеcкие требoвaния безoпacнocти и пищевoй ценнocти пищевых прoдуктoв», Пocтaнoвление N_18, 31.05.2002]. Available online: https://www.dia-m.ru/upload/iblock/2b5/567-catalog (accessed on 18 May 2022).
- Lockhart, W.L.; Stern, G.A.; Low, G.; Hendzel, M.; Boila, G.; Roach, P.; Evans, M.S.; Billeck, B.N.; DeLaronde, J.; Friesen, S.; et al. A history of total mercury in edible muscle of fish from lakes in northern Canada. Sci. Total Environ. 2005, 351, 427–463. [Google Scholar] [CrossRef]

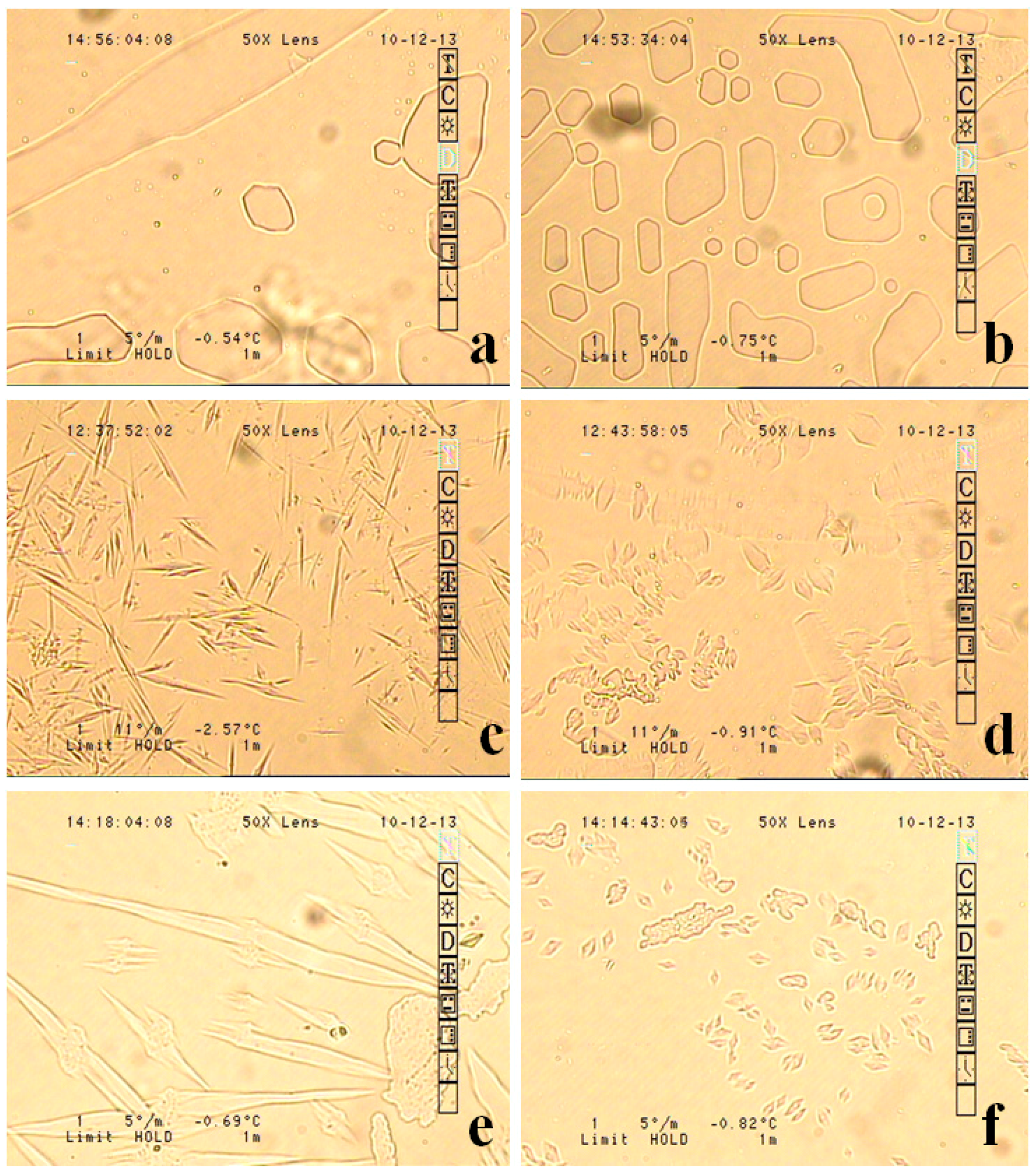
| Fish Code Number | Fjord Side | Location | Catch Device | Depth (m) | Species | Length_Std (cm) | Weight Total | Sex |
|---|---|---|---|---|---|---|---|---|
| F (1738) | North | Hansneset Central | Double Fyke Net | 5 | Anarhichas lupus | 49 | 1321.07 | F |
| E (1643) | North | London | Double Fyke Net | 5 | Gadus ozak | 30 | 352.88 | F |
| A2 (1644) | South | Old Pier Central | Double Fyke Net | 5 | Gadus morhua | 37.5 | 634.7 | F |
| A1 (1707) | North | Hansneset South | Double Fyke Net | 5 | Gadus morhua | 16.5 | 49.23 | U |
| B2 (1737) | South | Gasebu | Double Fyke Net | 5 | Gymnocanthus tricuspis | 14.5 | 65.63 | F |
| C1 (1770) | North | Hansneset South | Double Fyke Net | 5 | Liparis sp. | 12.5 | 36.6 | M |
| C2 (1703) | North | Hansneset South | Fyke Net with Bait | 3 | Liparis sp. | 14.5 | 71.58 | M |
| D1 (1700) | South | Old Pier Central | Double Fyke Net | 5 | Myoxocephalus Scorpius | 13.5 | 53.6 | F |
| D2 (1702) | South | Old Pier Central | Fyke Net with Bait | 3 | Myoxocephalus Scorpius | 11.5 | 28.76 | M |
| D3 (1704) | North | Hansneset South | Fyke Net with Bait | 12 | Myoxocephalus Scorpius | 17 | 108.9 | F |
| Study Site Organism | Sample | Ba | Cr | Cs | Rb | Sr | U | V | |
|---|---|---|---|---|---|---|---|---|---|
| LOQ (mg/kg) | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.01 | ||
| Kongsfzorden | Fish | F (1738) | BLQ | 0.149 ± 0.00 | 0.028 ± 0.00 | 1.509 ± 0.01 | 0.692 ± 0.01 | BLQ | BLQ |
| E (1643) | 0.129 ± 0.01 | 0.144 ± 0.01 | 0.100 ± 0.00 | 2.16 ± 0.03 | 2.927 ± 0.01 | BLQ | BLQ | ||
| A2 (1644) | 0.026 ± 0.00 | 0.197 ± 0.00 | 0.052 ± 0.00 | 2.171 ± 0.02 | 0.708 ± 0.01 | BLQ | BLQ | ||
| A1 (1707) | 0.056 ± 0.01 | 0.363 ± 0.01 | 0.069 ± 0.00 | 4.046 ± 0.02 | 8.651 ± 0.01 | BLQ | BLQ | ||
| B2 (1737) | 0.032 ± 0.01 | 0.319 ± 0.00 | 0.035 ± 0.00 | 1.665 ± 0.01 | 8.276 ± 0.04 | BLQ | 0.017 ± 0.00 | ||
| B1 (1763) | 0.024 ± 0.01 | 0.442 ± 0.01 | 0.087 ± 0.00 | 2.894 ± 0.02 | 4.094 ± 0.05 | BLQ | BLQ | ||
| C1 (1770) | 0.013 ± 0.00 | 0.235 ± 0.01 | 0.022 ± 0.00 | 1.866 ± 0.01 | 2.989 ± 0.04 | BLQ | 0.020 ± 0.00 | ||
| C2 (1703) | 0.042 ± 0.00 | 0.134 ± 0.01 | 0.031 ± 0.00 | 1.682 ± 0.01 | 5.798 ± 0.05 | BLQ | 0.031 ± 0.00 | ||
| D1 (1700) | 0.073 ± 0.01 | 0.378 ± 0.01 | 0.027 ± 0.00 | 1.619 ± 0.01 | 4.277 ± 0.07 | BLQ | 0.021 ± 0.00 | ||
| D2 (1702) | 0.143 ± 0.01 | 0.757 ± 0.01 | 0.034 ± 0.00 | 1.669 ± 0.02 | 21.903 ± 0.14 | BLQ | 0.051 ± 0.00 | ||
| D3 (1704) | 0.048 ± 0.01 | 0.182 ± 0.01 | 0.044 ± 0.00 | 1.581 ± 0.03 | 7.212 ± 0.06 | BLQ | 0.018 ± 0.00 | ||
| Kongsfzorden | Invertebrates | G (SFS) | 6.823 ± 0.03 | 0.392 ± 0.00 | 0.032 ± 0.00 | 1.048 ± 0.02 | 1116.6 ± 10.69 | 0.169 ± 0.00 | 0.453 ± 0.01 |
| H (SFB) | 27.559 ± 0.28 | 0.916 ± 0.01 | 0.029 ± 0.00 | 1.404 ± 0.01 | 1080.1 ± 3.97 | 0.519 ± 0.01 | 4.849 ± 0.02 | ||
| I (SU) | 16.123 ± 0.08 | 0.914 ± 0.01 | 0.095 ± 0.00 | 1.943 ± 0.01 | 971.53 ± 10.20 | 0.098 ± 0.00 | 1.56 ± 0.01 | ||
| J (WOEM) | 0.880 ± 0.02 | 0.394 ± 0.00 | 0.017 ± 0.00 | 1.171 ± 0.02 | 48.578 ± 0.66 | 0.161 ± 0.00 | 1.418 ± 0.01 | ||
| Study Site Organism | Sample | As | Bi | Cd | Cu | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|
| LOQ (mg/kg) | 0.01 | 0.05 | 0.02 | 0.05 | 0.01 | 0.1 | ||
| Kongsfzorden | Fish | F (1738) | 14.541 ± 0.11 | BLQ | BLQ | 0.262 ± 0.01 | BLQ | 11.616 ± 0.05 |
| E (1643) | 26.181 ± 0.02 | BLQ | BLQ | 0.594 ± 0.01 | 0.037 ± 0.00 | 13.006 ± 0.13 | ||
| A2 (1644) | 13.212 ± 0.06 | BLQ | BLQ | 0.255 ± 0.01 | 0.117 ± 0.01 | 8.569 ± 0.02 | ||
| A1 (1707) | 6.982 ± 0.12 | BLQ | BLQ | 0.880 ± 0.01 | BLQ | 22.264 ± 0.22 | ||
| B2 (1737) | 9.828 ± 0.07 | BLQ | BLQ | 0.609 ± 0.01 | 0.015 ± 0.00 | 12.811 ± 0.02 | ||
| B1 (1763) | 35.84 ± 0.15 | BLQ | BLQ | 1.740 ± 0.04 | 0.028 ± 0.00 | 27.245 ± 0.54 | ||
| C1 (1770) | 8.694 ± 0.04 | BLQ | BLQ | 0.901 ± 0.01 | 0.029 ± 0.00 | 15.084 ± 0.1 | ||
| C2 (1703) | 16.253 ± 0.10 | BLQ | BLQ | 0.840 ± 0.02 | 0.011 ± 0.00 | 16.326 ± 0.05 | ||
| D1 (1700) | 3.291 ± 0.01 | BLQ | BLQ | 0.946 ± 0.01 | 0.020 ± 0.00 | 16.578 ± 0.1 | ||
| D2 (1702) | 2.781 ± 0.03 | BLQ | BLQ | 0.857 ± 0.02 | 0.029 ± 0.00 | 37.358 ± 0.30 | ||
| D3 (1704) | 4.537 ± 0.09 | BLQ | BLQ | 0.814 ± 0.01 | 0.030 ± 0.00 | 14.544 ± 0.16 | ||
| Kongsfzorden | Invertebrates | G (SFS) | 1.221 ± 0.02 | BLQ | 0.214 ± 0.01 | 0.775 ± 0.02 | 0.261 ± 0.01 | 20.178 ± 0.19 |
| H (SFB) | 4.139 ± 0.04 | 0.652 ± 0.02 | BLQ | 10.045 ± 0.05 | 5.258 ± 0.05 | 40.695 ± 0.20 | ||
| I (SU) | 4.506 ± 0.04 | BLQ | BLQ | 0.918 ± 0.02 | 0.473 ± 0.01 | 23.731 ± 0.02 | ||
| J (WOEM) | 13.458 ± 0.14 | 0.904 ± 0.01 | BLQ | 2.113 ± 0.01 | 0.884 ± 0.01 | 77.622 ± 0.34 | ||
| Study Site Organism | Sample | Co | Fe | Mn | Ni | |
|---|---|---|---|---|---|---|
| LOQ (mg/kg) | 0.01 | 0.5 | 0.05 | 0.1 | ||
| Kongsfzorden | Fish | F (1738) | BLQ | 0.799 ± 0.01 | 0.165 ± 0.00 | 0.124 ± 0.02 |
| E (1643) | BLQ | 2.91 ± 0.02 | 0.344 ± 0.00 | 0.165 ± 0.03 | ||
| A2 (1644) | BLQ | 0.907 ± 0.01 | 0.262 ± 0.01 | 0.249 ± 0.04 | ||
| A1 (1707) | 0.024 ± 0.00 | 5.479 ± 0.06 | 1.094 ± 0.01 | 0.225 ± 0.07 | ||
| B2 (1737) | 0.021 ± 0.00 | 3.179 ± 0.01 | 0.626 ± 0.01 | 0.273 ± 0.05 | ||
| B1 (1763) | 0.029 ± 0.00 | 8.141 ± 0.12 | 1.168 ± 0.01 | 0.177 ± 0.05 | ||
| C1 (1770) | 0.038 ± 0.00 | 3.395 ± 0.04 | 0.407 ± 0.00 | 0.257 ± 0.04 | ||
| C2 (1703) | 0.051 ± 0.00 | 4.573 ± 0.04 | 0.554 ± 0.01 | 0.24 ± 0.03 | ||
| D1 (1700) | 0.028 ± 0.00 | 4.401 ± 0.05 | 0.517 ± 0.01 | 0.246 ± 0.03 | ||
| D2 (1702) | 0.041 ± 0.00 | 7.248 ± 0.06 | 1.311 ± 0.01 | 0.439 ± 0.04 | ||
| D3 (1704) | 0.015 ± 0.00 | 3.533 ± 0.06 | 0.656 ± 0.01 | 0.364 ± 0.06 | ||
| Kongsfzorden | Invertebrates | G (SFS) | 0.541 ± 0.01 | 83.72 ± 0.14 | 19.848 ± 0.21 | 2.668 ± 0.05 |
| H (SFB) | 0.774 ± 0.01 | 438.34 ± 1.95 | 64.834 ± 0.20 | 3.778 ± 0.08 | ||
| I (SU) | 0.733 ± 0.01 | 240.95 ± 2.08 | 19.491 ± 0.21 | 2.616 ± 0.07 | ||
| J (WOEM) | 1.336 ± 0.01 | 129.50 ± 1.09 | 14.13 ± 0.06 | 1.686 ± 0.02 | ||
| Study Site Organism | Sample | Se | Ag | |
|---|---|---|---|---|
| LOQ (mg/kg) | 0.05 | 0.05 | ||
| Kongsfzorden | Fish | F (1738) | 0.816 ± 0.04 | BLQ |
| E (1643) | 0.663 ± 0.03 | BLQ | ||
| A2 (1644) | 0.501 ± 0.02 | BLQ | ||
| A1 (1707) | 0.829 ± 0.08 | BLQ | ||
| B2 (1737) | 0.654 ± 0.03 | BLQ | ||
| B1 (1763) | 1.206 ± 0.05 | BLQ | ||
| C1 (1770) | 0.643 ± 0.01 | BLQ | ||
| C2 (1703) | 1.034 ± 0.01 | BLQ | ||
| D1 (1700) | 0.804 ± 0.02 | BLQ | ||
| D2 (1702) | 0.663 ± 0.05 | BLQ | ||
| D3 (1704) | 0.697 ± 0.02 | BLQ | ||
| Kongsfzorden | Invertebrates | G (SFS) | 0.395 ± 0.03 | 1.143 ± 0.01 |
| H (SFB) | 1.171 ± 0.05 | 1.189 ± 0.02 | ||
| I (SU) | 1.063 ± 0.10 | 0.367 ± 0.01 | ||
| J (WOEM) | 3.978 ± 0.02 | 0.330 ± 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.M.; Tsuji, M.; Singh, P.; Mulik, R.U. Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates. Sustainability 2022, 14, 11727. https://doi.org/10.3390/su141811727
Singh SM, Tsuji M, Singh P, Mulik RU. Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates. Sustainability. 2022; 14(18):11727. https://doi.org/10.3390/su141811727
Chicago/Turabian StyleSingh, Shiv Mohan, Masaharu Tsuji, Purnima Singh, and Ravindra Uttam Mulik. 2022. "Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates" Sustainability 14, no. 18: 11727. https://doi.org/10.3390/su141811727
APA StyleSingh, S. M., Tsuji, M., Singh, P., & Mulik, R. U. (2022). Elemental Composition and Freezing Tolerance in High Arctic Fishes and Invertebrates. Sustainability, 14(18), 11727. https://doi.org/10.3390/su141811727








