An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Plan
2.2. Microbiological Evaluation
Swabs and Plates
2.3. Microbial Isolation and Identification
2.4. Cleaning Procedure
- -
- Use of pre-treated Puli-scrub cloths in TT, treated at higher temperatures (90 °C) than “GREEN” treatment (60 °C);
- -
- The use of eco-labelled products for the cleaning of floors and surfaces in TT, with higher tank dosages (1%) compared to the products of the experimental protocol “GREEN” (dosage 0.3%).
- -
- Use of eco-labelled textiles for the cleaning of floors and surfaces in the experimental protocol “GREEN”. Specifically, the Micro-Activa (Filmop), ultra-microfibre cloth used for the cleaning of floors passed the test conducted by an independent laboratory following the ISO 23231 for the release of microplastics in the water of washing and rinsing. Furthermore, the Ultra Cloth (Diversey) used for the cleaning of furniture, taps, and sanitaria has been certified as an Eco Nordic Swan product. These products are thoroughly tested to evaluate the human safety of their use, granting at the same time a low environmental impact (strict criteria concerning raw materials, productive cycle, and distribution chain).
- -
| Operation | Traditional Protocol | |||||
|---|---|---|---|---|---|---|
| Working Procedure | Dilution | Product | Equipment | Trolley | Machine | |
| FLOORS—Hand washing | Mop wash with flat fringe | D.U. 1.0% pre-impregnation in the machine | Pine Ecolabel (Sutter) | Puli-Scrub (Filmop) | Morgan Top-Down Maxi 7020 (Filmop) | |
| FLOORS—Manual disinfection | Mop wash with flat fringe | D.U. 1.0% pre-impregnation in the machine | Ondaklor (Sutter) | Puli-Scrub (Filmop) | Morgan Top-Down Maxi 7020 (Filmop) | |
| FLOORS—Mechanical washing | Scrubbing machine wash, normal mode | D.U. 1.0% tank dosing | Pine Ecolabel (Sutter) | Bluematic 50 CB BT (Magris) | ||
| FLOORS—Mechanical disinfection | Scrubbing machine wash, normal mode | D.U. 3.0% tank dosing | Ondaklor (Sutter) | Bluematic 50 CB BT (Magris) | ||
| Dusting of furniture and surfaces | Cloth and trigger | 100% (P.U.) | Diamond Ecolabel (Sutter) | My Micro Cloth (Diversey) | Morgan Top-Down Maxi 7020 (Filmop) | |
| Furniture disinfection | Cloth and trigger | D.U. 3.0% dosage in bottle with trigger | Ondaklor (Sutter) | My Micro Cloth (Diversey) | Morgan Top-Down Maxi 7020 (Filmop) | |
| Toilet cleaning | Cloth and trigger | 100% (P.U.) | Ruby Ecolabel (Sutter) | My Micro Cloth (Diversey) | Morgan Top-Down Maxi 7020 (Filmop) | |
| Toilet disinfection | Cloth and trigger | D.U. 3.0% dosage in bottle with trigger | Ondaklor (Sutter) | My Micro Cloth (Diversey) | Morgan Top-Down Maxi 7020 (Filmop) | |
| Sanitary and taps descaling | Cloth and trigger | 100% (U.R.) | Xtra-Calc Plus (Sutter) | My Micro Cloth (Diversey) | Morgan Top-Down Maxi 7020 (Filmop) | |
| Textile reconditioning—Detergent | Machine wash at 90 °C | 6 g/kg | Enzy Extra (Sutter) | PWM520 18 kg (Miele) | ||
| Textile reconditioning—Alkalizing | Machine wash at 90 °C | 10 g/kg | Alka Power (Sutter) | PWM520 18 kg (Miele) | ||
| Textile reconditioning—Disinfectant | Machine wash at 90 °C | 6 g/kg | Per Active (Sutter) | PWM520 18 kg (Miele) | ||
| Textile pre-impregnation—Floor cleaner | Machine wash at 90 °C | 10 g/kg | Pine Ecolabel (Sutter) | Puli-Scrub (Filmop) | PWM520 18 kg (Miele) | |
| Textile pre-impregnation—Surface disinfectant | Machine wash at 90 °C | 10 g/kg | Ondaklor (Sutter) | Puli-Scrub (Filmop) | PWM520 18 kg (Miele) | |
| Operation | Green Protocol | |||||
|---|---|---|---|---|---|---|
| Working Procedure | Dilution | Product | Equipment | Trolley | Machine | |
| FLOORS—Hand washing | Mop wash with flat fringe | D.U. 0.3% pre-impregnation in the machine | Pine Easy Ecolabel (Sutter) | Micro-Activa (Filmop) | Alpha 2672 (Filmop) | |
| FLOORS—Manual disinfection | Mop wash with flat fringe | D.U. 3.0% pre-impregnation in the machine | Ondaklor (Sutter) | Micro-Activa (Filmop) | Alpha 2672 (Filmop) | |
| FLOORS—Mechanical washing | Scrubbing machine wash, ECO mode | D.U. 0.3% tank dosing | Pine Easy Ecolabel (Sutter) | Bluematic 50 CB BT (Magris) | ||
| FLOORS—Mechanic disinfection | Scrubbing machine wash, ECO mode | D.U. 3.0% tank dosing | Ondaklor (Sutter) | Bluematic 50 CB BT (Magris) | ||
| Dusting of furniture and surfaces | Cloth and trigger | D.U. 6.0% dosage in bottle with trigger | Diamond Easy Ecolabel (Sutter) | Ultra Cloth (Diversey) | Alpha 2672 (Filmop) | |
| Furniture disinfection | Cloth and trigger | D.U. 3.0% dosage in bottle with trigger | Ondaklor (Sutter) | Ultra Cloth (Diversey) | Alpha 2672 (Filmop) | |
| Toilet cleaning | Cloth and trigger | D.U. 6.0% dosage in bottle with trigger | Ruby Easy Ecolabel (Sutter) | Ultra Cloth (Diversey) | Alpha 2672 (Filmop) | |
| Toilet disinfection | Cloth and trigger | D.U. 3.0% dosage in bottle with trigger | Ondaklor (Sutter) | Ultra Cloth (Diversey) | Alpha 2672 (Filmop) | |
| Sanitary and taps descaling | Cloth and trigger | D.U. 3.0% dosage in bottle with trigger | Descaler Plus (Sutter) | Ultra Cloth (Diversey) | Alpha 2672 (Filmop) | |
| Textile reconditioning—Detergent | Machine wash at 60 °C | 6 g/kg | Enzy Extra (Sutter) | PWM520 18 kg (Miele) | ||
| Textile reconditioning—Alkalizing | Machine wash at 60 °C | 10 g/kg | Alka Power (Sutter) | PWM520 18 kg (Miele) | ||
| Textile reconditioning—Disinfectant | Machine wash at 60 °C | 6 g/kg | Per Active (Sutter) | PWM520 18 kg (Miele) | ||
| Textile pre-impregnation—Floor cleaner | Machine wash at 60 °C | 3 g/kg | Pine Easy Ecolabel (Sutter) | Micro-Activa (Filmop) | PWM520 18 kg (Miele) | |
| Textile pre-impregnation—Surface disinfectant | Machine wash at 60 °C | 10 g/kg | Ondaklor (Sutter) | Micro-Activa (Filmop) | PWM520 18 kg (Miele) | |
2.5. Standard/GREEN Protocol Products
2.6. LCA Analysis
- the “upstream” phase included:
- ○
- the extraction of raw materials and their transformation;
- ○
- the transport of raw materials and semi-finished products to suppliers;
- ○
- the production of consumables, i.e., chemicals (detergents), textiles (fringes and cloths), accessories used, and their primary (plastic) and secondary (cardboard) packaging;
- ○
- the production of capital goods, or goods whose expected lifetime exceeds 3 years, i.e., machinery and equipment.
- The “core” phase included:
- ○
- the transport of consumables from producers to the building where the cleaning service is provided;
- ○
- the implementation of the service through the use of chemical and textile products, equipment, and machinery;
- ○
- the production of fuels needed for transport;
- ○
- the production of electrical and thermal energy used in the building for the implementation of the service;
- ○
- water consumption for the dilution of chemical products and by washing machine.
- The “downstream” phase included:
- ○
- the transport and treatment of solid waste and of waste water generated by the processes of the “core” phase.
2.7. Statistical Analysis
3. Results and Discussion
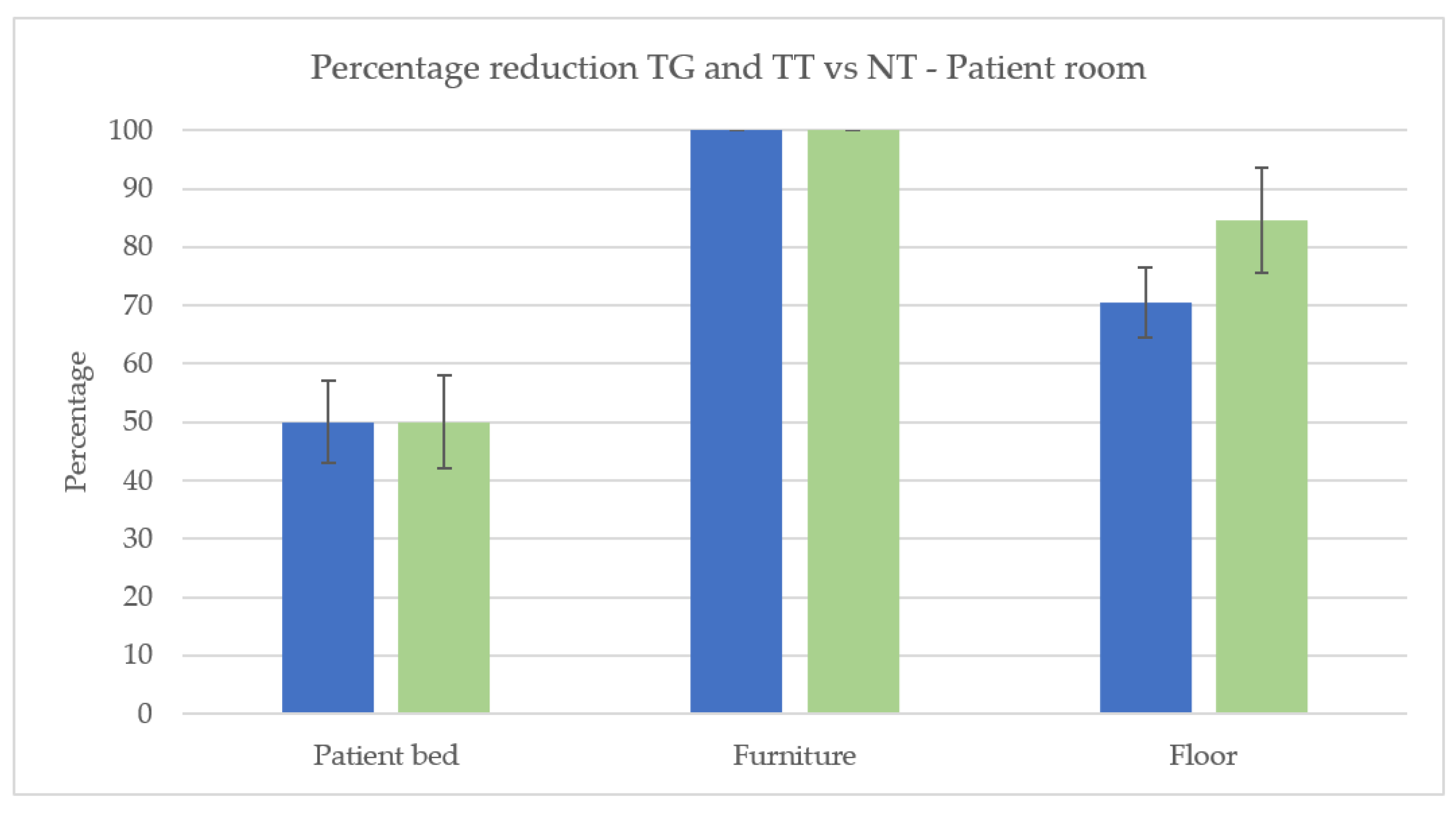
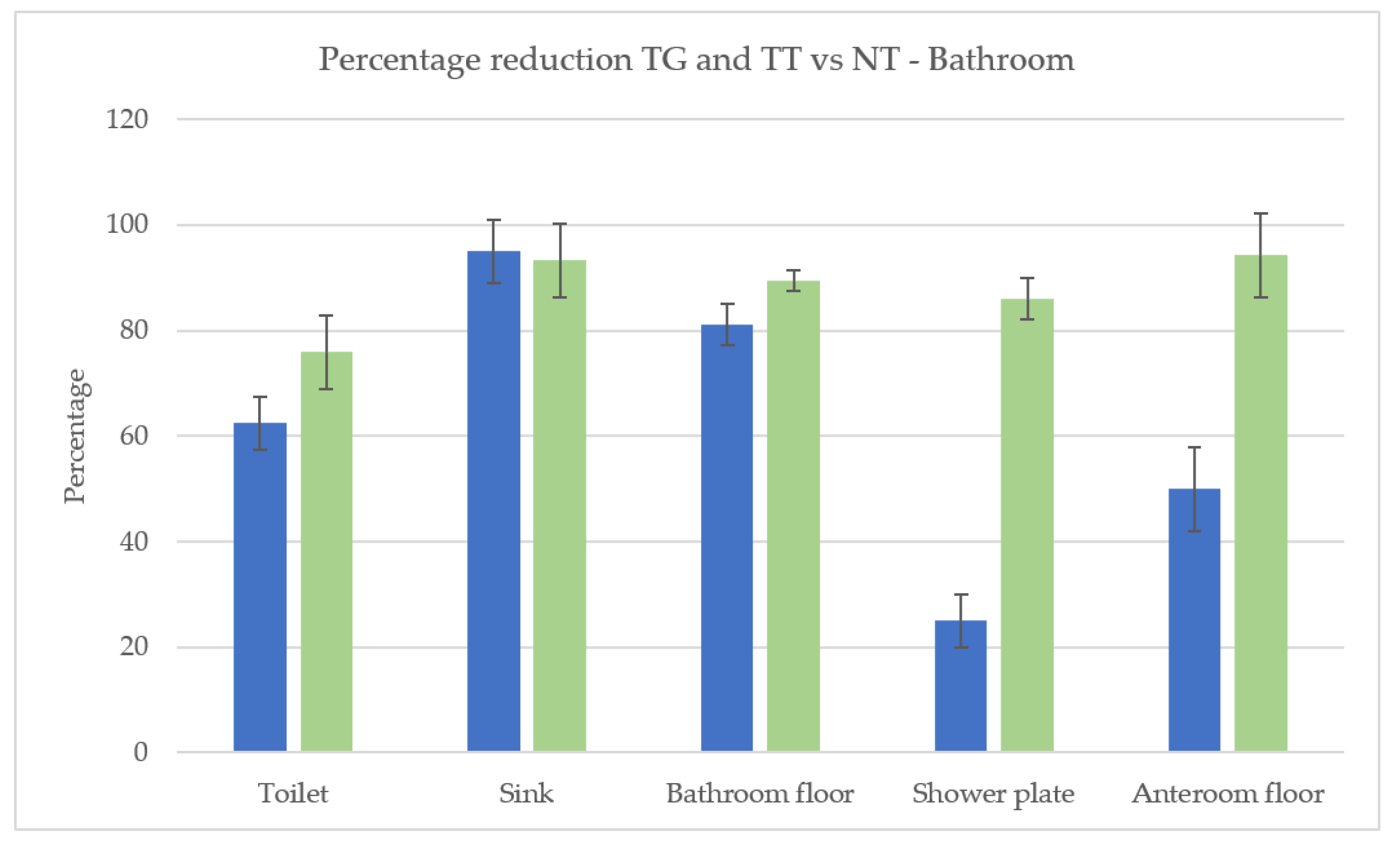
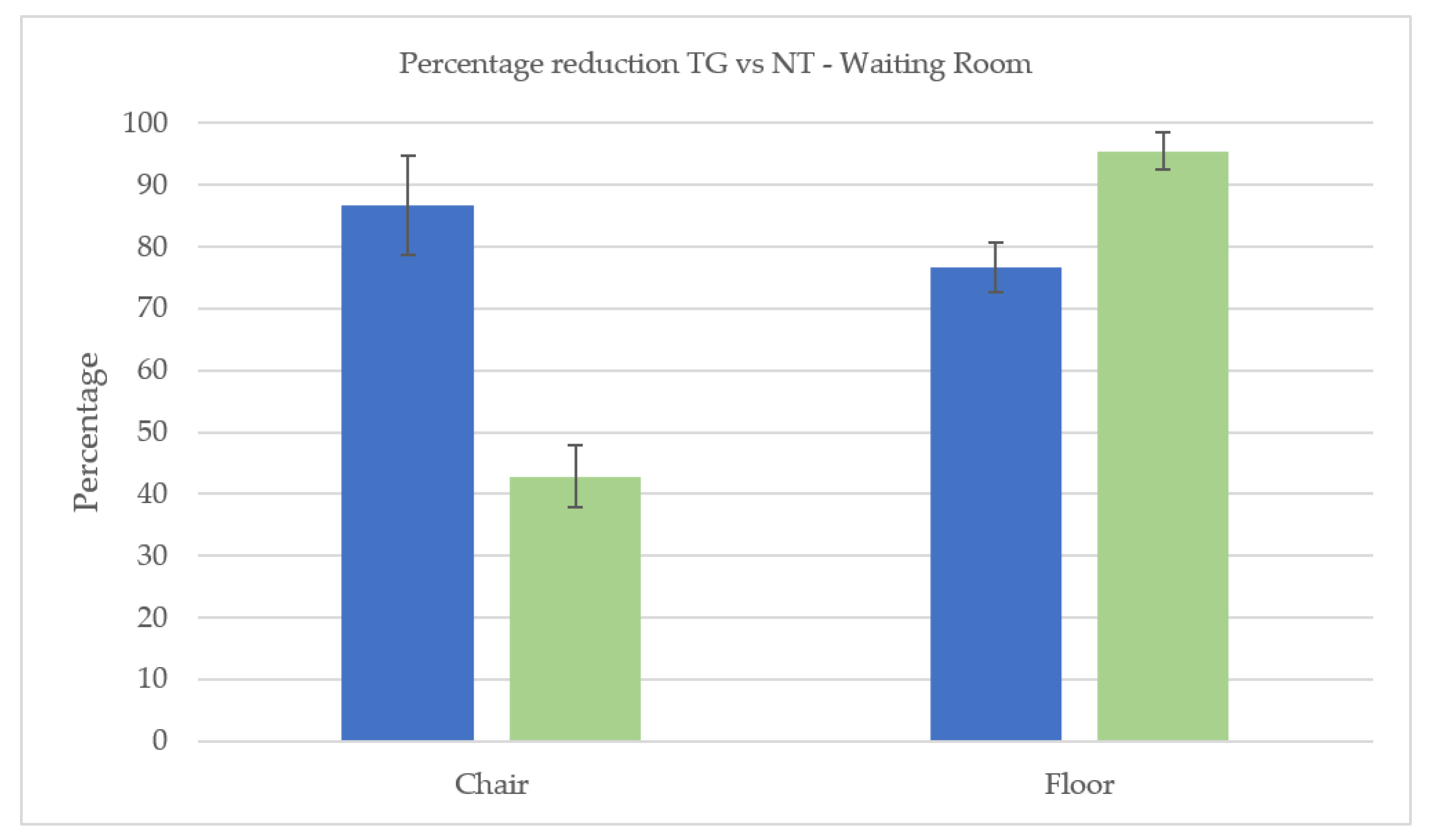
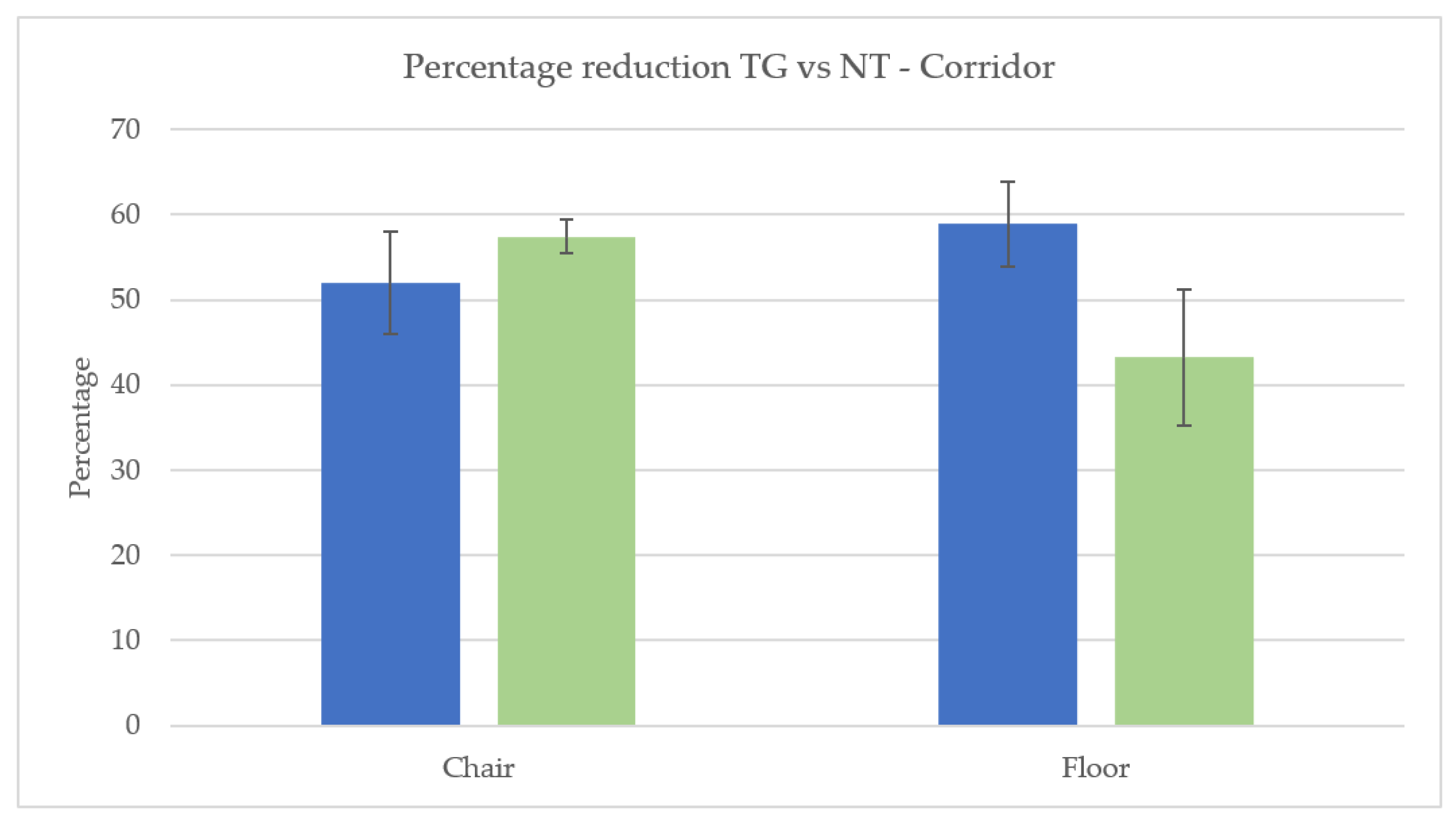
3.1. Cleaning Effectiveness
3.2. Microbial Isolation and Identification
- -
- Staph. hominis (27)
- -
- Staph. cohnii ssp. Cohnii (6)
- -
- Staph. sciuri (2)
- -
- Staph. capitis (15)
- -
- Aerococcus urinae (11)
- -
- S. agalactiae (3)
- -
- Lactococcus lactis spp. Cremoris (2)
- -
- Aerococcus viridans (4)
3.3. LCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, J. Sustainable development: Meaning, history, principles, pillars, and implications for human action: Literature review. Cogent Soc. Sci. 2019, 5, 1653531. [Google Scholar] [CrossRef]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design–A review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Helling, R.K. The Role of LCA in Sustainable Development. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Oxford, UK, 2017; pp. 237–242. [Google Scholar] [CrossRef]
- 302 Final Communication from the Commission to the Council and the European Parliament Integrated Product Policy Building on Environmental Life-Cycle Thinking. Available online: http://europa.eu.int/comm/environment/ipp/ippsum.pdf (accessed on 10 August 2022).
- Friedrich, A.W. Control of hospital acquired infections and antimicrobial resistance in Europe: The way to go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.S.; Zafar, A.; Waqas, M.; Hassan, S.G.; Haq, A.U.; Tariq, T.; Batool, S.; Irshad, M.; Hasan, M.; Shu, X. Phyto-reflexive Zinc Oxide Nano-Flowers Synthesis: An advanced Photocatalytic Degradation and Infectious therapy. J. Mater. Res. Technol. 2021, 13, 2375–2391. [Google Scholar] [CrossRef]
- Zafar, A.; Tariq, T.; Hasan, M.; Nazar, M.; Rasheed, M.N.; Mahmood, N.; Shu, X. Green-maturation of Cobalt-Oxide nano-sponges for reinforced bacterial apoptosis. Colloids Interface Sci. Commun. 2021, 45, 100531. [Google Scholar] [CrossRef]
- Henry, K.; Baronian, K.; Morris, M.; Galilee, C. Comparing the Current Chemical Cleaning Regime and Chemical-Free Cleaning at the University of Canterbury: A Report and Practical Microbiological Experiment for the Sustainability Office, University of Canterbury. 2011. Available online: https://www.canterbury.ac.nz/media/documents/sustain/Comparing_Chemical_and_Non_Chemical_Cleaning.pdf (accessed on 10 August 2022).
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Bhattacharya, S.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Ling, M.L.; Merkens, W.; et al. Chemical disinfection in healthcare settings: Critical aspects for the development of global strategies. GMS Hyg. Infect. Control. 2020, 15, 36. [Google Scholar]
- La Contaminazione Microbiologica Delle Superfici Negli Ambienti Lavorativi. 2017. Available online: www.inail.it (accessed on 10 August 2022).
- Linee Guida Sugli Standard di Sicurezza e di Igiene del Lavoro nel Reparto Operatorio Istituto Superiore per LA Prevenzione e la Sicurezza del Lavoro Dipartimento Igiene del Lavoro. Available online: http://www.ispesl.it (accessed on 10 August 2022).
- Dancer, S. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Galvin, S.; Dolan, A.; Cahill, O.; Daniels, S.; Humphreys, H. Microbial monitoring of the hospital environment: Why and how? J. Hosp. Infect. 2012, 82, 143–151. [Google Scholar] [CrossRef]
- Flouchi, R.; Elmniai, A.; El Far, M.; Touzani, I.; El Hachlafi, N.; Fikri-Benbrahim, K. Microbiological Monitoring of the Environment Using the “Association Rules” Approach and Disinfection Procedure Evaluation in a Hospital Center in Morocco. J. Environ. Public Health 2021, 2021, 7682042. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.; Obee, P.; Cooper, R.; Burton, N.; Lewis, M. The effectiveness of existing and modified cleaning regimens in a Welsh hospital. J. Hosp. Infect. 2007, 66, 352–359. [Google Scholar] [CrossRef]
- Griffith, C.; Cooper, R.; Gilmore, J.; Davies, C.; Lewis, M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Infect. 2000, 45, 19–28. [Google Scholar] [CrossRef]
- Nilsen, S.K.; Dahl, I.; Jhrgensen, O.; Schneider, T. Micro-Fibre and Ultra-Micro-Fibre Cloths, their Physical Characteristics, Cleaning Effect, Abrasion on Surfaces, Friction, and Wear Resistance. 2002. Available online: www.elsevier.com/locate/buildenv (accessed on 2 August 2022).
- Smith, D.; Gillanders, S.; Holah, J.; Gush, C. Assessing the efficacy of different microfibre cloths at removing surface micro-organisms associated with healthcare-associated infections. J. Hosp. Infect. 2011, 78, 182–186. [Google Scholar] [CrossRef]
- Glover, R.E.; Smith, R.R.; Jones, M.V.; Jackson, S.K.; Rowlands, C.C. An EPR investigation of surfactant action on bacterial membranes. FEMS Microbiol. Lett. 1999, 177, 57–62. [Google Scholar] [CrossRef]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef]
- Copello, G.; Teves, S.; Degrossi, J.; D’Aquino, M.; Desimone, M.; Díaz, L.E. Proving the antimicrobial spectrum of an amphoteric surfactant-sol-gel coating: A food-borne pathogen study. J. Ind. Microbiol. Biotechnol. 2008, 35, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Cabassud, A.C.; Aptel, P. Bactericidal Properties of Peracetic Acid and Hydrogen Peroxide, Alone and in Combination, and Chlorine and Formaldehyde against Bacterial Water Strains. Available online: www.nrcresearchpress.com (accessed on 2 August 2022).
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/37456.html (accessed on 10 August 2022).
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/38498.html (accessed on 10 August 2022).
- ISO 14067:2018; Greenhouse Gases—Carbon Footprint of Products—Requirements and Guidelines for Quantification. 27. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71206.html (accessed on 10 August 2022).
- Product Category Rules Construction Products and Construction Services 2012:01, Version 2.2; Environdec (Stockholm, Sweden). 2017. Available online: www.environdec.com (accessed on 10 August 2022).
- Rutala, W.A.; Gergen, M.F.; Sickbert-Bennett, E.E.; Anderson, D.J.; Weber, D.J.; CDC Prevention Epicenters Program. Antimicrobial activity of a continuously active disinfectant against healthcare pathogens. Infect. Control Hosp. Epidemiol. 2019, 40, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Lo, C.-W.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; et al. SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein. Viruses 2021, 13, 942. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, R.; Buratto, M.; Marzola, M.; Trioschi, G.; Bandera, B.; Buffone, C.; Vogli, L.; Marconi, P. An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures. Sustainability 2022, 14, 11465. https://doi.org/10.3390/su141811465
Fontana R, Buratto M, Marzola M, Trioschi G, Bandera B, Buffone C, Vogli L, Marconi P. An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures. Sustainability. 2022; 14(18):11465. https://doi.org/10.3390/su141811465
Chicago/Turabian StyleFontana, Riccardo, Mattia Buratto, Marco Marzola, Giulia Trioschi, Beatrice Bandera, Cesare Buffone, Luciano Vogli, and Peggy Marconi. 2022. "An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures" Sustainability 14, no. 18: 11465. https://doi.org/10.3390/su141811465
APA StyleFontana, R., Buratto, M., Marzola, M., Trioschi, G., Bandera, B., Buffone, C., Vogli, L., & Marconi, P. (2022). An Evaluation of Hospital Cleaning Regimes—Microbiological Evaluation and LCA Analysis after Traditional and Sustainable/Green Procedures. Sustainability, 14(18), 11465. https://doi.org/10.3390/su141811465










