Abstract
The slaughterhouse industry produces large amounts of highly polluted wastewater which needs to be treated before being discharged water. Thus, this work was conducted to investigate the feasibility of treating slaughterhouse wastewater using combined chemical coagulation and electro-Fenton methods. We studied the effect of process parameters such as polyaluminum chloride (PAC) concentration (25, 50, 75, 100 mg/L) for chemical coagulation and hydrogen peroxide concentration (500, 1000, 1500, 2000, 2500, 3000, 4000 mg/L), the pH of the solution (3, 5, 7, 10), and the reaction time (5, 10, 15, 30, 45, 75, 120 min) and the voltage (10, 20, 30, 40 V) on the removal of chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), total Kjeldahl nitrogen (TKN), and fecal coliforms (FC). The optimum removal efficiency for the electro-Fenton process was obtained at PAC = 75 mg/L, reaction time = 75 min, pH = 3, H2O2 = 2500 mg/L, and V = 20 V, which resulted in the removal efficiency of 89.55% for COD, 88.88% for BOD, 91.27% for TSS, 69.23% for TKN, and 100% for FC. The findings demonstrated that combined chemical coagulation and electro-Fenton processes effectively and efficiently treat slaughterhouse wastewater. The results of this research can be used by competent authorities to increase the efficiency of slaughterhouse wastewater treatment and to protect the environment.
1. Introduction
With the development of urbanization and associated requirements, along with the advancements in industries and increasing demands, pollutants are discharged into the environment that cause health issues in the short or long term. Among them, protein production industries, such as slaughterhouses and associated wastewater, can cause notable damages to the environment [1]. Industrial slaughterhouses are one of the leading centers of meat products producing a high amount of wastewater with a high organic load, residual blood, animal feces, and fats [2]. In these units, the water consumption per dead animal is different depending on the animal, and the method used is 1–8 m3 [3]. The unsanitary disposal of slaughterhouse wastewater can cause serious adverse effects on the region’s public health and contamination of soil, air, agricultural products, and water [1,4]. Organic compounds in wastewater resulted in irreversible public health risks as they are primarily mutagenic, toxic, and endocrine-disruptors or have carcinogenic potential for humans, animals, and aquatic environments. Even low concentrations of organic pollutants are toxic and perilous [5,6]. The characteristics of slaughterhouse wastewater are different, which must be discharged into receiving sources based on the standards allowed by the Environmental Protection Agency [7]. Meanwhile, fats comprise about 40% of the total COD in beef slaughterhouse sewage; however, less than 1% of soluble COD and more than 67% of suspended COD in mixed effluent effluents were reported (poultry slaughterhouses and aviaries). While insoluble organics could prevent biological processes [8].
Indicator organisms are not potentially a health risk and usually not are pathogens. These indicators show the possible presence of pathogens. Due to the pathogenic bacteria’s capability to contaminate water, it is essential to control their availability to avoid health risks. FC as an index of the bacteria should be evaluated constantly [9,10].
The basic control of these units’ contamination is establishing wastewater treatment plants and strictly monitoring their operation. Different methods for treating slaughterhouse wastewater are activated sludge, stabilization ponds, anaerobic reactors, electrocoagulation, and soluble air flotation [11]. Aerobic treatment processes have limited applications due to the high energy consumption for aeration and the production of notable amounts of sludge. However, the anaerobic treatment of abattoir wastewater is often performed and mainly interrupted because of the accumulation of suspended solids and floating fats in the reactors, resulting in reducing methanogenic activity and biomass leaching. Although biological processes are occasionally operational and cost-effective, they are less favored due to the requirements of large areas, the long time of hydraulic retention, as well as excellent output biomass concentrations [1,12].
One of the new and widely used approaches to the elimination of contaminants is chemical coagulation and advanced oxidation processes [13]. Several recent studies have been carried out on the coagulation process and various coagulants [14,15]. In wastewater treatment, the chemical coagulation process is mainly used to remove colloidal materials that create turbidity and color. A key characteristic of wastewater coagulation is removing suspended solids (SS) besides organic matter. However, the highest efficiency of COD removal during the chemical coagulation process of slaughterhouse wastewater was 45–75%, achieved by adding aluminum salts, iron, and polymer compounds [15,16]. The PAC is also a pre-polymerized coagulant, which has recently been broadly used in water treatment in the United States, Canada, China, Italy, France, and the UK [17,18]. Innovative oxidation procedures use oxidizing, particularly hydroxyl radicals [19]. In advanced oxidation processes, wastewater compounds are decomposed rather than concentrated or transferred to another phase, resulting in no secondary waste [20].
Different Fenton processes are among the advanced oxidation processes. Divalent iron ions and hydrogen peroxide are used simultaneously in a Fenton process to decompose and eliminate contaminants [21]. In the Fenton process, a hydroxide radical is produced for oxidation while hydrogen peroxide and divalent iron ions exist. If electric current and iron electrodes are used instead of divalent iron ions in the Fenton process to produce divalent iron ions, the process is termed electro-Fenton [22]. This process has attracted researchers because of its lower relative cost, simple implementation and operation, and high efficiency [23,24]. Furthermore, the electro-Fenton method produces excess sludge with relatively good deposition properties [25]. There is an integration of Fenton and electrocoagulation processes for increasing the organic matter degradability in high-strength wastewater [26,27]. The electro-Fenton process efficiency depends on the oxygen diffusion rate, pH, temperature of the solution, the intensity of the applied current, the concentration of Fe2+, the concentration of hydrogen peroxide, state of charge, and distance between the electrodes. This technique investigates the removal of numerous organic contaminants, such as drugs, dyes, pesticides, insecticides, leachates, phenolic compounds, and similar compounds [28]. Generally, the electro-Fenton process is a valuable technology in wastewater treatment. Although slaughterhouse wastewater, sustainable environmental protection, and the need for extensive studies on various treatment methods are important, few studies have been performed in this regard. Research has revealed that hybrid processes are more efficient in treating slaughterhouse wastewater [29]. In the previous literature, it was found that hybrid processes such as the combined use of chemical coagulation and electro-Fenton for slaughterhouse wastewater treatment have not been reported. Thus, this study was conducted to assess the feasibility of treating wastewater of livestock slaughterhouses by integrating chemical coagulation processes and the electro-Fenton process.
2. Material and Methods
2.1. Sewage Sampling
Sewage sampling was carried out from the Pak Dam Slaughterhouse in Zahedan Desert from 30 cm from the surface of the sewage storage tank, respecting homogeneous conditions. The samples were immediately transferred to the laboratory and the initial parameters of TSS (Total suspended solids in water and wastewater) [30], TKN (the sum of organic nitrogen and ammonia nitrogen) [31], FC (based on the multi-tube fermentation method is performed in presumptive phase, confirmed phase, completed phase), and BOD (in this method, the required amount of oxygen for the oxidation of sewage organic matter is obtained by bacteria) were determined. By measuring the required amount of oxygen, the concentration of organic substances in wastewater that bacteria can oxidize is obtained (biodegradability) were analyzed. The amount of BOD is usually expressed based on five days at a temperature of 20 degrees Celsius) [32], and COD (COD is the amount of oxygen consumed by organic matter from boiling acid potassium dichromate solution, which represents the part of organic substances that can be decomposed under the test conditions) [33]. To measure COD, we mixed 2.5 mL of the sample with 1.5 mL of digestion solution and 3.5 mL of reagent and put it in the COD reactor at a temperature of 150 degrees Celsius for 2 h after cooling down. The samples were measured at a wavelength of 600 nm [34]. Samples were then stored in the laboratory refrigerator. As our research progressed, finding the optimal point for each variable, a 5 L sample was prepared to perform 115 experiments. The parameters studied in this research are provided in Table 1.

Table 1.
Research variables and their range.
2.2. Coagulation Test in Slaughterhouse Sewage
Chemical coagulation was conducted in the jar test apparatus (VELP TLT6 model), at room temperature, with specific concentrations of PAC, and normal pH of the sample, under rapid mixing settings (300 rpm) for 1 min, gentle mixing settings (50 rpm) for 20 min, and the settling time of 1 h. A sample was taken to evaluate and analyze the effect of poly aluminum chloride coagulant (at different doses) on the FC, TKN, TSS, BOD, and COD test factors. The coagulant concentration yielding the best removal efficiency was chosen as the optimal coagulant concentration. All of the experiments were done conforming to the Standard Methods for the Examination of Water and Wastewater [35].
2.3. Implementing the Electro-Fenton Process
After settling, the supernatant was separated from the top and located in the reactor. Then, the pH of the sample was set to the considered range. The H2O2 catalyst was added to the sample in definite amounts to initiate the electro-Fenton reactions. Finally, by making an electric current, the electrolysis of the solution was started to perform the electro-Fenton oxidation reaction [36,37]. Then, the COD, BOD, TSS, TKN, and FC were calculated using the necessary sample from the reactor at the specified times. The electro-Fenton schematic diagram is displayed in Figure 1.
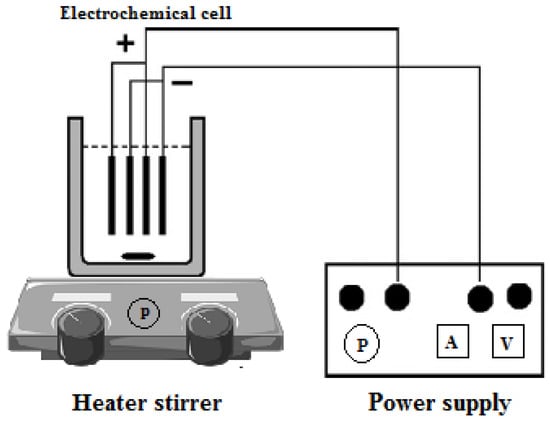
Figure 1.
The schematic view of the electro-Fenton system.
2.4. Measurement of BOD in Wastewater Samples
This is the main instrument for measuring biodegradable organic matter and is commonly employed for wastewater measurements. The organic matter concentration in the wastewater oxidizable by bacteria (biodegradability) is obtained by measuring the amount of oxygen required. The oxy-direct aqualytic sensor system is a six-sample system that facilitates precise measurement of BOD based on barometry. By respirometric approaches, it is possible to directly measure oxygen consumed by microorganisms from the air or oxygen-rich environment at a fixed temperature in a closed container and flow conditions. The system’s pressure drop is directly proportional to the amount of BOD. It is measured by the BOD aqualytic sensor and exhibited directly in mg/L. BOD was measured in the laboratory using the BOD meter (WTW BSB-Controller Model 620 T). The BOD meter was placed in the incubator to keep the temperature conditions constant during the measurement.
2.5. COD Test
For the COD measurement, the sample (2.5 mL) was mixed with reagent (3.5 mL) and digestion solution (1.5 mL) and put in a COD reactor for two hours at 150 °C. After cooling, the absorbance was read at 600 nm with the DR5000 spectrophotometer (DR/5000, HACH, USA).
2.6. TKN Test
TKN is the sum of organic and ammoniacal nitrogen. After adding 50 mL of the diluted sample to borate buffer (3 mL), the pH was set to 9.5 with NaOH (6 N). The sample was transferred to a 100 mL Kjeldahl vessel and then transported to the digestion phase, where the outlet from the distillation unit determined the Kjeldahl nitrogen. Then, 10 mL of digestion reagent was added to the container comprising the sample along with five or six glass pearls. After washing the samples, the digested sample was transferred to a micro-Kjeldahl distillation tool. A boric acid reagent was used to determine the end of the titration. The collected sample was titrated with 0.02 normal sulfuric acid. All of these operations were also conducted for a blank sample [36].
2.7. TSS Test
To measure the amount of TSS in the effluent sample, the perfectly uniform sample (50 mL) was filtered using filter paper, which was dried at 103 °C to 105 °C and weighed in a flat Buchner funnel. The filter paper was dried entirely and weighed from 103 °C to 105 °C. Using a Buchner funnel, wastewater (50 mL) was filtered using a weighed filter, and the filter was dried and weighed at 103 °C to 105 °C. The TSS value was then obtained using Equation (1).
where W2 (mg) is the weight of the filter paper containing the unfiltered sample, W1 (mg) shows the filter containing the filtered sample, and V (L) is the sample size.
2.8. Testing for FC
The standard test was conducted to identify the coliform group based on the stages of presumptive, confirmed, and completed multi-tube fermentation. When several tubes with different concentrations of samples are used in the fermentation test, the test results are the most probable number. Based on presumptive formulas, this number provides an estimate of the average density of coliforms in the studied sample. [38]. The accuracy of each test depends on the number of tubes used. Adequate information will be obtained when the highest concentrations of the sample in some or all tubes show the gas formation and the lowest sample concentrations do not show gas formation in all or most of the tubes. Bacterial density in samples can be determined with the help of specific formulas or by using tables based on the number of positive tubes in several different concentrations. The number of volumes removed from the sample (or the dilution coefficients for the samples) depends on the desired accuracy of the work results. The MPN table is prepared based on the assumption of Poisson distribution (random distribution) [38].
3. Results and Discussion
3.1. Characteristics of the Studied Wastewater
In each sampling, wastewater was assessed concerning TSS, BOD, TKN, COD, and FC parameters. Characteristics of wastewater obtained from the slaughtering process are displayed in Table 2.

Table 2.
The properties of raw samples from the slaughterhouse.
Researchers worldwide consider slaughterhouse wastewater unsafe because it is composed of proteins, fats, high organic content, fibers, pharmaceuticals, and veterinary pathogens. Table 2 shows that the effluent from Zahedan Slaughterhouse contained TSS, BOD, COD, TKN, and FC for 1108.6 ± 102.6, 2710 ± 159.6, 5238.8 ± 416.6, 211.4 ± 6.8 mg/L, 9.2 × 106. The high blood quantity is the main source of pollution in slaughterhouse wastewater [39]. Thus, it is essential to have an efficient treatment system so that the high blood content of organic matter is removed, and it should be according to the standards and requirements approved by environmental legislation for preventing ecosystem damage. Blood, a critical pollutant dissolved in slaughterhouse wastewater, shows a COD of 375,000 mg/L [40]. Also, slaughterhouse wastewater encompasses high concentrations with a high FC load in the wastewater.
3.2. Effect of Coagulating Agent Concentration on Electro-Fenton Process Efficiency
The removal efficiency of the parameters FC, TKN, TSS, BOD, and COD as a coagulating agent used is shown in Figure 2.
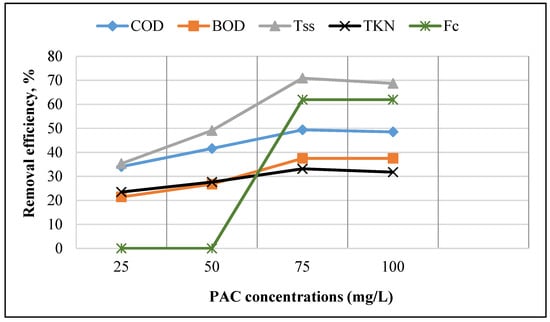
Figure 2.
Different concentrations of PAC affect the removal of FC, TKN, TSS, BOD, and COD.
Figure 2 indicates that as the coagulant concentration increases, the removal efficiency of FC, TKN, TSS, COD, and BOD increases. When coagulant concentration increased to 75 mg/L, the removal efficiency was increased by 61.95, 33.17, 70.93, 37.5, and 49.38% for FC, TKN, and TSS, BOD, and COD, respectively. Nevertheless, by increasing the initial concentration of the samples to 100 mg/L, the removal efficiencies decreased to 31.79, 68.72, and 48.54% for TKN, TSS, and COD, respectively. Since the best efficiency was achieved at the PAC’s 75 mg/L concentration, this was considered the optimal concentration for further tests. Bazrafshan et al. used a PAC concentration of 25–100 mg in their study, indicating that the removal efficiency was improved with a rising coagulant dose [32]. However, in the present study, the removal efficiency improved by elevating the coagulation dose to a specific concentration, but then the removal efficiency decreased. This reduction in efficiency by increasing the concentration of coagulant may be related to the re-instabilization of clots generated in the sample and the accumulation of coagulant. Zakeri et al., using a PAC at concentrations of 25–100 mg/L, found that by increasing the PAC concentration by 100 mg /L, the percentages of TSS (55.8) and BOD5 (42.8), and COD (49.5) removal increased [41].
3.3. Impact of Time Changes on Electro-Fenton Process Efficiency
The FC, TKN, TSS, BOD, and COD of wastewater discharged from the chemical coagulation phase and entering the electro-Fenton phase was 3.5 × 106, 141 (mg/L), 308 (mg/L), 1600 (mg/L), and (2688 mg/L). The time changes based on the removal rates of FC, TKN, TSS, BOD, and COD with constant variables pH = 3, H2O2 = 2000 mg/L, and Voltage = 20 V, which indicates that increasing the duration of the electro-Fenton process increases the removal efficiency up to an equilibrium level around 120 min. (Figure 3).
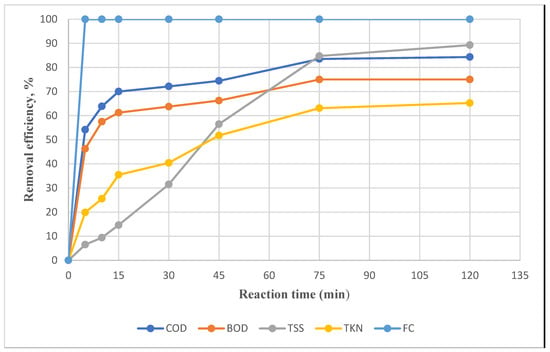
Figure 3.
Impact of time on removal efficiency in the electro-Fenton process.
Considering Figure 3, with increased duration of the electro-Fenton process, the removal efficiency increased so that FC was removed by 100% at t = 5 min. During the first 15 min of the electro-Fenton process, the removal rate is highest, decreasing over time. However, it still has an upward trend and reaches a maximum at t = 75 min. The diagram shows that the removal values of FC, TKN, TSS, BOD, and COD are 100, 63.12, 84.74, 75, and 83.51, respectively, over 75 min. Thus, compared to the time of 120 min and the removal values of 100%, 65.24%, 89.28%, 75%, and 84.33%, there is a slight difference in the removal of contaminants over 45 min. “Organic pollutants with their resistant compounds are likely to cause this, compounds that cannot be broken down by the oxidants produced during the electro-Fenton process [42]. Heidari et al. and Yang et al. found an increase in removal efficiency with increasing time and obtained the optimal time of 34.49 and 45 min, respectively [43,44]. Classifying the electro-Fenton process into two phases concerning the material’s decomposition rate is possible. In the first phase, hydroxyl radicals with high reactivity and considerable oxidizing ability are produced by the presence of Fe2+ and H2O2. In the second phase, the removal efficiency is reduced over time, decreasing the concentrations of Fe2+ and H2O2, producing oxidizing agents with low oxidizing ability, such as HO2 *, and increasing the pH, which causes the production of Fe (OH)n clots with the slow dissolution of the absorbed organic matter [45].
3.4. Impact of pH Changes on Electro-Fenton Process Efficiency
After the coagulation and sedimentation stage, the resulting effluent is fed into the electro-Fenton reactor to obtain the ideal pH. The values of parameters measured for the sample entered at the time of the ideal pH was FC (3.5 × 106) number, TKN (149 mg/L), TSS (330 mg/L), BOD (1750 mg/L), and COD (2937 mg/L). Figure 4 shows the impact of pH changes on the removal of FC, TSS, TKN, COD, and BOD.
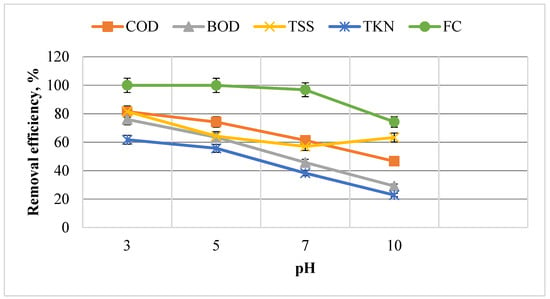
Figure 4.
Impact of pH changes on the removal of FC, TKN, TSS, BOD, and COD with the constant variables of H2O2 = 2000 mg, Voltage = 20 V, and t = 75 min.
As shown in Figure 4, the removal efficiency decreases with increasing pH, and the greatest removal efficiency is achieved at pH = 3. The removal percentages of FC, TKN, TSS, BOD, and COD at pH = 3 are 100, 61.74, 81.51, 76, and 81.47%, respectively. Jurate Virkutyte studied TOC and nitrate removal from aqueous media via the electro-Fenton process and found the optimum pH to be 2.2 [46]. Generally, the Fenton process occurs under moderately acidic conditions [37]. An increase in pH from 3 to 10 reduces removal efficiency due to reduced OH * oxidation potential. These values are 2.8 to 1.9 V at a pH of 3 to 7 [47]. At pH > 3, especially above 5, unstable H2O2 in solution is converted to water and oxygen with a constant coefficient. This conversion factor at pH of 7 and 10.5 is min and , respectively [48,49]. At pHs above 7, the hydroxyl radical is rapidly converted to *O−, which reacts even more slowly than OH * [47]. At higher pH, the ferrous ion precipitates as Fe(OH)3, thus inhibiting the reaction between Fe2+ and H2O2. Therefore, the reproduction of Fe2+ is reduced, which is essential for the efficiency of the process. When the pH is highly alkaline, decomposition of H2O2 is prevented due to the absence of H+. Thus, the production of hydroxyl radicals is reduced without significantly decomposing the contaminants. Besides, Fe(OH)3 produced at high pH accelerates the decomposition of H2O2 into O2 and H2O, which decreases the generation of OH radicals and, ultimately, the electro-Fenton process efficiency [50]. Fard et al. employed the Fenton as a post-treatment of a UASB reactor to remove TCOD and phosphate from the slaughterhouse. Under optimum conditions, pH = 3, 1000 mg/L of H2O2 and 400 mg/L of Fe (II), the removal efficiencies of TCOD and phosphate reached 95.41 and 85.29%, but The combined method removed TCOD and phosphate up to 98.6 and 90.5%, respectively [51].
3.5. Effect of Voltage Changes on Electro-Fenton Process Efficiency
The FC, TKN, TSS, BOD, and COD features of the reactor inlet effluent to obtain the optimal electro-Fenton voltage was 3.5 × 106, 140, 295, 1550, and 2507 mg/L, respectively. The voltage effect on the removal efficiency in the electro-Fenton process is shown in Figure 5.
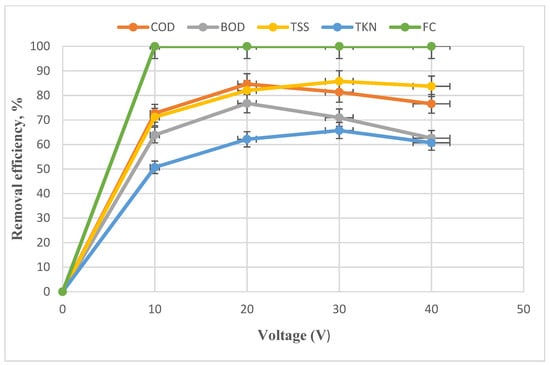
Figure 5.
Impact of voltage changes on the removal of FC, TKN, BOD, COD, and TSS with the constant variables of H2O2 = 2000 mg/L, t = 75 min, and pH = 3.
As shown in Figure 5, the pollutants’ removal efficiency increases with elevating voltage up to 20 V. Nevertheless, the BOD and COD removal efficiency decreased by elevating the voltage to 30 V. Then, with increasing the voltage to 40 V, there was a significant reduction in the efficiency of the process, except for FC. In the studies by Li, by increasing the voltage, the removal efficiency decreased concerning the optimal removal efficiency [52]. The important point in process efficiency is that energy consumption increases with increasing voltage, which is not economically favored. Voltage is a chief reason for oxygen reduction, which results in hydrogen peroxide production at the cathode. At higher voltages, the amount of the produced hydrogen peroxide is increased. Hence, the number of highly active hydroxyl radicals responsible for decomposition increases [53,54]. Moreover, the higher electrical production of Fe2+ ferrous ions than Fe3+ ferric ions (Equation (2)) causes an increase in the efficiency of Fenton cycle reactions by raising the voltage. At high voltages, the electro-Fenton process efficiency will decrease because of the competitive reactions of the electrode in the electrolyte cell. Oxygen discharge at the anode (Equation (3)) and hydrogen decreases at the cathode (Equation (4)) occur at high voltages. These reactions prevent the main reactions (Equation (5)), thus reducing the efficiency of the electro-Fenton [55,56,57].
Fe3+ + e− → Fe2+
2H2O → 4H+ + O2 + 4e−
2H+ +2e−→ H2
H2O → OH * + H+ + e−
3.6. Impact of the Hydrogen Peroxide Concentration Changes on Electro-Fenton Process Efficiency
Here, the effect of H2O2 concentration was investigated along with other optimal parameters obtained from the previous steps to determine its optimum concentration. FC (3.5 × 106), TKN (143 mg/L), TSS (298 mg/L), BOD (1700 mg/L), and COD (2767 mg/L) represent the characteristics of the effluent entering the reactor at this stage. The H2O2 impact on the removal efficiency in the electro-Fenton process is shown in Figure 6.
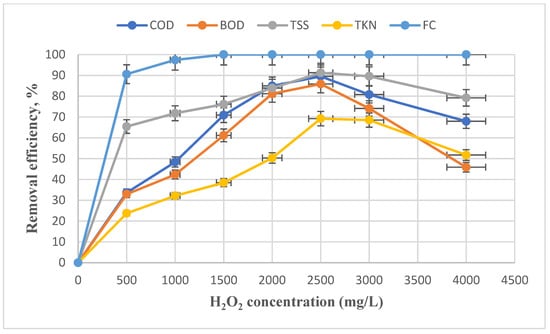
Figure 6.
Effect of concentration changes in hydrogen peroxide on removing FC, TKN, BOD, COD, and TSS with constant parameters of Voltage = 30 V, t = 75 min, and pH = 3.
In the electro-Fenton process, it is necessary to determine the optimal concentration of H2O2 for the removal efficiency and especially economic applicability related to H2O2 costs. The hydrogen peroxide effect on removal efficiency in the electro-Fenton process is shown in Figure 6. It is observed that with rising H2O2 concentration, the removal efficiency is enhanced. At an H2O2 concentration of 2500 mg/ L, the removal efficiencies for FC, TKN, TSS, BOD, and COD were 100, 69.23, 91.27, 85.88, and 89.55%, respectively, indicating the maximum removal of contaminants at that concentration. Thus, the removal efficiency decreased with increasing concentration. A similar result was observed by Nidheesh and Rajan and Xavier et al. [58,59]. The difference in the optimal concentration of our study with these studies is the type and nature of the pollutants and the higher contamination load of the slaughterhouse effluent. It should be stated that in these studies, the removal efficiency decreased by raising the hydrogen peroxide concentration above the optimal concentration. With the extra amounts of H2O2, hydroxyl radicals (HO2 *) are generated with less reactivity than OH * (Equations (6) and (7)). Moreover, the OH * radical is neutralized or repelled by Fe2+ (Equation (8)) [60,61]. It is noteworthy that the oxidation potential of hydroperoxyl radicals (1.25 eV) is lower than H2O2 (1.3 eV) [62].
H2O2 + OH * → H2O +HO2 *
H2O2 + Fe3+ → Fe2+ + H+ +HO2 *
Fe2+ + OH * → Fe3+ + OH¯
Thus, a reduced ability to access active oxidizing species at higher H2O2 concentrations results in the reduced removal of contaminants [63]. Excess H2O2 acts as an OH * scavenger. Therefore, its increased concentration leads to a reduced concentration of the hydroxyl raptor. Furthermore, excess H2O2 remaining in the solution following purification results in toxicity [64,65].
Generally, we observed that increasing the reaction time at t = 75 min did not change the removal efficiency due to the presence of resistant organic compounds in the sample. Moreover, decreased removal efficiency was found due to the increased concentration of PAC (more than 75 mg/L due to clot destabilization), H2O2 (>2500 mg/L caused by radical abduction of OH * by excess H2O2), pH (>3 due to the occurrence of adverse reactions), and voltage (>20 V due to the production of hydrogen peroxide).
Table 3 compares the efficiency of other methods with the electro-Fenton process for the destruction of slaughterhouse wastewater.

Table 3.
The efficiency of different methods for the degradation of industrial wastewater.
4. Conclusions
The combined chemical coagulation and electro-Fenton processes were studied by measuring the influent and effluent concentrations of COD, BOD, TSS, TKN, and FC of slaughterhouse wastewater. Under optimum conditions, the highest and lowest removal efficiency was related to FC (100%) and TKN (69.23%). In this study, effluent characteristics for FC, TKN, TSS, BOD, COD were 0, 44, 26, 240, and 289 mg/L, respectively. These values are still far from environmental standards. However, the process has very suitable convenience and speed. On the other hand, under optimal conditions, the final FC of the treated wastewater is zero, which is appropriate for release into the environment. Electricity was used as a source of clean energy in this process. Thus, no secondary pollutants were generated in the entire process. No harmful reagents are used in the electro-Fenton process; it is considered an eco-friendly water and wastewater treatment technique.
Author Contributions
E.B.: Designed the study, performed the analysis, and drafted the manuscript; H.R.Z.: Investigation, Conceptualization, Methodology, Visualization; M.G.A.V. and Z.D.: Contributed to study design, and data interpretation, revised the manuscript and provided critical comments; L.M., A.M. and A.M.K.: Conceptualization, Writing—review and editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The funding of this project (ID: 6687) was supported by Zahedan University of Medical Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the cooperation of Zahedan University of Medical Sciences.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ng, M.; Dalhatou, S.; Wilson, J.; Kamdem, B.P.; Temitope, M.B.; Paumo, H.K.; Djelal, H.; Assadi, A.A.; Nguyen-Tri, P.; Kane, A. Characterization of Slaughterhouse Wastewater and Development of Treatment Techniques: A Review. Processes 2022, 10, 1300. [Google Scholar] [CrossRef]
- Fatima, F.; Du, H.; Kommalapati, R. Treatment of Poultry Slaughterhouse Wastewater with Membrane Technologies: A Review. Water 2021, 13, 1905. [Google Scholar] [CrossRef]
- Louvet, J.; Homeky, B.; Casellas, M.; Pons, M.; Dagot, C. Monitoring of slaughterhouse wastewater biodegradation in a SBR using fluorescence and UV–Visible absorbance. Chemosphere 2013, 91, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.; Ma, N.L.; Rupani, P.F.; Sultana, N.; Yaakob, M.A.; Mohamed, R.M.S.R.; Soon, C.F. Biowastes of slaughterhouses and wet markets: An overview of waste management for disease prevention. Environ. Sci. Pollut. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Dong, F.; Lin, Q.; Li, C.; He, G.; Deng, Y. Impacts of pre-oxidation on the formation of disinfection byproducts from algal organic matter in subsequent chlor(am)ination: A review. Sci. Total Environ. 2020, 754, 141955. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Valladão, A.; Torres, A.; Freire, D.; Cammarota, M. Profiles of fatty acids and triacylglycerols and their influence on the anaerobic biodegradability of effluents from poultry slaughterhouse. Bioresour. Technol. 2011, 102, 7043–7050. [Google Scholar] [CrossRef]
- Rodrigues, C.; Cunha, M. Assessment of the microbiological quality of recreational waters: Indicators and methods. Euro-Mediterr. J. Environ. Integr. 2017, 2, 25. [Google Scholar] [CrossRef]
- Javaid, M.; Qasim, H.; Zia, H.Z.; Bashir, M.A.; Qayyoum, A.; Samiullah, K.; Hashem, M.; Morsy, K.; Dajem, S.B.; Alshehri, M.A.; et al. Bacteriological composition of groundwater and its role in human health. J. King Saud Univ. -Sci. 2022, 34, 102128. [Google Scholar] [CrossRef]
- Musa, M.; Idrus, S. Physical and Biological Treatment Technologies of Slaughterhouse Wastewater: A Review. Sustainability 2021, 13, 4656. [Google Scholar] [CrossRef]
- Baker, B.R.; Mohamed, R.; Al-Gheethi, A.; Aziz, H.A. Advanced technologies for poultry slaughterhouse wastewater treatment: A systematic review. J. Dispers. Sci. Technol. 2020, 42, 880–899. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Fernandes, F.; Madeira, L.; Luz, S.; Albuquerque, A.; Simões, R.; Beltrán, F.; Jerónimo, E.; Rivas, J. Treatment of slaughterhouse wastewater by acid precipitation (H2SO4, HCl and HNO3) and oxidation (Ca(ClO)2, H2O2 and CaO2). J. Environ. Manag. 2019, 250, 109558. [Google Scholar] [CrossRef]
- Gökçek, B.; Özdemir, S. Optimization of the Coagulation–Flocculation Process for Slaughterhouse Wastewater Using Response Surface Methodology. CLEAN—Soil Air Water 2020, 48, 2000033. [Google Scholar] [CrossRef]
- Abouelenien, F.; Trabik, Y.A.; Shukry, M.; El-Sharnouby, M.; Sayed, S.; Gaber, A.; Elsaidy, N.R. A Pilot Model for the Treatment of Slaughterhouse Wastewater Using Zeolite or Psidium-Leaf Powder as a Natural Coagulant, Followed by Filtration with Rice Straw, in Comparison with an Inorganic Coagulant. Processes 2022, 10, 887. [Google Scholar] [CrossRef]
- Reátegui-Romero, W.; Tuesta-Tinoco, S.A.; De la Cruz, C.E.O.; Huamán-Ccopa, J.A.; King-Santos, M.E.; Estrada-Huamaní, E.F.; Bulege-Gutierrez, W.; Yuli-Posadas, R.A.; Fernández-Guzmán, V. Electrocoagulation in batch mode for the removal of the chemical oxygen demand of an effluent from slaughterhouse wastewater in Lima Peru: Fe and Al electrodes. Desalination Water Treat. 2020, 201, 206–218. [Google Scholar] [CrossRef]
- Islam, M.R.; Mostafa, M.G. Removal Of A Reactive Dye From Synthetic Wastewater Using Pac And FeCl3 Coagulants. J. Life Earth Sci. 2021, 13, 39–44. Available online: http://banglajol.info.index.php/JLES (accessed on 15 August 2022).
- Asselin, M.; Drogui, P.; Benmoussa, H.; Blais, J.-F. Effectiveness of electrocoagulation process in removing organic compounds from slaughterhouse wastewater using monopolar and bipolar electrolytic cells. Chemosphere 2008, 72, 1727–1733. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, L.; Igwegbe, C.A.; Rahdar, S.; Banach, A.M. Application of response surface methodology in the degradation of Reactive Blue 19 using H2O2/MgO nanoparticles advanced oxidation process. Int. J. Ind. Chem. 2018, 9, 241–253. [Google Scholar] [CrossRef]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A. Application of Heterogeneous Catalytic Ozonation Process with Magnesium Oxide Nanoparticles for Toluene Degradation in Aqueous Environments. Health Scope 2016, 5, e40439. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-T.; Teng, Y.-J.; Dai, B.-H.; Tsao, I.-Y.; Lin, W.-C.; Wang, K.-W.; Hsu, L.-C.; Chang, Y.-C.; Li, C.-T.; Nguyen, H.T.T.; et al. Novel high-entropy ceramic/carbon composite materials for the decomposition of organic pollutants. Mater. Chem. Phys. 2021, 275, 125274. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current advances and trends in electro-Fenton process using heterogeneous catalysts–A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Nadais, H.; Li, X.; Alves, N.; Couras, C.; Andersen, H.R.; Angelidaki, I.; Zhang, Y. Bio-electro-Fenton process for the degradation of Non-Steroidal Anti-Inflammatory Drugs in wastewater. Chem. Eng. J. 2018, 338, 401–410. [Google Scholar] [CrossRef]
- Alavi, N.; Dehvari, M.; Alekhamis, G.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Application of electro-Fenton process for treatment of composting plant leachate: Kinetics, operational parameters and modeling. J. Environ. Health Sci. Eng. 2019, 17, 417–431. [Google Scholar] [CrossRef]
- Dindaş, G.B.; Çalışkan, Y.; Çelebi, E.E.; Tekbaş, M.; Bektaş, N.; Yatmaz, H.C. Treatment of pharmaceutical wastewater by combination of electrocoagulation, electro-fenton and photocatalytic oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103777. [Google Scholar] [CrossRef]
- Mohajeri, S.; Hamidi, A.A.; Isa, M.H.; Zahed, M.A. Landfill Leachate Treatment through Electro-Fenton Oxidation. Pollution 2019, 5, 199–209. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: Electrical energy consumption and biodegradability improvement. J. Environ. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Asfaha, Y.G.; Tekile, A.K.; Zewge, F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean. Eng. Technol. 2021, 4, 100261. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Farzadkia, M.; Ownagh, K.A.; Mahvi, A.H. Slaughterhouse Wastewater Treatment by Combined Chemical Coagulation and Electrocoagulation Process. PLoS ONE 2012, 7, e40108. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Ahmadi, S. Removal COD of landfill leachate using a coagulation and activated tea waste (ZnCL2) adsorption. IJISET-Int. J. Innov. Sci. Eng. Technol. 2017, 4, 339–347. Available online: www.ijiset.com (accessed on 15 August 2022).
- Bazrafshan, E.; Mostafapour, F.K.; Alizadeh, M.; Farzadkia, M. Dairy wastewater treatment by chemical coagulation and adsorption on modified dried activated sludge: A pilot-plant study. Desalin. Water Treat. 2015, 57, 8183–8193. [Google Scholar] [CrossRef]
- Bartram, J.; Ballance, R. (Eds.) Water Quality Monitoring: A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programs; Chapman and Hall: London, UK, 1996; Available online: https://apps.who.int/iris/handle/10665/41851 (accessed on 15 August 2022).
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalination Water Treat. 2015, 57, 9203–9215. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A. Standard Methods for The Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Lotfi, K.; Bonakdari, H.; Ebtehaj, I.; Delatolla, R.; Zinatizadeh, A.A.; Gharabaghi, B. A novel stochastic wastewater quality modeling based on fuzzy techniques. J. Environ. Health Sci. Eng. 2020, 18, 1099–1120. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhou, Z. Electro-Fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Farzadkia, M.; Vanani, A.F.; Golbaz, S.; Sajadi, H.S.; Bazrafshan, E. Characterization and evaluation of treatability of wastewater generated in Khuzestan livestock slaughterhouses and assessing of their wastewater treatment systems. Glob. Nest J. 2016, 18, 108–118. [Google Scholar]
- Husam, A.-N.; Nassar, A. Slaughterhouses Wastewater Characteristics in the Gaza Strip. J. Water Resour. Prot. 2019, 11, 844–851. [Google Scholar] [CrossRef]
- Chowdhury, W.; Nabi, N.; Arefin, A.; Rashid, F.; Islam, M.T.; Gudimetla, P.; Muyeen, S. Recycling slaughterhouse wastes into potential energy and hydrogen sources: An approach for the future sustainable energy. Bioresour. Technol. Rep. 2022, 19, 101133. [Google Scholar] [CrossRef]
- Zakeri, H.R.; Yousefi, M.; Mohammadi, A.A.; Baziar, M.; Mojiri, S.A.; Salehnia, S.; Hosseinzadeh, A. Chemical coagulation-electro fenton as a superior combination process for treatment of dairy wastewater: Performance and modelling. Int. J. Environ. Sci. Technol. 2021, 18, 3929–3942. [Google Scholar] [CrossRef]
- Rastgar, M.; Karkooti, A.; Sohrabi, A.; Karami, P.; Nazemifard, N.; Sadrzadeh, M. Osmotic dewatering accelerates inherent sluggish kinetics of electro-Fenton process: Toward sustainable removal of organic contaminants. Chem. Eng. J. 2020, 394, 125043. [Google Scholar] [CrossRef]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of diazinon from aqueous solutions by electro-Fenton process: Effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Sep. Sci. Technol. 2020, 56, 2287–2299. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Wang, J.; Yuan, R.; Wang, F.; Zhou, B. Efficient degradation of diisobutyl phthalate in aqueous solution through electro-Fenton process with sacrificial anode. J. Environ. Chem. Eng. 2020, 8, 104057. [Google Scholar] [CrossRef]
- Atmaca, E. Treatment of landfill leachate by using electro-Fenton method. J. Hazard. Mater. 2009, 163, 109–114. [Google Scholar] [CrossRef]
- Virkutyte, J.; Jegatheesan, V. Electro-Fenton, hydrogenotrophic and Fe2+ ions mediated TOC and nitrate removal from aquaculture system: Different experimental strategies. Bioresour. Technol. 2009, 100, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Shemer, H.; Linden, K.G. Degradation and by-product formation of diazinon in water during UV and UV/H2O2 treatment. J. Hazard. Mater. 2006, 136, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lemley, A.T. Kinetic Model and Optimization of 2,4-D Degradation by Anodic Fenton Treatment. Environ. Sci. Technol. 2001, 35, 4509–4514. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.; Gilarranz, M.; Casas, J.; Rodriguez, J. Application of Fenton oxidation to cosmetic wastewaters treatment. J. Hazard. Mater. 2007, 143, 128–134. [Google Scholar] [CrossRef]
- Fard, M.B.; Mirbagheri, S.A.; Pendashteh, A. Removal of TCOD and phosphate from slaughterhouse wastewater using Fenton as a post-treatment of an UASB reactor. J. Environ. Health Sci. Eng. 2020, 18, 413–422. [Google Scholar] [CrossRef]
- Li, J.; Song, D.; Du, K.; Wang, Z.; Zhao, C. Performance of graphite felt as a cathode and anode in the electro-Fenton process. RSC Adv. 2019, 9, 38345–38354. [Google Scholar] [CrossRef]
- Xia, G.; Lu, Y.; Xu, H. An energy-saving production of hydrogen peroxide via oxygen reduction for electro-Fenton using electrochemically modified polyacrylonitrile-based carbon fiber brush cathode. Sep. Purif. Technol. 2015, 156, 553–560. [Google Scholar] [CrossRef]
- Malakootian, M.; Moridi, A. Efficiency of electro-Fenton process in removing Acid Red 18 dye from aqueous solutions. Process Saf. Environ. Prot. 2017, 111, 138–147. [Google Scholar] [CrossRef]
- Ghanbarloua, H.; Nasernejada, B.; Fini, M.N.; Simonsen, M.E.; Muff, J. Synthesis of an iron-graphene based particle electrode for pesticide removal in three-dimensional heterogeneous electro-Fenton water treatment system. Chem. Eng. J. 2020, 395, 125025. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Srinophakun, P.; Amat, S.; Saisriyoot, M. Decolorization of molasses-based distillery wastewater by means of pulse electro-Fenton process. J. Environ. Chem. Eng. 2017, 5, 2305–2312. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Rajan, R. Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon. RSC Adv. 2016, 6, 5330–5340. [Google Scholar] [CrossRef]
- Xavier, S.; Gandhimathi, R.; Nidheesh, P.V.; Ramesh, S.T. Comparison of homogeneous and heterogeneous Fenton processes for the removal of reactive dye Magenta MB from aqueous solution. Desalination Water Treat. 2013, 53, 109–118. [Google Scholar] [CrossRef]
- Sruthi, T.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P. Stabilized landfill leachate treatment using heterogeneous Fenton and electro-Fenton processes. Chemosphere 2018, 210, 38–43. [Google Scholar] [CrossRef]
- Mohanty, N.R.; Wei, I.W. Oxidation of 2,4-Dinitrotoluene Using Fenton’s Reagent: Reaction Mechanisms and Their Practical Applications. Hazard. Waste Hazard. Mater. 1993, 10, 171–183. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Li, S.; Guo, S.; Shen, L.; Zhou, T.; Zhong, H.; Wu, L.; Meng, Q.; Zhang, Y. Oxygen-Vacancy-Enhanced Peroxidase-like Activity of Reduced Co3O4 Nanocomposites for the Colorimetric Detection of H2O2 and Glucose. Inorg. Chem. 2020, 59, 3152–3159. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Iron(3) oxide-based nanoparticles as catalysts in advanced organic aqueous oxidation. Water Res. 2008, 42, 492–498. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical Pathway and Kinetics of Phenol Oxidation by Fenton’s Reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, X.; Li, X.; Yuan, Y. Performance of aerobic granular sludge in a sequencing batch bioreactor for slaughterhouse wastewater treatment. Bioresour. Technol. 2015, 190, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Puat, N.N.A.; Alazaiza, M.Y.D.; Hung, Y.-T. Poultry Slaughterhouse Wastewater Treatment Using Submerged Fibers in an Attached Growth Sequential Batch Reactor. Int. J. Environ. Res. Public Health 2018, 15, 1734. [Google Scholar] [CrossRef] [PubMed]
- Bingo, M.N.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Performance evaluation of an integrated multi-stage poultry slaughterhouse wastewater treatment system. J. Water Process Eng. 2021, 43, 102309. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Treatment of poultry slaughterhouse wastewater using tubular microfiltration membrane with fly ash as key precursor. J. Water Process Eng. 2020, 37, 101361. [Google Scholar] [CrossRef]
- del Real-Olvera, J.; Rustrian-Portilla, E.; Houbron, E.; Landa-Huerta, F.J. Adsorption of organic pollutants from slaughterhouse wastewater using powder of Moringa oleifera seeds as a natural coagulant. Desalination Water Treat. 2015, 57, 9971–9981. [Google Scholar] [CrossRef]
- Garduño-Pineda, L.; Solache-Ríos, M.J.; Martínez-Miranda, V.; Linares-Hernández, I.; Teutli-Sequeira, E.A.; Castillo-Suárez, L.A.; Soto, M.E. Photolysis and heterogeneous solar photo-Fenton for slaughterhouse wastewater treatment using an electrochemically modified zeolite as catalyst. Sep. Sci. Technol. 2021, 57, 822–841. [Google Scholar] [CrossRef]
- Davarnejad, R.; Nasiri, S. Slaughterhouse wastewater treatment using an advanced oxidation process: Optimization study. Environ. Pollut. 2017, 223, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).