Biomass Allocation, Root Spatial Distribution, and the Physiological Response of Dalbergia odorifera Seedlings in Simulated Shallow Karst Fissure-Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of the Plant Growth Index
2.3. Determination of Root Morphology and Root Weight Density
2.4. Determination of Plant Physiological Indexes
2.5. Statistical Analysis
3. Results
3.1. D. odorifera Plant Height and Ground Diameter Response to Different SKF-S Conditions
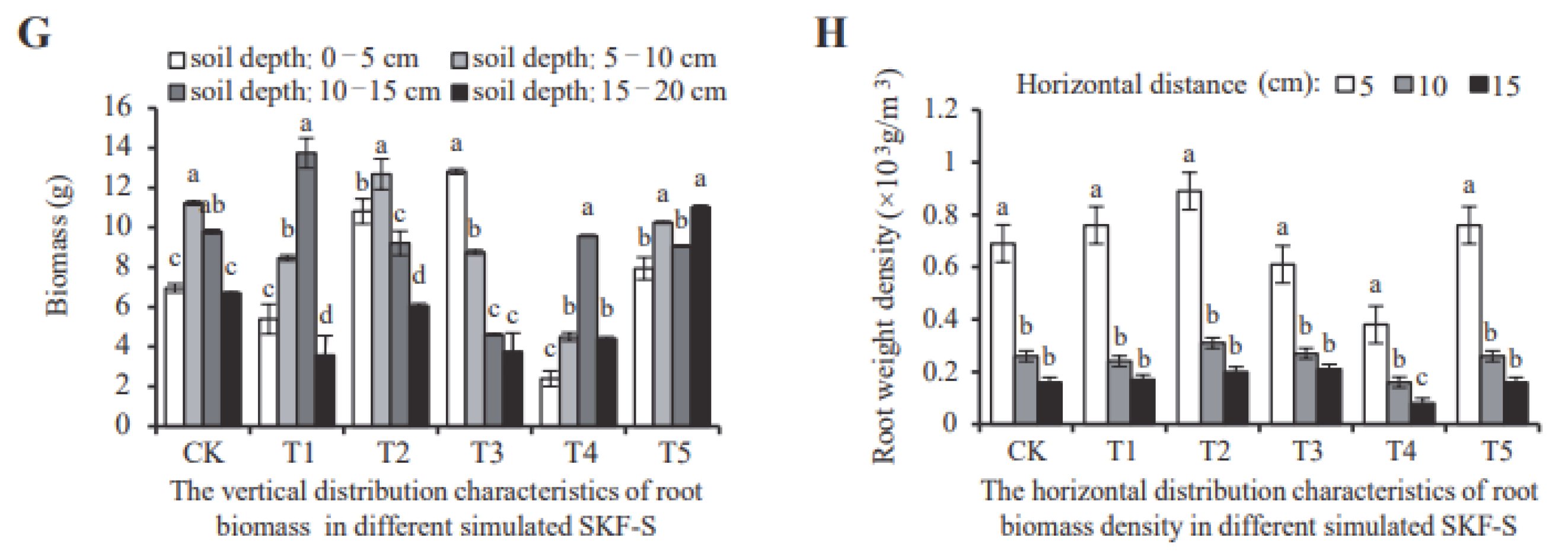
3.2. D. odorifera Biomass Allocation Response to Different SKF-S Conditions
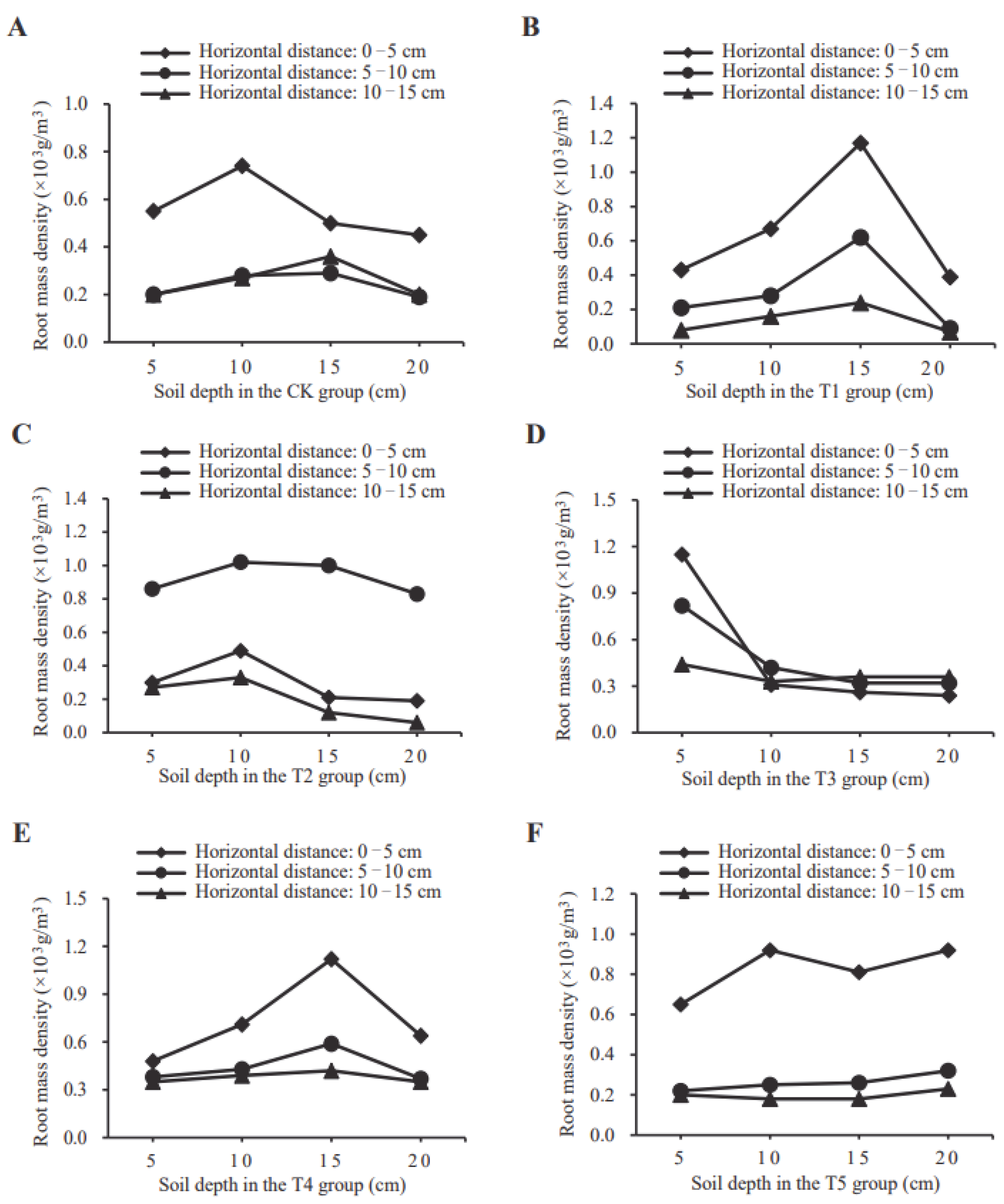
3.3. D. odorifera Root Spatial Distribution Response to Different SKF-S Conditions
3.4. Response of D. odorifera Root Morphological Characteristics to Different SKF-S Conditions
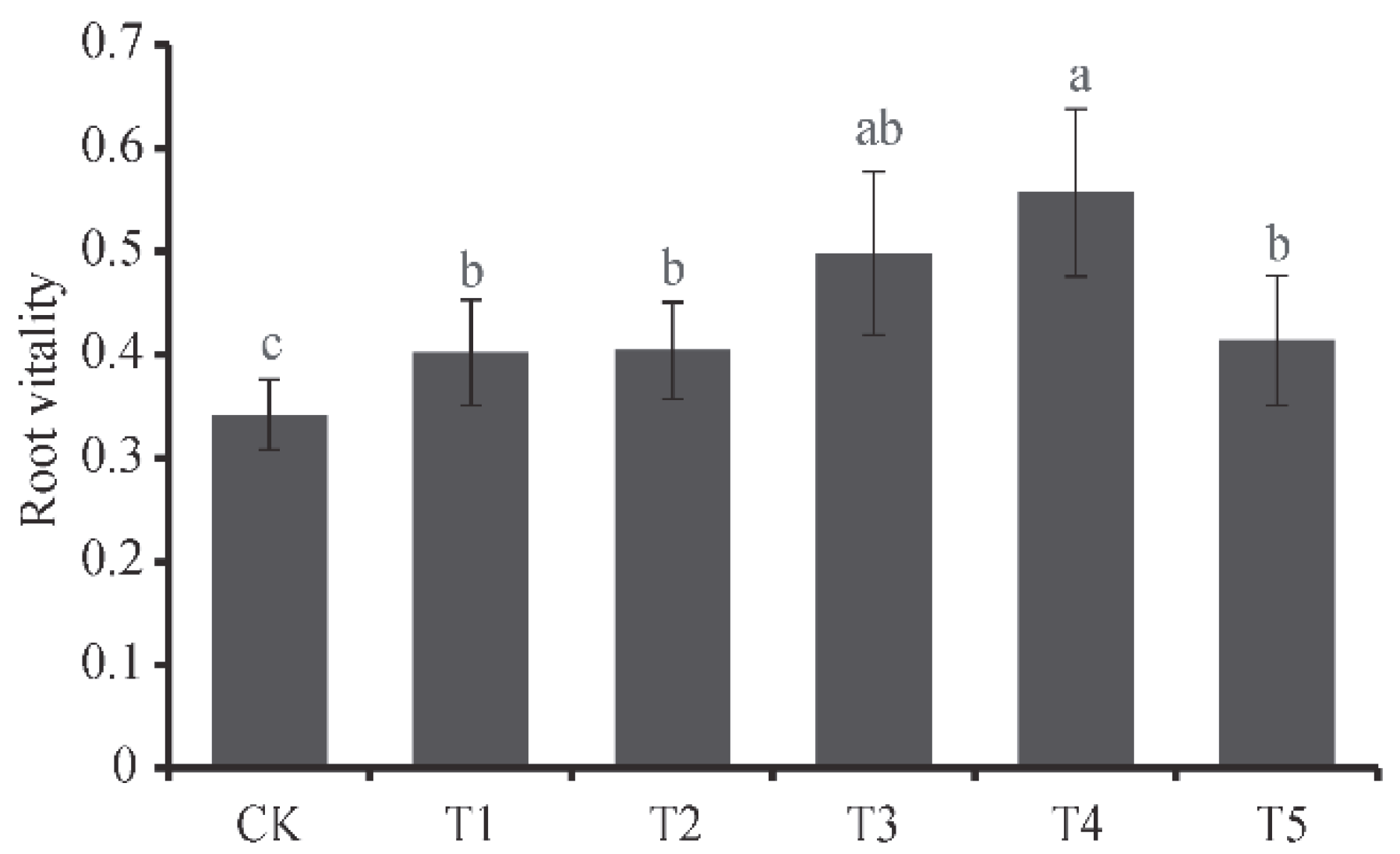
3.5. Response of D. odorifera Root System Physiological Indexes tο Different SKF-S Conditions
4. Discussion
4.1. Response of D. odorifera Root Growth and Biomass Allocation to Different Simulated SKF-S Conditions
4.2. Response of D. odorifera Root System Spatial Distribution to Different Simulated SKF-S Conditions
4.3. Response of D. odorifera Root Morphology to Different Simulated SKF-S Conditions
4.4. Response of D. odorifera Physiology to Different Simulated SKF-S Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Wang, S.-J.; Li, R.-L.; Sun, C.-X.; Zhang, D.-F.; Li, F.-Q.; Zhou, D.-Q.; Xiong, K.-N.; Zhou, Z.-F. How types of carbonate rock assemblages constrain the distribution of karst rocky desertified land in Guizhou Province, PR China: Phenomena and mechanisms. Land Degrad. Dev. 2004, 15, 123–131. [Google Scholar] [CrossRef]
- Li, S.; Ren, H.; Xue, L.; Chang, J.; Yao, X. Influence of bare rocks on surrounding soil moisture in the karst rocky desertification regions under drought conditions. Catena 2014, 116, 157–162. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Long, W.; Leng, X.; Wang, J.; Yao, X.; Li, S. Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply. Forests 2018, 9, 667. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Q.; Jin, L.; Wang, X. Geometric morphology and soil properties of shallow karst fissures in an area of karst rocky desertification in SW China. Catena 2019, 174, 48–58. [Google Scholar] [CrossRef]
- Wang, S.-J.; Liu, Q.-M.; Zhang, D.-F. Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Qi, X.; Wang, K.; Zhang, C. Effectiveness of ecological restoration projects in a karst region of southwest China assessed using vegetation succession mapping. Ecol. Eng. 2013, 54, 245–253. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Green, S.M.; Dungait, J.; Wen, X.; Tang, Y.; Guo, Z.; Yang, Y.; Sun, X.; Quine, T.A. Nitrogen functional gene activity in soil profiles under progressive vegetative recovery after abandonment of agriculture at the Puding Karst Critical Zone Observatory, SW China. Soil Biol. Biochem. 2018, 125, 93–102. [Google Scholar] [CrossRef]
- Delzon, S. New insight into leaf drought tolerance. Funct. Ecol. 2015, 29, 1247–1249. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xiong, K.; Yu, Y.; Min, X. Changes of leaf functional traits in karst rocky desertification ecological environment and the driving factors. Glob. Ecol. Conserv. 2020, 24, e01381. [Google Scholar] [CrossRef]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.-F.; Kitajima, K. Leaf trait variations associated with habitat affinity of tropical karst tree species. Ecol. Evol. 2017, 8, 286–295. [Google Scholar] [CrossRef]
- Qi, D.; Wieneke, X.; Zhou, X.; Jiang, X.; Xue, P. Succession of plant community composition and leaf functional traits in responding to karst rocky desertification in the Wushan County in Chongqing, China. Community Ecol. 2017, 18, 157–168. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Choat, B.; Sterck, F.; Maenpuen, P.; Katabuchi, M.; Zhang, S.; Tomlinson, K.W.; Oliveira, R.S.; Zhang, Y.; Shen, J.; et al. Hydraulic prediction of drought-induced plant dieback and top-kill depends on leaf habit and growth form. Ecol. Lett. 2021, 24, 2350–2363. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Jansen, S.; Cao, K. Topography strongly affects drought stress and xylem embolism resistance in woody plants from a karst forest in Southwest China. Funct. Ecol. 2021, 35, 566–577. [Google Scholar] [CrossRef]
- Ni, J.; Luo, D.H.; Xia, J.; Zhang, Z.H.; Hu, G. Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 2015, 6, 799–810. [Google Scholar] [CrossRef]
- Wu, Y.; Song, L.; Liu, W.; Liu, W.; Li, S.; Fu, P.; Shen, Y.; Wu, J.; Wang, P.; Chen, Q.; et al. Fog Water Is Important in Maintaining the Water Budgets of Vascular Epiphytes in an Asian Tropical Karst Forests during the Dry Season. Forests 2018, 9, 260. [Google Scholar] [CrossRef]
- Fu, P.-L.; Liu, W.-J.; Fan, Z.-X.; Cao, K.-F. Is fog an important water source for woody plants in an Asian tropical karst forest during the dry season? Ecohydrology 2016, 9, 964–972. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Johnson, D.M.; Domec, J.-C.; Berry, Z.C.; Schwantes, A.; McCulloh, K.A.; Woodruff, D.R.; Polley, H.W.; Wortemann, R.; Swenson, J.J.; Mackay, D.; et al. Co-occurring woody species have diverse hydraulic strategies and mortality rates during an extreme drought. Plant Cell Environ. 2018, 41, 576–588. [Google Scholar] [CrossRef]
- Crouchet, S.E.; Jensen, J.; Schwartz, B.F.; Schwinning, S. Tree Mortality after a Hot Drought: Distinguishing Density-Dependent and -Independent Drivers and Why It Matters. Front. For. Glob. Chang. 2019, 2, 21. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef]
- Arnold, P.A.; Kruuk, L.E.B.; Nicotra, A.B. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Weemstra, M.; Freschet, G.T.; Stokes, A.; Roumet, C. Patterns in intraspecific variation in root traits are species-specific along an elevation gradient. Funct. Ecol. 2021, 35, 342–356. [Google Scholar] [CrossRef]
- Salmela, M.J. Patterns of genetic diversity vary among shoot and root functional traits in Norway spruce Picea abies along a latitudinal gradient. Oikos 2021, 139, 1143–1157. [Google Scholar] [CrossRef]
- Kumordzi, B.B.; Aubin, I.; Cardou, F.; Shipley, B.; Violle, C.; Johnstone, J.; Anand, M.; Arsenault, A.; Bell, F.W.; Bergeron, Y.; et al. Geographic scale and disturbance influence intraspecific trait variability in leaves and roots of North American understorey plants. Funct. Ecol. 2019, 33, 1771–1784. [Google Scholar] [CrossRef]
- Colom, S.M.; Baucom, R.S. Below-ground competition favors character convergence but not character displacement in root traits. New Phytol. 2021, 229, 3195–3207. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, Y.; Song, X.; Zhang, C.; Ren, M.; Du, Y. Seasonal Differences in Water-Use Sources of Impatiens hainanensis (Balsaminaceae), a Limestone-Endemic Plant Based on “Fissure-Soil” Habitat Function. Sustainability 2021, 13, 8721. [Google Scholar] [CrossRef]
- Chang, J.; Zhu, J.; Xu, L.; Su, H.; Gao, Y.; Cai, X.; Peng, T.; Wen, X.; Zhang, J.; He, N. Rational land-use types in the karst regions of China: Insights from soil organic matter composition and stability. Catena 2018, 160, 345–353. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Guo, X.; Yang, H.; Zhang, Z.; Zhang, K. Effects of topographic factors on runoff and soil loss in Southwest China. Catena 2018, 160, 394–402. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X.; Zhou, Z.; Shao, C.; Su, S. Influence of Heterogeneous Karst Microhabitats on the Root Foraging Ability of Chinese Windmill Palm (Trachycarpus fortunei) Seedlings. Int. J. Environ. Res. Public Health 2020, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dai, Q.; Wang, X.; Jin, L.; Mei, L. Response of shallow karst fissure soil quality to secondary succession in a degraded karst area of southwestern China. Geoderma 2019, 348, 76–85. [Google Scholar] [CrossRef]
- Wang, J.; Zou, B.; Liu, Y.; Tang, Y.; Zhang, X.; Yang, P. Erosion-creep-collapse mechanism of underground soil loss for the karst rocky desertification in Chenqi village, Puding county, Guizhou, China. Environ. Earth Sci. 2014, 72, 2751–2764. [Google Scholar] [CrossRef]
- Nie, Y.-P.; Chen, H.-S.; Wang, K.-L.; Tan, W.; Deng, P.-Y.; Yang, J. Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 2011, 341, 399–412. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, G.; Xiong, K.; Cai, L. Dynamic variations in soil moisture in an epikarst fissure in the karst rocky desertification area. J. Hydrol. 2020, 591, 125587. [Google Scholar] [CrossRef]
- Peng, X.; Dai, Q.; Ding, G.; Shi, D.; Li, C. Impact of vegetation restoration on soil properties in near-surface fissures located in karst rocky desertification regions. Soil Tillage Res. 2020, 200, 104620. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Q.; Yang, Y.; Yan, L.; Yi, X. Epikarst shallow fissure soil systems are key to eliminating karst drought limitations in the karst rocky desertification area of SW China. Ecohydrology 2021, 15, e2372. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef]
- Wariss, H.M.; Yi, T.-S.; Wang, H.; Zhang, R. Characterization of the complete chloroplast genome of Dalbergia odorifera (Leguminosae), a rare and critically endangered legume endemic to China. Conserv. Genet. Resour. 2017, 10, 527–530. [Google Scholar] [CrossRef]
- Ma, R.K.; Luo, J.; Qiao, M.J.; Fu, Y.L.; Zhu, P.C.; Wei, P.L.; Liu, Z.G. Chemical composition of extracts from Dalbergia odorifera heartwood and its correlation with color. Ind. Crop. Prod. 2022, 180, 114728. [Google Scholar] [CrossRef]
- Li, M.; You, Y.; Tan, X.; Wen, Y.; Yu, S.; Xiao, N.; Shen, W.; Huang, X. Mixture of N2-fixing tree species promotes organic phosphorus accumulation and transformation in topsoil aggregates in a degraded karst region of subtropical China. Geoderma 2022, 413, 115752. [Google Scholar] [CrossRef]
- Zhou, X.G.; Sun, D.J.; Wen, Y.G.; Wang, L.; Ming, A.A.; Jia, H.Y.; Zhu, H.G.; Zhao, Y.Y.; Huang, Y.J.; Liang, J. Biomass, Productivity and Their Dynamic Changes of Different Restoration Stands in Karst Rocky Desertification. Guangxi Sci. 2022, 29, 98–107. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 2004, 166, 293–302. [Google Scholar] [CrossRef]
- Luo, Z.-B.; Calfapietra, C.; Scarascia-Mugnozza, G.; Liberloo, M.; Polle, A. Carbon-based secondary metabolites and internal nitrogen pools in Populus nigra under Free Air CO2 Enrichment (FACE) and nitrogen fertilisation. Plant Soil 2008, 304, 45–57. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Qu, W.J.; Li, X.F. Experimental Guidance for Plant Physiology; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Poorter, H.; Jagodzinski, A.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Freschet, G.T.; Swart, E.; Cornelissen, J.H.C. Integrated plant phenotypic responses to contrasting above- and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- He, C.X.; Huang, Y.Q.; Li, X.K.; Wang, X.Y.; Wang, Q. The ecophysiological traits of three karst rockey desert restoration species. Guihaia 2007, 27, 53–61. [Google Scholar]
- Ni, L.K.; Gu, D.X.; He, W.; Huang, Y.Q.; Chen, Z.Y. Research advances in plant ecological adaptability in karst area. Chin. J. Ecol. 2019, 38, 2210–2217. [Google Scholar] [CrossRef]
- Savi, T.; Petruzzellis, F.; Moretti, E.; Stenni, B.; Zini, L.; Martellos, S.; Lisjak, K.; Nardini, A. Grapevine water relations and rooting depth in karstic soils. Sci. Total Environ. 2019, 692, 669–675. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Abenavoli, M.R.; Leone, M.; Sunseri, F.; Bacchi, M.; Sorgonà, A. Root Phenotyping For Drought Tolerance in Bean Landraces From Calabria (Italy). J. Agron. Crop Sci. 2016, 202, 1–12. [Google Scholar] [CrossRef]



| Media | pH | Moisture (%) | Total Nitrogen (g·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) |
|---|---|---|---|---|---|
| 100% Loam | 6.23 | 33.50 | 2.18 | 3.72 | 55.33 |
| 50% Loam + 50% Gravel | 8.16 | 4.17 | 1.10 | 5.17 | 60.67 |
| 100% Gravel | 9.41 | 0.56 | 0.11 | 0.14 | 6.43 |
| Media | Groups | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Gravel layer (cm) | 0 | 5 | 10 | 15 | 20 | The fan-shaped simulation column |
| Loam layer (cm) | 20 | 15 | 10 | 5 | 0 | |
| Processing Group | 6 Months Later | 12 Months Later | ||
|---|---|---|---|---|
| Plant Height Relative Growth | Ground Diameter Relative Growth | Plant Height Relative Growth | Ground Diameter Relative Growth | |
| CK | 1.735 ± 0.058 a | 2.359 ± 0.133 a | 1.618 ± 0.063 a | 3.081 ± 0.091 a |
| T1 | 1.687 ± 0.093 a | 1.927 ± 0.056 b | 1.689 ± 0.047 a | 2.536 ± 0.081 b |
| T2 | 1.823 ± 0.075 a | 1.980 ± 0.051 b | 1.755 ± 0.117 a | 2.593 ± 0.106 b |
| T3 | 1.779 ± 0.064 a | 1.964 ± 0.045 b | 1.659 ± 0.083 a | 2.485 ± 0.125 b |
| T4 | 0.899 ± 0.071 b | 0.886 ± 0.032 c | 0.747 ± 0.039 b | 1.586 ± 0.049 c |
| T5 | 1.744 ± 0.077 a | 2.157 ± 0.124 a | 1.657 ± 0.043 a | 2.947 ± 0.076 a |
| Part | Groups | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Leaf (g) | 119.62 ± 5.74 a | 97.63 ± 4.11 ab | 118.59 ± 13.89 a | 75.83 ± 3.62 b | 49.43 ± 1.60 c | 108.16 ± 4.52 a |
| Stem (g) | 230.15 ± 11.53 a | 178.15 ± 13.88 ab | 214.11 ± 8.22 a | 112.96 ± 10.31 b | 34.19 ± 4.85 c | 218.14 ± 7.58 a |
| Level 1 Root (g) | 40.71 ± 2.68 a | 30.39 ± 1.67 b | 34.51 ± 1.23 ab | 14.83 ± 1.02 c | 14.83 ± 1.72 c | 41.42 ± 1.78 a |
| Level 2 Root (g) | 19.44 ± 1.33 ab | 20.99 ± 1.12 ab | 24.09 ± 3.19 a | 12.93 ± 1.17 b | 5.51 ± 0.32 c | 24.92 ± 1.45 a |
| Level 3 Root (g) | 29.73 ± 2.04 b | 29.42 ± 1.75 b | 39.55 ± 2.90 a | 35.74 ± 4.92 a | 12.69 ± 0.77 c | 31.64 ± 2.91 ab |
| Total Biomass (g) | 439.65 ± 22.91 a | 356.58 ± 18.97 b | 430.85 ± 9.57 a | 252.29 ± 7.26 c | 116.65 ± 4.00 d | 424.28 ± 9.41 a |
| Part | Groups | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Leaf (%) | 27.21 ± 0.71 b | 27.38 ± 0.57 b | 27.52 ± 1.10 b | 30.06 ± 1.01 b | 42.37 ± 1.14 a | 25.49 ± 0.52 b |
| Stem (%) | 52.35 ± 1.94 a | 49.96 ± 0.78 a | 49.69 ± 0.56 a | 44.77 ± 0.41 b | 29.31 ± 0.64 c | 51.41 ± 0.79 a |
| Level 1 Root (%) | 9.26 ± 0.77 a | 8.52 ± 0.43 b | 8.01 ± 0.50 b | 5.88 ± 0.57 c | 12.71 ± 0.45 a | 9.76 ± 0.36 ab |
| Level 2 Root (%) | 4.42 ± 0.27 a | 5.89 ± 0.48 a | 5.59 ± 0.27 a | 5.13 ± 0.26 a | 4.72 ± 0.18 a | 5.87 ± 0.22 a |
| Level 3 Root (%) | 6.76 ± 0.19 bc | 8.25 ± 0.44 ab | 9.18 ± 0.21 a | 14.17 ± 0.25 a | 10.88 ± 1.19 a | 7.46 ± 0.54 b |
| Total Root Systems (%) | 20.44 ± 0.76 b | 22.66 ± 0.94 ab | 22.78 ± 0.46 ab | 25.18 ± 0.73 ab | 28.31 ± 1.01 a | 23.09 ± 0.42 ab |
| Category | Groups | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Total Root Surface Area (cm2) | 1261.21 ± 47.61 b | 1253.35 ± 40.30 b | 1364.97 ± 53.49 ab | 918.7 ± 27.17 c | 996.94 ± 33.50 c | 1412.22 ± 68.98 a |
| Root Tip Number | 12,655 ± 239.17 ab | 12,099 ± 213.19 ab | 13,084 ± 255.11 a | 9981 ± 117.30 b | 7243 ± 109.98 c | 13,793 ± 305.26 a |
| Group | Root Length of Different Diameter Classes (cm) | ||||
|---|---|---|---|---|---|
| d < 0.5 mm | 0.5 mm ≤ d < 1 mm | 1 mm ≤ d < 3 mm | d ≥ 3 mm | Total Root Length | |
| CK | 381.77 ± 6.98 b (38%) | 579.25 ± 14.93 a (57%) | 36.78 ± 3.27 bc (4%) | 15.60 ± 2.59 b (2%) | 1013.4 |
| T1 | 418.21 ± 10.09 ab (50%) | 360.27 ± 14.0 b (44%) | 30.15 ± 1.55 bc (4%) | 14.14 ± 1.29 bc (2%) | 822.77 |
| T2 | 486.28 ± 7.62 a (69%) | 164.16 ± 7.17 c (23%) | 45.92 ± 3.31 b (7%) | 24.73 ± 1.17 a (4%) | 703.09 |
| T3 | 365.76 ± 4.77 b (75%) | 63.11 ± 3.29 d (13%) | 61.60 ± 3.36 a (13%) | 25.69 ± 2.72 a (5%) | 489.16 |
| T4 | 227.41 ± 5.21 c (80%) | 39.46 ± 1.52 e (11%) | 16.84 ± 1.73 c (5%) | 11.54 ± 1.43 c (3%) | 345.25 |
| T5 | 465.16 ± 6.96 ab (64%) | 212.89 ± 2.19 c (29%) | 33.63 ± 3.07 bc (5%) | 13.84 ± 0.30 bc (2%) | 725.52 |
| Category | Groups | |||||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | T5 | |
| Soluble sugar (mg·g−1) | 61.28 ± 5.33 a | 55.42 ± 4.69 ab | 53.45 ± 4.28 b | 36.48 ± 5.03 c | 32.29 ± 2.83 c | 42.07 ± 5.14 bc |
| Soluble protein (mg·g−1) | 65.32 ± 4.23 a | 57.5 ± 3.35 b | 44.3 ± 2.51 c | 33.3 ± 2.69 d | 17.6 ± 0.29 e | 43.53 ± 2.51 c |
| SOD (U·g−1) | 182.23 ± 7.73 c | 205.93 ± 4.24 bc | 212.47 ± 3.93 bc | 225.68 ± 3.73 b | 299.61 ± 4.59 a | 231.14 ± 3.26 b |
| POD (U·g−1) | 380.62 ± 5.07 a | 357.18 ± 7.55 a | 383.33 ± 12.26 a | 351.27 ± 10.21 a | 362.10 ± 4.03 a | 350.42 ± 15.41 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Ni, Z.; Yang, Z. Biomass Allocation, Root Spatial Distribution, and the Physiological Response of Dalbergia odorifera Seedlings in Simulated Shallow Karst Fissure-Soil Conditions. Sustainability 2022, 14, 11348. https://doi.org/10.3390/su141811348
Yu S, Ni Z, Yang Z. Biomass Allocation, Root Spatial Distribution, and the Physiological Response of Dalbergia odorifera Seedlings in Simulated Shallow Karst Fissure-Soil Conditions. Sustainability. 2022; 14(18):11348. https://doi.org/10.3390/su141811348
Chicago/Turabian StyleYu, Shuzhong, Zhouyou Ni, and Zhende Yang. 2022. "Biomass Allocation, Root Spatial Distribution, and the Physiological Response of Dalbergia odorifera Seedlings in Simulated Shallow Karst Fissure-Soil Conditions" Sustainability 14, no. 18: 11348. https://doi.org/10.3390/su141811348
APA StyleYu, S., Ni, Z., & Yang, Z. (2022). Biomass Allocation, Root Spatial Distribution, and the Physiological Response of Dalbergia odorifera Seedlings in Simulated Shallow Karst Fissure-Soil Conditions. Sustainability, 14(18), 11348. https://doi.org/10.3390/su141811348






