Impact of Vermicomposting on Greenhouse Gas Emission: A Short Review
Abstract
:1. Introduction
2. Vermicomposting
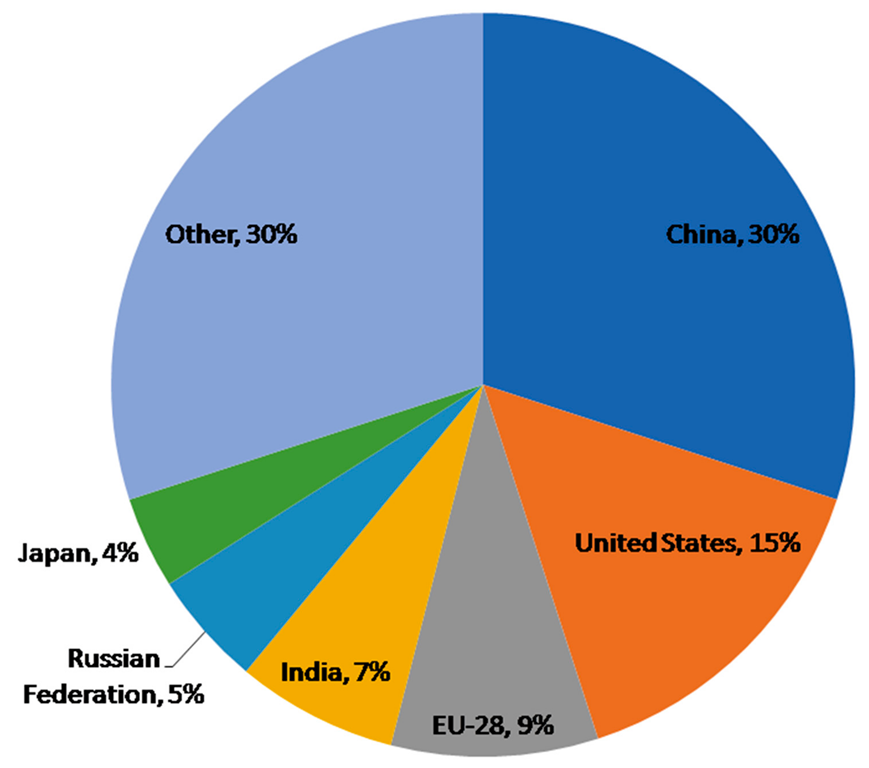
3. Greenhouse Gas Emissions by Organic Waste Management
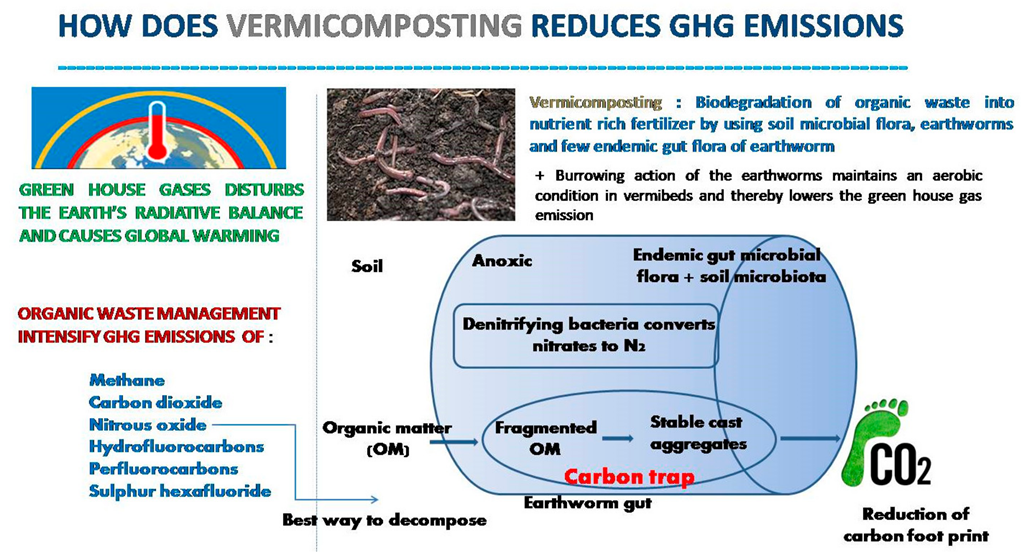
3.1. Mitigating GHGs Emission during the Vermicomposting of Waste
3.2. Effect of Feeding Ratio on Greenhouse Gas Emission (GHG)
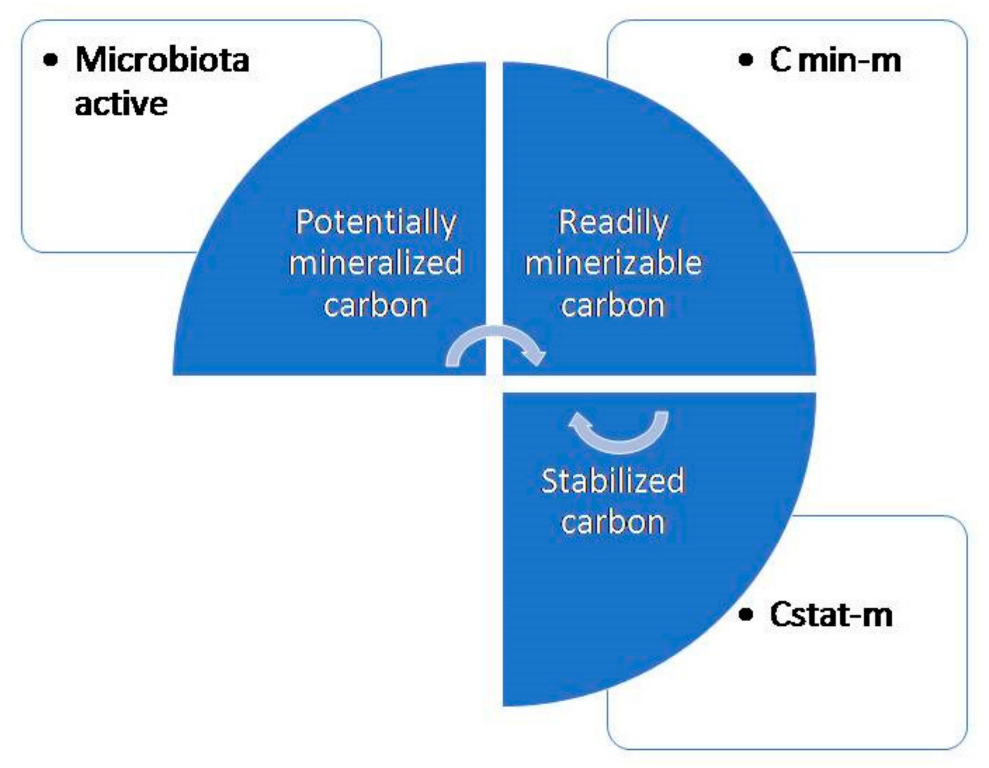
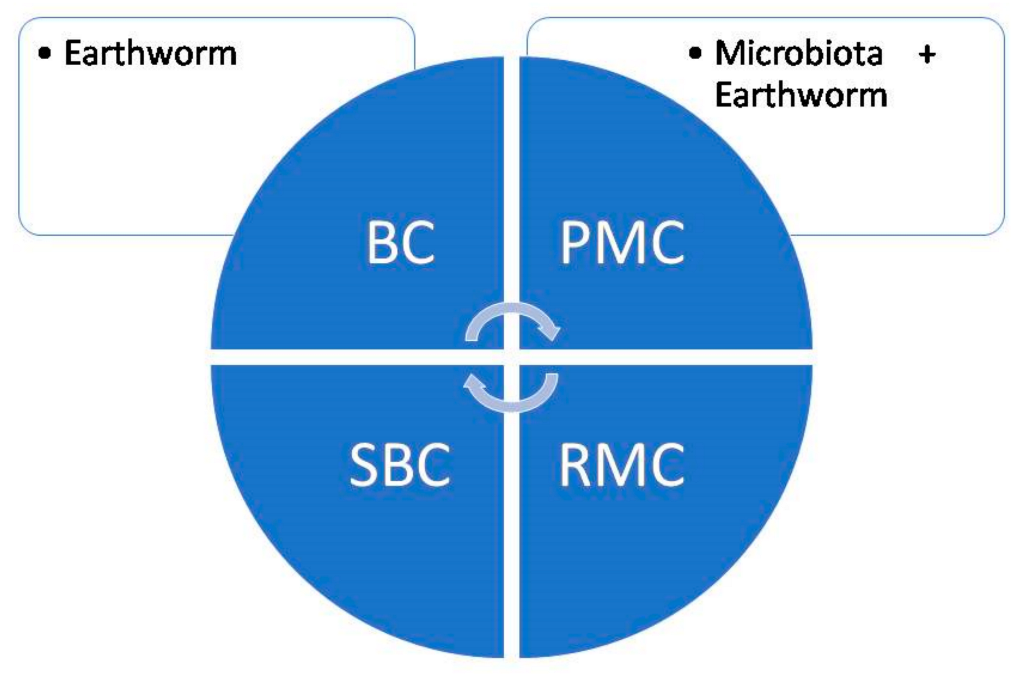
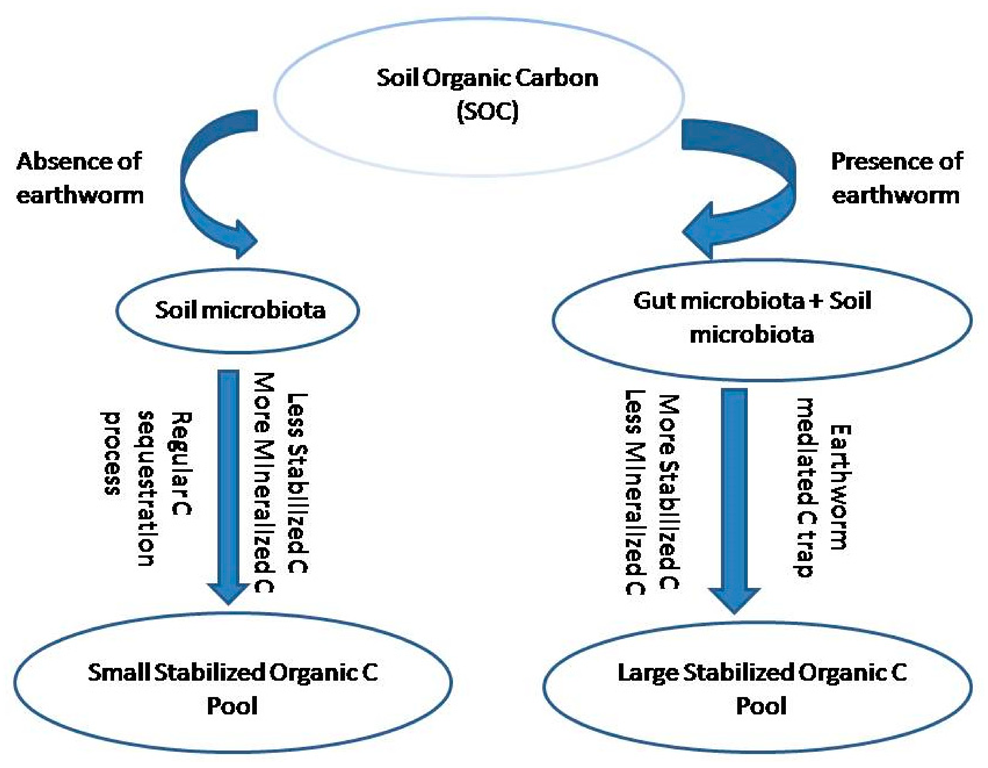
3.3. Role of Earthworms in Soil Carbon Sequestration
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathison, C.; Wiltshire, A.; Dimri, A.P.; Falloon, P.; Jacob, D.; Kumar, P.; Moors, E.; Ridley, J.; Siderius, C.; Stoffel, M.; et al. Regional projections of North Indian climate for adaptation studies. Sci. Total Environ. 2013, 468, S4–S17. [Google Scholar] [CrossRef] [PubMed]
- Biggs, E.M.; Gupta, N.; Saikia, S.D.; Duncan, J.M. The tea landscape of Assam: Multi-stakeholder insights into sustainable livelihoods under a changing climate. Environ. Sci. Pol. 2018, 82, 9–18. [Google Scholar] [CrossRef]
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Curr. Sci. 2002, 82, 510–518. [Google Scholar]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 54, 779–791. [Google Scholar] [CrossRef]
- Frederickson, J.; Howell, G. Large-scale vermicomposting: Emission of nitrous oxide and effects of temperature on earthworm populations: The 7th international symposium on earthworm ecology·Cardiff·Wales. Pedobiologia 2003, 47, 724–730. [Google Scholar]
- Brown, G.G. How do earthworms affect microfloral and faunal community diversity. Plant Soil. 1995, 170, 209–231. [Google Scholar] [CrossRef]
- Swati, A.; Hait, S. Greenhouse Gas Emission During Composting and Vermicomposting of Organic Wastes—A Review. Clean 2003, 46, 1700042. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Xu, X.; Jiang, X.; Zheng, B.; Liu, X.; Pan, X.; Kardol, P. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag. 2014, 34, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shaaban, M.; Zhao, J.; Hao, R.; Hu, R. Effect of the earthworm gut-stimulated denitrifiers on soil nitrous oxide emissions. Eur. J. Soil Biol. 2015, 70, 104–110. [Google Scholar] [CrossRef]
- Lubbers, I.M.; Van Groenigen, K.J.; Brussaard, L.; Van Groenigen, J.W. Reduced greenhouse gas mitigation potential of no-tillage soils through earthworm activity. Sci. Rep. 2015, 5, 13787. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Sinha, R.K.; Wang, W. Emission of greenhouse gases from home aerobic composting, anaerobic digestion and vermicomposting of household wastes in Brisbane (Australia). Waste Manag. Res. 2011, 29, 540–548. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Zang, B.; Zhang, Z. The Maturity and CH4, N2O, NH3 Emissions from Vermicomposting with Agricultural Waste. Compost Sci. Util. 2017, 25, 262–271. [Google Scholar] [CrossRef]
- Nigussie, A.; Kuyper, T.W.; Bruun, S.; de Neergaard, A. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J. Clean. Prod. 2016, 136, 429–439. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ali, M.H.; Samal, K. Assessment of compost maturity-stability indices and recent development of composting bin. Energy Nex. 2022, 6, 100062. [Google Scholar] [CrossRef]
- Hobson, A.M.; Frederickson, J.; Dise, N.B. CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manag. 2005, 25, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xia, H. Role of earthworms’ mucus in vermicomposting system: Biodegradation tests based on humification and microbial activity. Sci. Total Environ. 2018, 610, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.; Abdullah, R.; Yaacob, J.S. Effect of vermicompost on growth, plant nutrient uptake and bioactivity of ex vitro pineapple (Ananas comosus var. MD2). Agronomy 2020, 10, 1333. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer and biocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotechnol. 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, R.K. Vermicomposting of organic wastes by earthworms: Making wealth from waste by converting ‘garbage into gold’for farmers. In Advanced Organic Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 93–120. [Google Scholar]
- Lv, B.; Zhang, D.; Cui, Y.; Yin, F. Effects of C/N ratio and earthworms on greenhouse gas emissions during vermicomposting of sewage sludge, Bioresour. Technol. 2018, 268, 408–414. [Google Scholar]
- Samal, K.; Naushin, Y.; Priya, K. Challenges in the implementation of Phyto Fuel System (PFS) for wastewater treatment and harnessing bio-energy. J. Environ. Chem. Eng. 2020, 8, 104388. [Google Scholar] [CrossRef]
- Garnier, P.; Makowski, D.; Hedde, M.; Bertrand, M. Changes in soil carbon mineralization related to earthworm activity depend on the time since inoculation and their density in soil. Sci. Rep. 2022, 12, 13616. [Google Scholar]
- Pereira, R.F.; Cardoso, E.J.B.N.; Oliveira, F.C.; Estrada-Bonilla, G.A.; Cerri, C.E.P. A novel way of assessing C dynamics during urban organic waste composting and greenhouse gas emissions in tropical region. Bioresour. Technol. Rep. 2018, 3, 35–42. [Google Scholar] [CrossRef]
- Rogelj, J.; den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 632–639. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Ren, X.; Zhao, J.; Huang, H.; Awasthi, S.K.; Lahori, A.H.; Li, R.; Zhou, L.; Zhang, Z. Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour. Technol. 2016, 219, 270–280. [Google Scholar] [CrossRef]
- Nigussie, A.; Bruun, S.; Kuyper, T.W.; de Neergaard, A. Delayed addition of nitrogen-rich substrates during composting of municipal wastes: Effects on nitrogen loss, greenhouse gas emissions and compost stability. Chemosphere 2017, 166, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Boden, T.; Marland, G.; Andres, R.J. National CO2 Emissions from Fossil-Fuel Burning, Cement Manufacture, and Gas Flaring; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2017; pp. 1751–2014. [Google Scholar]
- Yang, F.; Li, G.X.; Yang, Q.Y.; Luo, W.H. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013, 93, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Barthod, J.; Rumpel, C.; Calabi-Floody, M.; Mora, M.L.; Bolan, N.S.; Dignac, M.F. Adding worms during composting of organic waste with red mud and fly ash reduces CO2 emissions and increases plant available nutrient contents. J. Environ. Manag. 2018, 222, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: A review. Environ. Sci. Pollut. Res. 2018, 25, 10577–10595. [Google Scholar] [CrossRef] [PubMed]
- Maulini-Duran, C.; Artola, A.; Font, X.; Sanchez, A. Gaseous emissions in municipal wastes composting: Effect of the bulking agent. Bioresour. Technol. 2014, 172, 260–268. [Google Scholar] [CrossRef]
- Santos, A.; Bustamante, M.A.; Tortosa, G.; Moral, R.; Bernal, M.P. Gaseous emissions and process development during composting of pig slurry: The influence of the proportion of cotton gin waste. J. Clean. Prod. 2016, 112, 81–90. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Muller, K.; Li, Y.; Fu, W.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2022, 7, 100092. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, M.H.; Samal, K. Pharmaceutical wastewater as emerging contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Dume, B.; Hanc, A.; Svehla, P.; Chane, A.; Nigussie, A. Carbon Dioxide and Methane Emissions during the Composting and Vermicomposting of Sewage Sludge under the Effect of Different Proportions of Straw Pellets. Environ. Sci. Proc. 2021, 8, 7. [Google Scholar] [CrossRef]
- Wust, P.K.; Horn, M.A.; Drake, H.L. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 2009, 75, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.K.; Horn, M.A.; Henderson, G.; Janssen, P.H.; Rehm, B.H.; Drake, H.L. Gut-associated denitrification and in vivo emission of nitrous oxide by the earthworm families Megascolecidae and Lumbricidae in New Zealand. Appl. Environ. Microbiol. 2009, 75, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Angst, G.; John, S.; Mueller, C.W.; Kogel-Knabner, I.; Rethemeyer, J. Tracing the sources and spatial distribution of organic carbon in subsoils using a multi-biomarker approach. Sci. Rep. 2016, 6, 29478. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.D.J.; Siu, M.T.; Marcus, A.H.; Harold, L.D. Emission of nitrous oxide and dinitrogen by diverse earthworm families from Brazil and resolution of associated denitrifying and nitrate-dissimilating taxa. FEMS Microbiol. Ecol. 2013, 83, 375–391. [Google Scholar]
- Angst, S.; Mueller, C.W.; Cajthaml, T.; Angst, G.; Lhotakova, Z.; Bartuska, M.; Špaldoňová, A.; Frouz, J. Stabilization of soil organic matter by earthworms is connected with physical protection rather than with chemical changes of organic matter. Geoderma 2017, 289, 29–35. [Google Scholar] [CrossRef]
- Lleo, T.; Albacete, E.; Barrena, R.; Font, X.; Artola, A.; Sanchez, A. Home and vermicomposting as sustainable options for biowaste management. J. Clean. Prod. 2013, 47, 70–76. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S.; Das, K. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Luth, R.P.; Germain, P.; Lecomte, M.; Landrain, B.; Li, Y.; Cluzeau, D. Earthworm effects on gaseous emissions during vermifiltration of pig fresh slurry. Bioresour. Technol. 2011, 102, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; van Groenigen, J.W. Greenhouse gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Potthoff, M.; Joergensenb, R.G.; Woltersc, V. Short-term effects of earthworm activity and straw amendment on the microbial C and N turnover in a remoistened arable soil after summer drought. Soil Biol. Biochem. 2001, 33, 583–591. [Google Scholar] [CrossRef]
- Bernard, L.; Chapuis-Lardy, L.; Razafimbelo, T.; Razafindrakoto, M.; Pablo, A.L.; Legname, E.; Poulain, J.; Brüls, T.; O’Donohue, M.; Brauman, A.; et al. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J. 2012, 6, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urmi, T.A.; Rahman, M.M.; Islam, M.M.; Islam, M.A.; Jahan, N.A.; Mia, M.A.B.; Siddiqui, M.H.; Kalaji, H.M. Integrated Nutrient Management for Rice Yield, Soil Fertility, and Carbon Sequestration. Plants 2022, 11, 138. [Google Scholar] [CrossRef]
- Gnanavelrajah, N.; Shrestha, R.P.; Schmidt-Vogt, D.; Samarakoon, L. Carbon stock assessment and soil carbon management in agricultural land-uses in Thailand. Land Degrad. Dev. 2008, 19, 242–256. [Google Scholar] [CrossRef]
- Russell, A.E.; Larid, D.A.; Parkin, T.B.; Mallarino, A.P. Impact of nitrogen fertilization and cropping system on carbon sequestration in Midwestern Mollisols. Soil Sci. Soc. Am. J. 2005, 69, 413–422. [Google Scholar] [CrossRef]
- Zhang, W.; Hendrix, P.F.; Dame, L.E.; Burke, R.A.; Wu, J.; Neher, D.A.; Li, J.; Shao, Y.; Fu, S. Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization. Nat. Commun. 2013, 4, 2576. [Google Scholar] [CrossRef] [PubMed]
- Fonte, S.J.; Kong, A.Y.Y.; Van Kessel, C.; Hendrix, P.F.; Six, J. Influence of earthworm activity on aggregate-associated carbon and nitrogen dynamics differs with agroecosystem management. Soil Biol. Biochem. 2007, 39, 1014–1022. [Google Scholar] [CrossRef]
- Hedde, M.; Bureau, F.; Delporte, P.C.; Ecillon, L.; Decaens, T. The effects of earthworm species on soil behaviour depend on land use. Soil Biol. Biochem. 2013, 65, 264e273. [Google Scholar] [CrossRef]
- Bossuyt, H.; Six, J.; Hendrix, P.F. Protection of soil carbon by microaggregates within earthworm cast. Soil Biol. Biochem. 2005, 37, 251–258. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Abbey, L.; Appah, P. Pot-grown swiss chard and kale responses to a variable rate of manure compost in mycorrhizal fungi inoculated medium. In Proceedings of the III International Symposium on Organic Greenhouse Horticulture 1164, Izmir, Turkiye, 11–14 April 2016; pp. 241–248. [Google Scholar]
- Nagavallemma, K.P.; Wani, S.P.; Lacroix, S.; Padmaja, V.V.; Vineela, C.; Rao, M.B.; Sahrawat, K.L. Vermicomposting, Recycling Wastes into Valuable Organic Fertilizer. Global Theme on Agroecosystems Report No. 8; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 2004; p. 20. [Google Scholar]
- Abbey, L.; Pham, T.H.; Annan, N.; Leke-Aladekoba, A.; Thomas, R.H. Chemical composition of kale as influenced by dry vermicast, potassium humate and volcanic minerals. Food Res. Int. 2018, 107, 726–737. [Google Scholar] [CrossRef] [PubMed]
- El-Goud, A.; Amal, K. Efficiency response of vermicompost and vermitea levels on growth and yield of eggplant (Solanum melongena L.). Alex. Sci. Exch. J. 2020, 41, 69–75. [Google Scholar]
- Shi-Wei, Z.; Fu-Zhen, H. The nitrogen uptake efficiency from 15N labeled chemical fertilizer in the presence of earthworm manure (cast). In Advances in Management and Conservation of Soil Fauna; Oxford and IBH Publishing Company: New Delhi, Bombay, India, 2019; pp. 539–542. [Google Scholar]
- Palaniappan, S.; Alagappan, M.; Rayar, S. Influence of substrate particle size on vermicomposting of pre-processed vegetable waste. Nat. Env. Pollut. Technol. 2018, 17, 277–286. [Google Scholar]
- Lin, S.; Gunupuru, L.R.; Ofoe, R.; Saleh, R.; Asiedu, S.K.; Thomas, R.H.; Abbey, L. Mineralization and nutrient release pattern of vermicast-sawdust mixed media with or without addition of Trichoderma viride. PLoS ONE 2021, 16, e0254188. [Google Scholar] [CrossRef]
| S. No | Parameters | Vermicomposting Reactor | Composting Reactor | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methane [CH4] | NitrousOxide [N2O] | Carbon Dioxide [CO2] | Duration Days | Methane [CH4] | Nitrous Oxide [N2O] | Carbon Dioxide [CO2] | Duration Days | |||
| 1 | Earthworm species
| 4.76 kg mg−1 0.033 (μg g−1 h−1) 2.28 (kg CO2-eq t¡1 DM) | 1.17 kg mg−1 0.012 (μg g−1 h−1) 5.76 (kg CO2-eq t¡1 DM) | 1675 kg mg−1 16.5 % decrease in CO2 emission N.M | 30–60 56 50 | 2.2 kg mg−1 0.024 (μg g−1 h−1) 10.52 (kg CO2-eq t¡1 DM) | 1.5 kg mg−1 0.007 (μg g−1 h−1) 12.29 (kg CO2-eq t¡1 DM) | 882 kg mg−1 519–730 (mg g−1) N.M | 30–60 56 50 | [11] [29] [12] |
| 2 | Waste characteristics
| 2.2 × 10−3 kg mg−1 4.76 kg mg−1 0.02–0.38 kg mg−1 | N.D 1.17 kg mg−1 0.12–1.5 kg mg−1 | N.M 1675 kg mg−1 N.M. | 240 30–60 84 | 1.4 kg mg−1 2.2 kg mg−1 0.05–6.6 kg mg−1 | 1.2 ppm kg mg−1 1.5 kg mg−1 0.005–0.37 kg mg−1 | N.M 882 kg mg−1 N.M. | 240 120 84 | [42] [11] [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, A.K.; Mishra, R.; Dutta, J.; Wani, Z.A.; Pant, S.; Siddiqui, S.; Alamri, S.A.; Alrumman, S.A.; Alkahtani, M.A.; Bisht, S.S. Impact of Vermicomposting on Greenhouse Gas Emission: A Short Review. Sustainability 2022, 14, 11306. https://doi.org/10.3390/su141811306
Panda AK, Mishra R, Dutta J, Wani ZA, Pant S, Siddiqui S, Alamri SA, Alrumman SA, Alkahtani MA, Bisht SS. Impact of Vermicomposting on Greenhouse Gas Emission: A Short Review. Sustainability. 2022; 14(18):11306. https://doi.org/10.3390/su141811306
Chicago/Turabian StylePanda, Amrita Kumari, Rojita Mishra, Joystu Dutta, Zishan Ahmad Wani, Shreekar Pant, Sazada Siddiqui, Saad Abdulrahman Alamri, Sulaiman A. Alrumman, Mohammed Ali Alkahtani, and Satpal Singh Bisht. 2022. "Impact of Vermicomposting on Greenhouse Gas Emission: A Short Review" Sustainability 14, no. 18: 11306. https://doi.org/10.3390/su141811306
APA StylePanda, A. K., Mishra, R., Dutta, J., Wani, Z. A., Pant, S., Siddiqui, S., Alamri, S. A., Alrumman, S. A., Alkahtani, M. A., & Bisht, S. S. (2022). Impact of Vermicomposting on Greenhouse Gas Emission: A Short Review. Sustainability, 14(18), 11306. https://doi.org/10.3390/su141811306







