Abstract
Intending to contribute to sustainable agriculture by “New Green Revolution,” we developed a large-grain/semidwarf isogenic line “Koshihikari sd1GW2” that incorporates both the large-grain gene GW2 and semidwarf gene sd1. GW2 homozygous B3F2 plant with the genomic background of Koshihikari was backcrossed twice with “Koshihikari sd1.” Koshihikari sd1GW2 fixed in BC5F3 was found to be 12.6 cm shorter than Koshihikari. Whole-genome sequencing proved one deletion in GW2 at 8,147,416 bp on chromosome 2 and the SNPs in sd1 at 38,267,510 bp on chromosome 1. The size of the DNA fragments integrated with each gene was determined as the distance between both ends of SNP clusters. Through the backcrossing from BC4 to BC5, the DNA fragment integrated with GW2 decreased by 148,139 bp. The thousand-grain weight of Koshihikari sd1GW2 (27.8 g) was 18% greater than that of Koshihikari (23.6 g), and the grain yield of Koshihikari sd1GW2 (42.6 kg/a) was 0.5% higher than that of Koshihikari (42.4 kg/a). Our results suggested that Koshihikari sd1GW2 will be less susceptible to lodging by typhoons, cyclones, and heavy rainfall, ordinarily a concern in heavier panicle weight cultivars. We successfully integrated GW2 with sd1 for the first time, specifically in the genome of the leading, globally produced Japonica cultivar Koshihikari.
1. Introduction
Climate change due to global warming continues to worsen on a global scale. Throughout Japan, disastrous rainfall and floods represented as “Heavy Rain in July, Heisei 30” [1] and large typhoons with wind speeds over 54 m/s, including Bualoi, Jebi, and Trami, which were comparable to the Isewan typhoon, the worst one in Japan’s history [2], occasionally occur every year [2,3]. A series of natural disasters caused by these abnormal weather patterns substantially damages the agriculture, forestry, and fishery industries (with monetary losses of approximately JPY 330.6 billion) [4]. Under these climate crises, rice must be robust and more resistant to lodging [5].
During the middle of the 20th century, rice plants were genetically altered to moderately reduced height, forming semidwarf rice cultivars, thereby enhancing lodging resistance to wind and rain at their full-ripe stage, improving light interception properties, enabling heavy manuring and dense planting, and thus markedly increasing productivity and nitrogen responsiveness [6]. As a consequence of the development of semidwarf cultivars, the worldwide rice yield doubled between the 1960s and 1990s, and the so-called “Green Revolution” is evaluated as the most remarkable agricultural contribution in human history [7,8,9]. Subsequent genetic analyses revealed that this epoch-making rice improvement could be attributed to a single gene, semidwarf 1 (sd1), suppressing plant height. It has since been clarified that sd1 is a deletion mutation of the GA20 oxidase gene in the gibberellin (GA) biosynthesis pathway (GA 20-oxidase, OsGA20ox2) [10,11,12] localized on chromosome 1 [13,14,15], which results in reduced synthesis of gibberellin GA4 by disruptions at a late stage of the GA pathway, thereby leading to the semidwarfness. The sd1 gene confers no detrimental effects on grain yield [16,17,18,19,20,21].
The japonica rice Koshihikari is a leading rice variety in Japan, accounting for 36.1% of the rice acreage in the country. Koshihikari is globally valued and produced in the United States and Australia. However, Koshihikari suffers severe lodging damage due to frequent heavy rains, floods, and strong typhoons; thus, the development of lodging-resistant Koshihikari has been a longstanding challenge. Tomita (2009) introgressed the semidwarf gene sd1 from Jukkoku to Koshihikari by backcrossing to Koshihikari eight times to develop a semidwarf form of Koshihikari, which was approximately 20 cm shorter than Koshihikari [22] and consisted more than 99.8% of the genome of Koshihikari, except for sd1 derived from Jukkoku [23]. This cultivar, named Hikarishinseiki (rice cultivar number 12273) [22,24], is the first cultivar of semidwarf isogenic Koshihikari with sd1 registered in Japan and the USA [25,26].
The ongoing situation in international trade is also fueling this need for the development of new rice cultivars. For example, the Trans-Pacific Partnership of 11 countries, which has hitherto maintained tariffs for essential items such as rice and wheat, instead of maintaining compulsory importing of specific amounts, has abolished tariffs on 82% of agricultural, forestry, and fishery products imported by Japan. In addition, the patent on the plant variety protection of the primary rice variety of Japan, Koshihikari, which was registered in 1956, has expired. Therefore, serious concerns regarding global competition with foreign Koshihikari, which has a good taste and is relatively inexpensive, have been raised under the current global trends of free trade agreements such as the Japan–EU Economic Partnership Agreement and Trade Agreement on Goods (TAG) negotiations.
The “Green Revolution” has undoubtedly contributed to substantial gains and stability in agricultural productivity. However, depending on the effects of just a single gene, sd1, rice productivity is currently reaching a plateau. Furthermore, in response to the exacerbation of climate change and market globalization, there is an imperative need to call for the development of new, high-yielding rice cultivars that can be produced at low costs. Consequently, further genetic modification, namely the “New Green Revolution”, will be desired to meet the predicted population growth and adverse effects of climate change.
In this regard, we focused on a large-grain variety, “Inochinoichi”, with a thousand-kernel weight of unpolished rice being 1.5 times that of Koshihikari, as a high-yielding genetic source. We identified a single large-grain allele through genotypic segregation in a ratio of one large-grain type to two heterozygous types to one small-grain type via each generation of backcrossing (BC1F2 (the second filial generation in the first backcross; filial means progeny of hybrid) to BC5F2) to a recurrent parent Koshihikari using a large-grain F2 (second filial generation) plant as a non-recurrent parent, which segregated from a cross between Koshihikari and Inochinoichi [27]. Furthermore, the large-grain gene, grain width and weight 2 (GW2), derived from japonica rice, was identified for the first time based on a whole-genome analysis of the developed large-grain Koshihikari-type isogenic line [27]. To contribute towards the “New Green Revolution”, in this study, we developed a large-grain/semidwarf isogenic line “Koshihikari sd1GW2”, which is designated as “the Koshihikari incorporating both the large-grain gene GW2 and the semidwarf gene sd1”. Koshihikari sd1GW2 is a high-yielding rice cultivar with enhanced resistance to lodging.
2. Materials and Methods
2.1. Development of Koshihikari sd1GW2
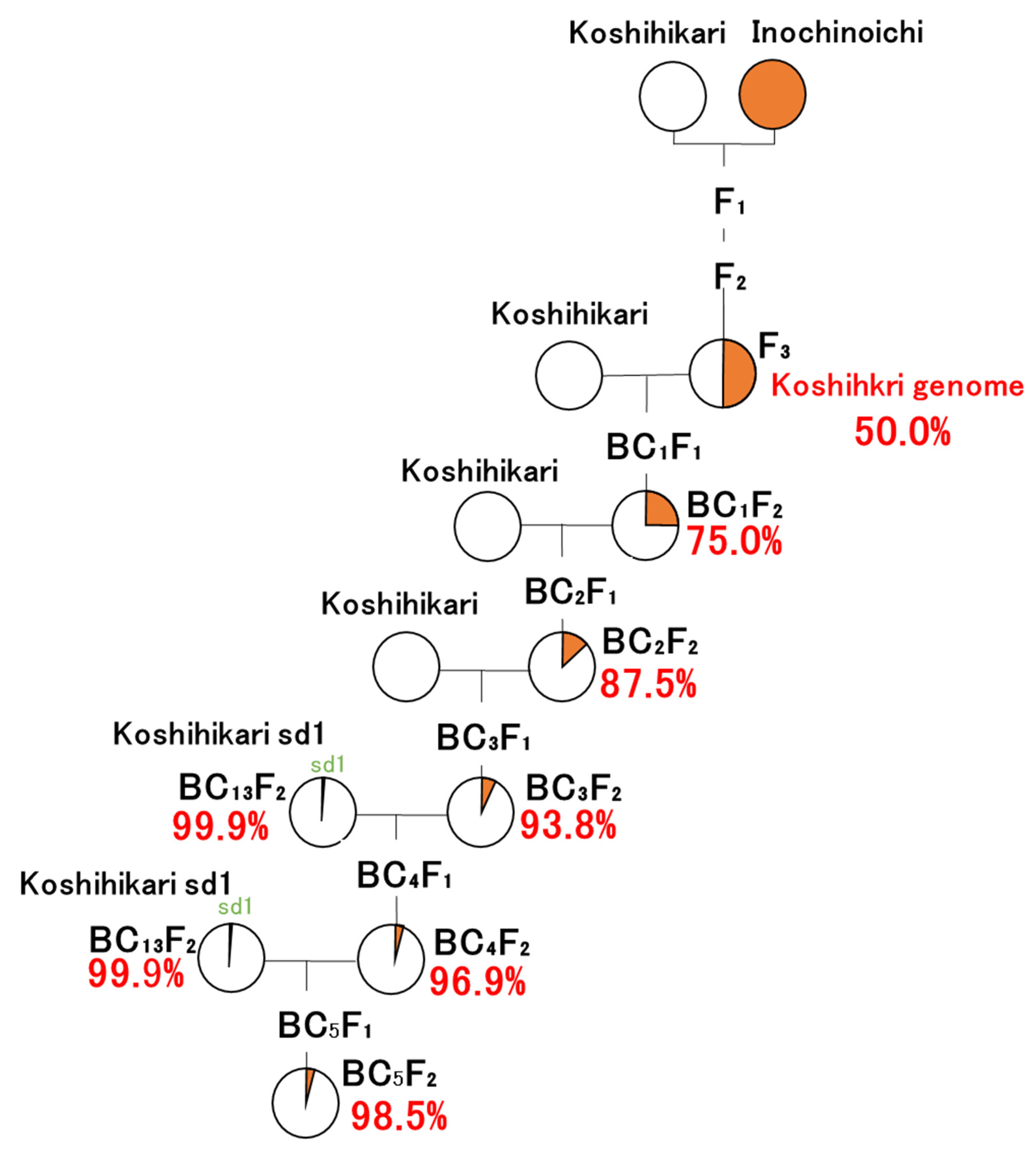
A Japanese leading rice variety, Koshishikri, was used as a recurrent parent to develop isogenic lines based on Koshishikri. The Japanese rice variety Inochinoichi was used as a source of the large-grain gene GW2, which was identified in a previous study [27]. In the present study, GW2 homozygous B3F2 plants segregated from Koshihikari × 3 ((Koshihikari × Inochinoichi) F3 GW2 type), in which a large-grain plant fixed in F3 of Koshihikari × Inochinoichi was backcrossed as the non-recurrent parent to a recurrent parent Koshihikari thrice, was backcrossed with “Koshihikari sd1” (Figure 1). Koshihikari sd1 is a semidwarf isogenic line developed via 13 backcrossings with a recurrent parent Koshihikari with a non-recurrent parent sd1 homozygous F4 plant, which was fixed with Koshihikari-type ear emergence period in the cross of Kanto No. 79 × Jukkoku [22]. Fifty plants of the Koshihikari sd1//Koshihikari × 3/((Koshihikari × Inochinoichi) F3 GW2 type) BC4F2 were grown, and all plants were measured for culm length, number of panicles, and grain size to determine phenotypes of large-grain and semidwarfness. We tested approximately 50 to 100 plants for each BCnF2 generation, which allowed the analysis of genetic segregation. We employed the chi-square goodness-of-fit test for genetic segregation in each BCnF2 generation.
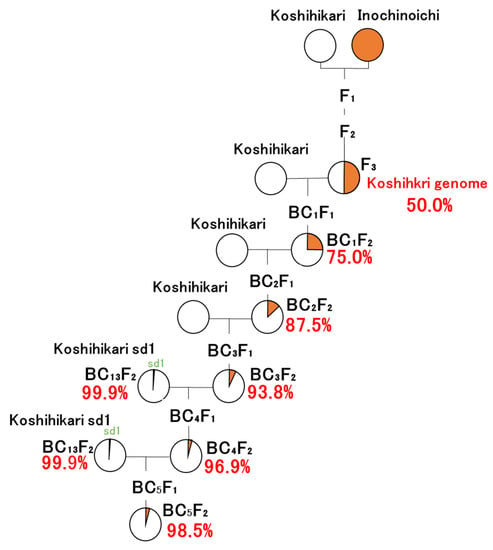
Figure 1.
Pyrogenetic process of Koshihikari sd1GW2 (Koshihikari sd1 × 2//Koshihikari × 3/((Koshihikari × Inochinoichi) F3 large-grain type) BC5F3) plants.
We then examined 93 plants of the Koshihikari sd1 × 2//Koshihikari × 3/((Koshihikari × Inochinoichi) F3) BC5F2, in which GW2sd1 homozygous BC4F2 segregant was second backcrossed with Koshihikari sd1 as the recurrent parent (Figure 1), and 66 BC5F3 progenies of GW2gw2sd1sd1 type BC5F2 segregant. We screened GW2sd1 homozygous plants from the BC5F2 and BC5F3 progeny based on the assessments of ear emergence day, culm length, grain size, number of panicles, and gene diagnosis for GW2 using the dCAP marker GW2-Hpa I.
Plant seed materials were sown and cultivated in a paddy field at Shizuoka University, Shizuoka, Japan, from 2013 to 2021. To accelerate backcross generation, plant materials BCnF1 and their next generation BCnF2 were grown every year from April to July and July to November, respectively. Finally, to test the performance of acquired genotypes, Koshihikari sd1GW2, Koshihikari sd1, Koshihikari GW2, and Koshihikari were grown from May to October. Seedlings were individually transplanted into a paddy field with a transplanting density of 22.2 seedlings/m2 (one seedling per 30 × 15 cm). The paddy field was fertilized with 4.0 kg of basal fertilizer containing nitrogen, phosphorus, and potassium (weight ratio, nitrogen:phosphorus:potassium = 2.6:3.2:2.6) at a rate of 4.3, 5.3, and 4.3 g/m2 across the field, respectively. The heading date was recorded as the date the first panicle had emerged from the flag leaf sheath for each plant. Culm length was measured as the length between the ground surface and the panicle base. Ten plants typical of each genotype were sampled twice at the same time after ripening for repeated measurements of yield-related traits. The sampled plants were air-dried and assessed for the following traits: panicle length, number of panicles, number of florets/panicles, proportion of fertile florets, total panicle number, and weight of unmilled rice/1000 grains. The yield of unpolished rice was calculated using the following equation: yield of unmilled rice (g/m2) = (number of panicles/m2) × (number of florets/panicle) × (proportion of fertile florets) × (weight of unmilled rice/grain) [28]. Lodging degree was determined based on the inclination angle of the plant: 0, standing; 1, almost 70; 2, almost 50; 3, almost 30; 4, almost 10; 5, lodged. Taste evaluation was based on a seven-grade organoleptic assessment by a panelist, and protein contents were determined using Infratec 1241 (VOSS Japan Ltd., Tokyo, Japan). The means of each trait of the developed isogenic lines were statistically evaluated using the t-test to determine if there is a significant difference between the means of Koshihikari.
GW2 homozygous B3F2 plants segregated from Koshihikari × 3 ((Koshihikari × Inochinoichi) F3 GW2 type), in which a large-grain plant fixed in F3 of Koshihikari × Inochinoichi was backcrossed as a non-recurrent parent to a recurrent parent Koshihikari three times, were backcrossed two times with “Koshihikari sd1”.
2.2. Whole-Genome Sequencing Analysis
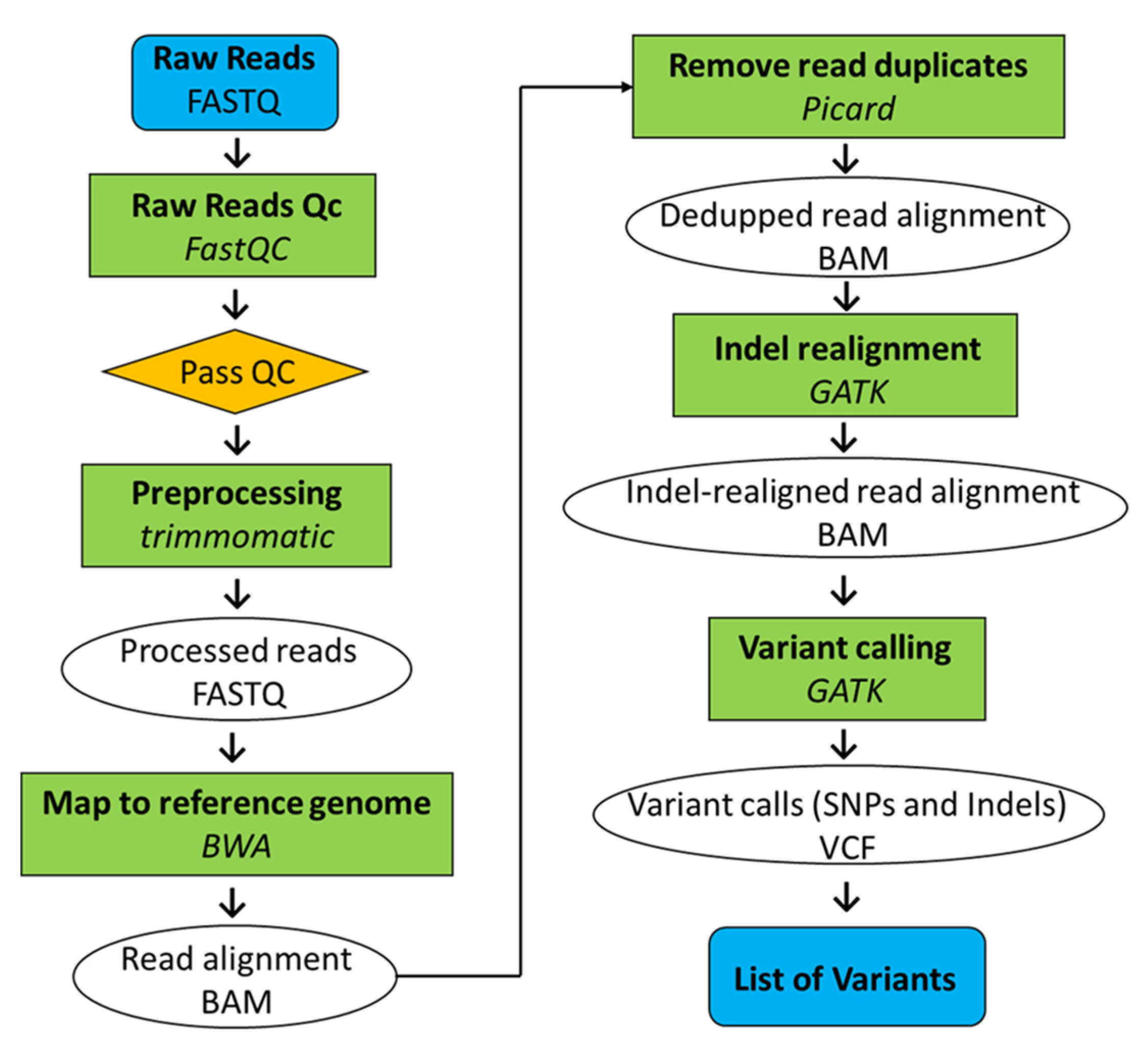
Whole-genome sequencing was conducted on both Koshihikari GW2 (BC4F3, BC6F3) and Koshihikari sd1GW2 (BC5F2), which was integrated with a large-grain gene GW2 and semidwarfing gene sd1, backcrossed five times into the genetic background of Koshihikari. The leaves were powdered using a mortar and pestle while frozen in liquid nitrogen. The genomic DNA was then extracted from each cultivar by the CTAB method. Genomic DNA was fragmented and simultaneously tagged, using the Nextera transposome (Illumina Inc., San Diego, CA, USA), so that the peak size of the fragments was approximately 500 bp. After purification of the transposome using DNA Clean & Concentrator (TM)-5 (Zymo Research, Irvine, CA, USA), adaptor sequences, including the sequencing primers, for fixation on the flow cell were synthesized at both ends of each fragment using polymerase chain reaction, and then, the DNA fragments were subjected to size selection using AMPure XP magnetic beads (Beckman Coulter, Brea, CA, USA). Finally, qualitative checks using Fragment Analyzer (Advanced Analytical Technologies, Heidelberg, Germany) and quantitative measurements using Qubit 2.0 Fluorometer (Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA) were performed to prepare a DNA library for next-generation sequencing (NGS). The sequencing was conducted in paired-end 2 × 100 bp on a HiSeq X next-gen sequencer, according to the manufacturer’s protocol (Illumina Inc., San Diego, CA, USA). The gained Illumina reads were firstly trimmed using Trimmomatic (version 0.39, Institut Pasteur, Paris, France) [29] (Figure 2). The sequencing adapters and sequences with low-quality scores on 3′ ends (Phred score (Q) < 20) were trimmed. The raw Illumina whole-genome sequencing reads were quality checked by performing a quality control with FastQC (version 0.11.9; Babraham Institute, Cambridge, UK). Mapping of reads from Koshihikari GW2 and Koshihikari sd1GW2 to the Koshihikari genome as a reference was conducted with Burrows-Wheeler Aligner software (version 0.7.17.tar.bz2; Appirits, Tokyo, Japan) [30]. Duplicated reads were removed using Picard (version 2.25.5; GitHub Inc., San Francisco, CA, USA), and secondary aligned reads were removed using SAMtools (version 1.10.2; SourceForge, San Diego, CA, USA) [31]. To identify genetic variations among strains, single nucleotide variant (SNV) detection (variant calling) and SNV matrix generation were performed using GATK (version 4.1.7.0; Broad Institute, Cambridge, MA, USA) [32].
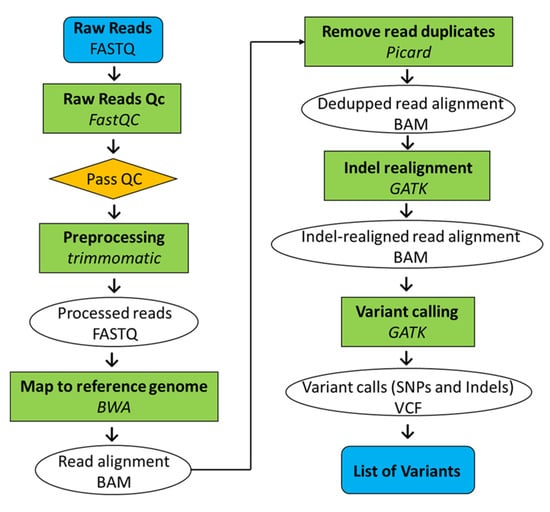
Figure 2.
Pipeline of whole-genome resequencing analysis.
3. Results
3.1. Inheritance of the Semidwarf Gene sd1 and the Large-Grain Gene Gw2 in the Genetic Background of Koshihikari
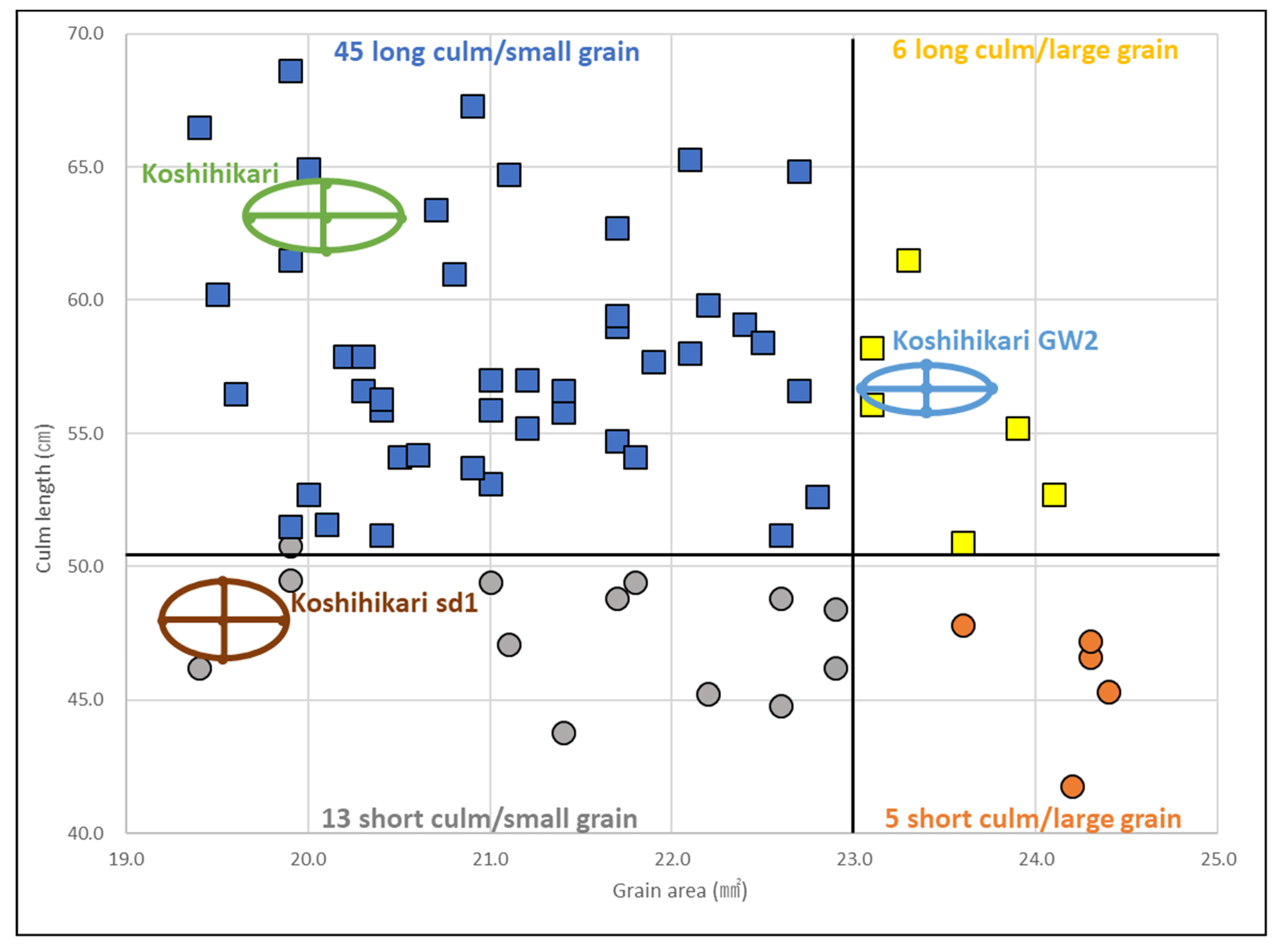
Figure 3 shows the distributions of the grain surface area and culm length of Koshihikari sd1//Koshihikari × 3/((Koshihikari × Inochinoichi) F3 GW2) BC4F2 plants, in which the large-grain type fixed F3 plant in Koshihikari × Inochinoichi was backcrossed as a non-recurrent parent thrice with a recurrent parent Koshihikari and a large-grain BC3F2 plant was used for the fourth backcross to “Koshihikari sd1” (Figure 1). sd1 homozygous semidwarf plants with culm length ranging from 41.8 to 50.5 cm and long-culm plants with culm length ranging from 50.6 to 68.6 cm were segregated in a 21:52 ratio (1 sd1sd1:3 (1Sd1Sd1 + 2Sd1Sd1) (χ2 = 0.02, 0.85 < p < 0.90)) (Figure 3). In addition, among the 21 sd1 homozygous semidwarf plants, GW2 homozygous large-grain plants with a grain area of 22.7–24.4 mm2, heterozygous plants with a grain area of 20.5–22.6 mm2, and gw2 homozygous small-grain plants with a grain area of 19.4–20.4 mm2 were segregated in a 5:10:3 ratio (1 GW2GW2:2 GW2gw2:1 gw2gw2 (χ2 = 2.0, 0.35 < p < 0.40)). Similarly, 52 long-culm plants were segregated into 6 large-grain type, 30 intermediate type, and 15 small-grain type plants (1 Gw2Gw2:2 Gw2gw2:1 gw2gw2 (χ2 = 1.6, 0.35 < p < 0.40)). Thus, as a whole, BC4F2 plants segregated into 45 long-culm/small-grain, 6 long-culm/large-grain, 13 semidwarf/small-grain, and 5 semidwarf/large-grain plants (9(Sd1Sd1gw2gw2 + 2Sd1Sd1GW2gw2 + 2Sd1sd1gw2gw2 + 4Sd1sd1GW2gw2):3(Sd1Sd1GW2Gw2 + 2Sd1sd1GW2Gw2):3(sd1sd1gw2gw2 + 2sd1sd1GW2gw2):1(sd1sd1GW2Gw2) (χ2 = 4.8, 0.15 < p < 0.20)). In the cross between the semidwarf and large-grain isogenic lines, sd1 (chromosome 1) and GW2 (chromosome 2) were inherited independently.
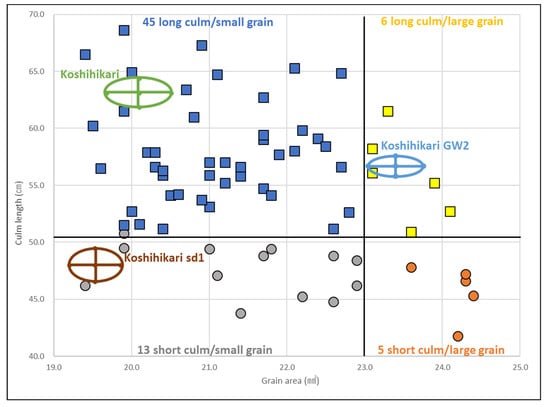
Figure 3.
Relationship between grain area and culm length in Koshihikari sd1//Koshihikari × 3/((Koshihikari × Inochinoichi) F3 GW2 type) BC4F2 plants. sd1 homozygous semidwarf plants with a culm length of 41.8–50.5 cm and long-culm plants with a culm length of 50.6–68.6 cm were segregated in a 21:52 ratio (1 sd1sd1:3(1Sd1Sd1 + 2Sd1Sd1) (χ2 = 0.02, 0.85 < p < 0.90)). In addition, among the 21 sd1 homozygous semidwarf plants, GW2 homozygous large-grain plants with a grain area of 22.7–24.4 mm2, heterozygous plants with a grain area of 20.5–22.6 mm2, and gw2 homozygous small-grain plants with a grain area of 19.4–20.4 mm2 segregated in a 5:10:3 ratio (1 GW2GW2:2 GW2gw2:1 gw2gw2 (χ2 = 2.0, 0.35 < p < 0.40)).
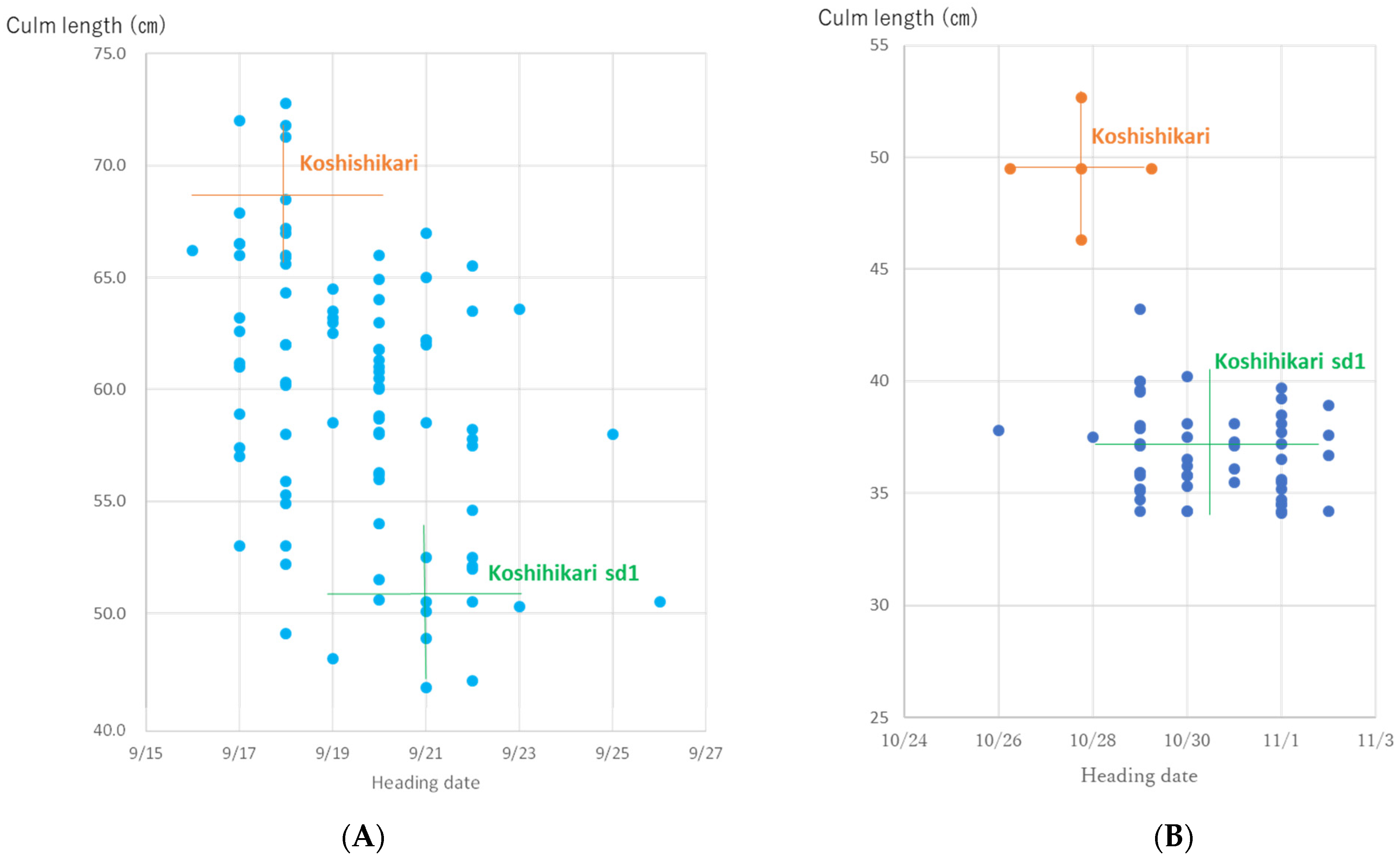
The relationship between the culm length and ear emergence period in BC5F2 plants is shown in Figure 4A. The culm length is distributed in the range of 42.5–72.0 cm. sd1 homozygous with short/thick/dark green flag leaves having a culm length of 42.5–54.9 cm, similar to Koshihikari sd1, the intermediate heterozygous having a culm length of 55.3–63.6 cm, and Sd1 homozygous with long/light-colored flag leaves having a culm length of 64.0–72.0 cm, similar to Koshihikari, were segregated with a theoretical one gene inheritance ratio of 12:22:10 (1 sd1 homozygous:2 Sd1sd1 heterozygous:1 Sd1 homozygous (χ2 = 0.18, 0.90 < p < 0.95)). Regarding grain size, large-grain GW2 homozygous plants with a grain area of 22.4–23.8 cm2, intermediate heterozygotes with a grain area of 21.0–22.3 cm2, and small-grain gw2 homozygous plants with a grain area of 19.0–20.9 cm2 were found to segregate in a ratio of 29:43:20 (1 GW2 homozygous:2 GW2gw2 heterozygous:1 gw2 homozygous) and fitted to the theoretical one gene inheritance ratio (χ2 = 4.5, 0.09 < p < 0.10). Thus, these BC5F2 plant were segregated as 50 long-culm/small-grain, 21 long-culm/large-grain, 15 semidwarf/small-grain, and 8 semidwarf/large-grain type plants (9(Sd1Sd1gw2gw2 + 2Sd1Sd1GW2gw2 + 2Sd1sd1gw2gw2 + 4Sd1sd1GW2gw2):3(Sd1Sd1GW2GW2 + 2Sd1sd1GW2Gw2):3(sd1sd1gw2gw2 + 2sd1sd1GW2gw2):1(sd1sd1GW2Gw2) (χ2 = 0.77, 0.80 < p < 0.90)); namely, GW2 and sd1 were independently inherited. For semidwarf and large-grain segregants in BC5F2, we conducted a genetic diagnosis for GW2 using the dCAP marker. As a result, three GW2sd1 homozygous plants were identified (Figure 5).
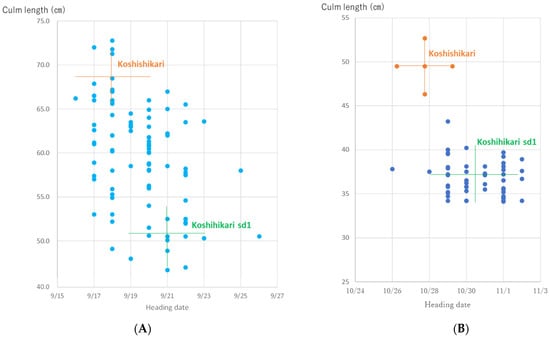
Figure 4.
Relationship between ear emergence period and culm length in Koshihikari sd1 × 2//Koshihikari × 3/((Koshihikari × Inochinoichi) F3 GW2 homozygous) BC5 plants. (A) sd1 homozygous with short/thick/dark green flag leaves having a culm length of 42.5–54.9 cm, similar to Koshihikari sd1, the intermediate heterozygous having a culm length of 55.3–63.6 cm, and Sd1 homozygous with long/light-colored flag leaves having a culm length of 64.0–72.0 cm, similar to Koshihikari, were segregated with a theoretical one gene inheritance ratio of 12:22:10 (1 sd1 homozygous:2 Sd1sd1 heterozygous:1 Sd1 homozygous (χ2 = 0.18, 0.90 < p < 0.95)). Regarding grain size, large-grain GW2 homozygous plants with a grain area of 28.2–31.3 cm2, intermediate heterozygotes, and small-grain gw2 homozygous plants with a grain area of 25.2 to 27.8 cm2 were found to segregate in a ratio of 29:43:20 (1 GW2 homozygous:2 GW2gw2 heterozygous:1 gw2 homozygous) and fitted to the theoretical one gene inheritance ratio (χ2 = 4.5, 0.09 < p < 0.10). (B) The culm length of BC5F3 plants ranged from 29.2 to 43.2 cm and was similar to that of Koshihikari sd1, which ranged from 32.5 to 39.2 cm. Therefore, these BC5F3 plants were fixed in sd1 homozygous.
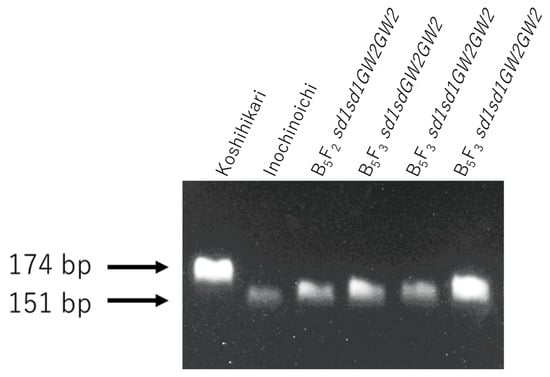
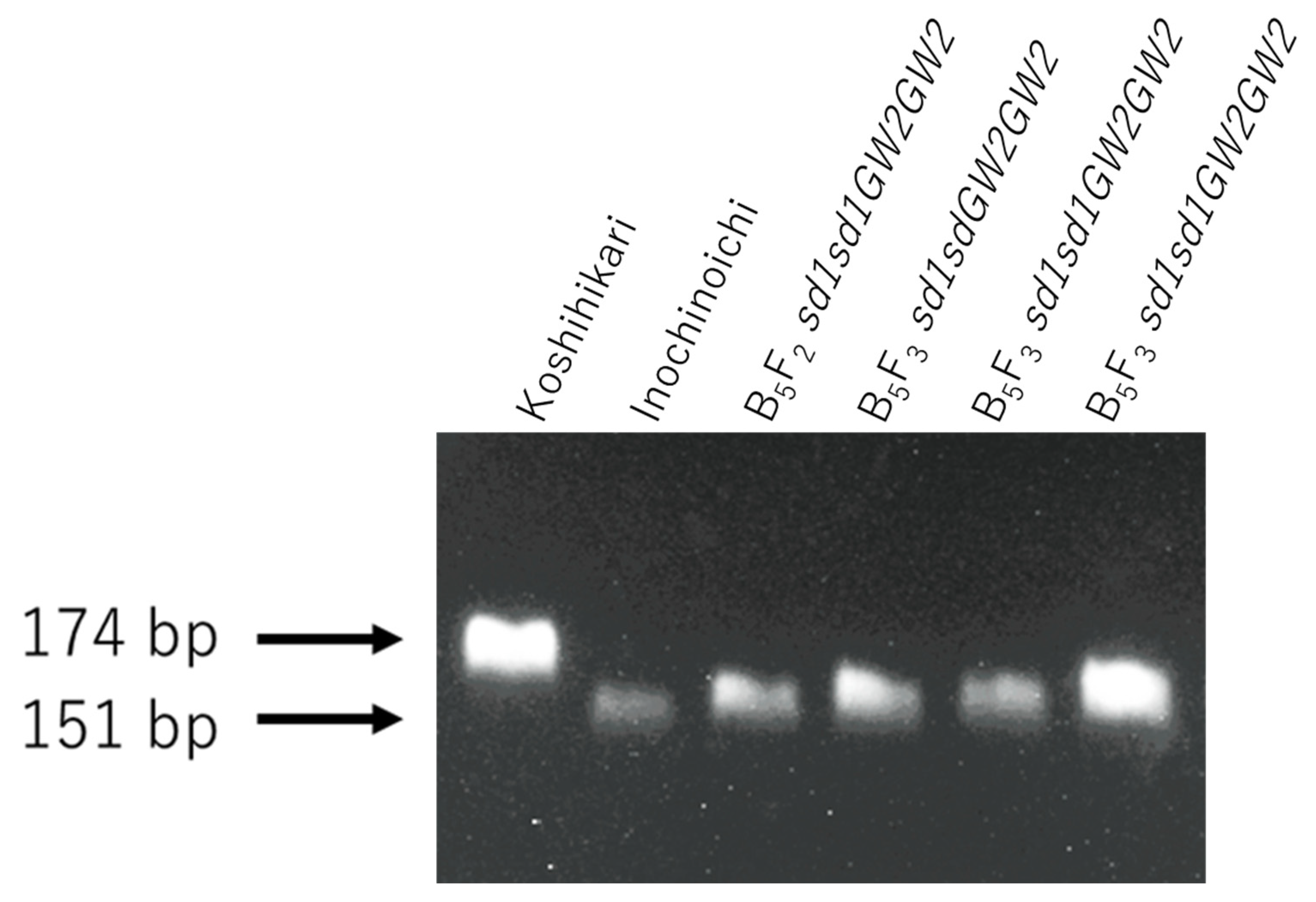
Figure 5.
Genetic screening of BC5F2 and BC5F3 plants using the dCAP marker GW2-Hpa I. Genetic diagnosis for GW2 using the dCAP marker was conducted for semidwarf and large-grain segregants in BC5F2 and the progeny BC5F3. As a result, three GW2sd1 homozygous BC5F3 plants were identified.
The relationship between the ear emergence period and culm length of the BC5F3 progeny of GW2sd1 homozygous BC5F2 plants (Figure 4A) is shown in Figure 4B. The culm length of BC5F3 plants ranged from 29.2 to 43.2 cm and was similar to that of Koshihikari sd1, which ranged from 32.5 to 39.2 cm (Figure 4B). Therefore, these BC5F3 plants were fixed in an sd1 homozygote. Genetic diagnosis of three arbitrarily selected plants using dCAP markers revealed that all plants were GW2 homozygous type (Figure 5).
3.2. Whole-Genome Sequencing of “Koshihikari sd1GW2”
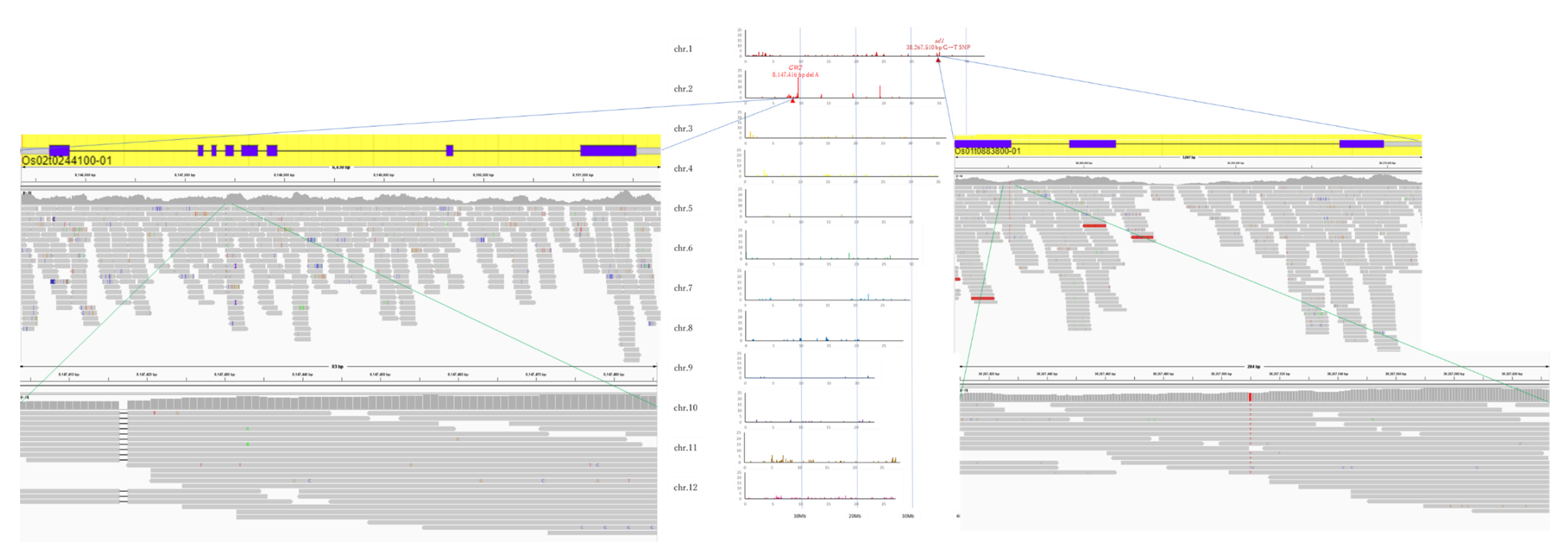
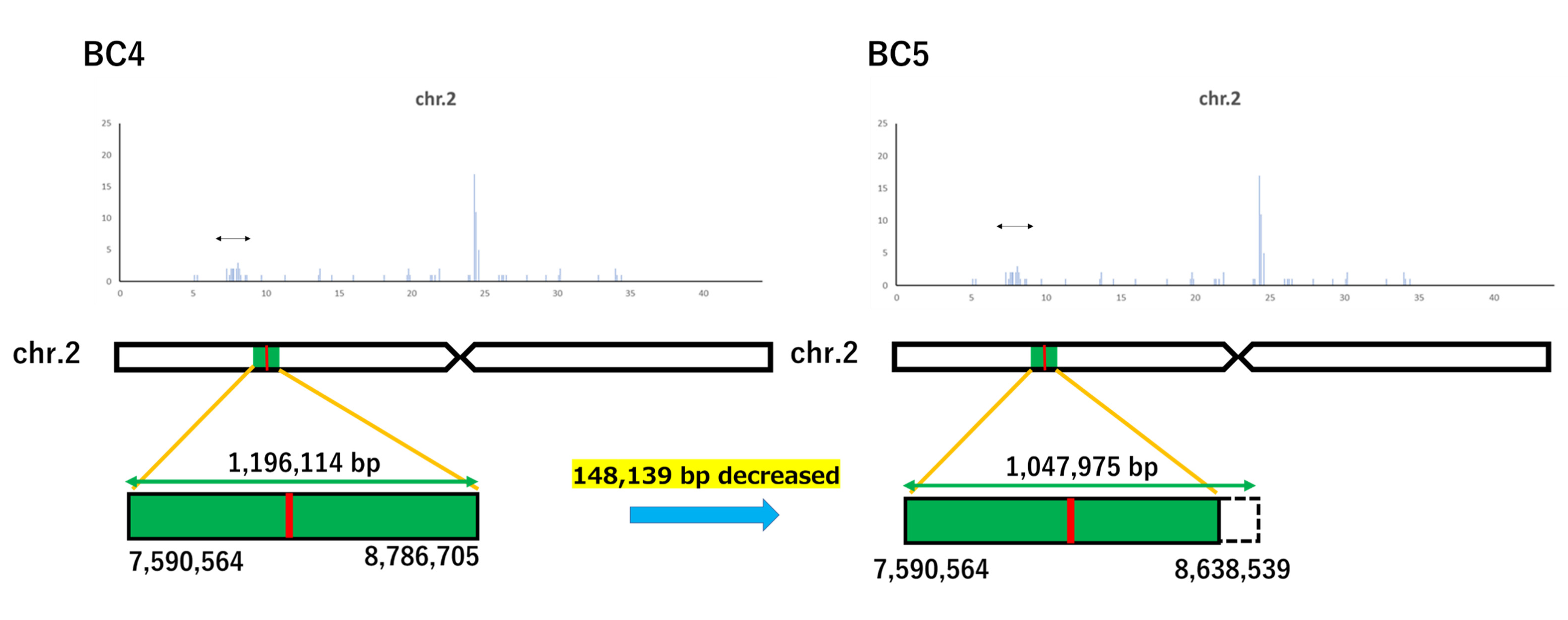
The gained reads of Koshihikari GW2 and Koshihikari sd1GW2 were mapped to the consensus sequence of Koshihikari as the reference, and the mean coverage was 48.41 and 17.55, respectively. We clarified that one deletion of adenine was detected in the GW2 gene at 8,147,416 bp on chromosome 2, and a single SNP from thymine to guanine was detected in the sd1 gene at 38,267,510 bp on chromosome 1 in an isogenic large-grain Koshihikari integrated with GW2 and sd1 (Figure 6). Except for the region around GW2 and sd1, the SNPs were less than 10 per 0.1 Mb. The results indicated that a large portion of the 12 chromosomes of rice was substituted to the genome of Koshihikari after continuous backcross targeting these two genes. The size of the DNA fragment integrated with GW2 was determined as the distance between both ends of the SNP cluster (Figure 7). Through the backcrossing from BC4 to BC5, the size of the DNA fragment integrated with GW2 decreased by 148,139 bp. There existed 166 annotated genes in the integrated DNA fragment. Among them were mutations in two genes, such as the hydroquinone glucosyltransferase gene (Table 1).
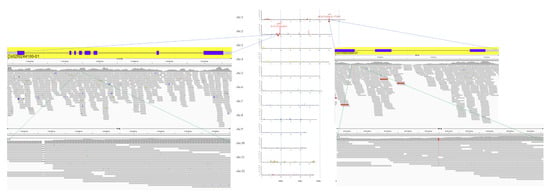
Figure 6.
Causative SNP for GW2 and sd1 in Koshihikari sd1GW2 (BC5F2). In Koshihikari sd1GW2 (BC5F2), one deletion of adenine was detected in the GW2 gene at 8,147,416 bp on chromosome 2, and a single SNP from thymine to guanine was detected in the sd1 gene at 38,267,510 bp on chromosome 1.
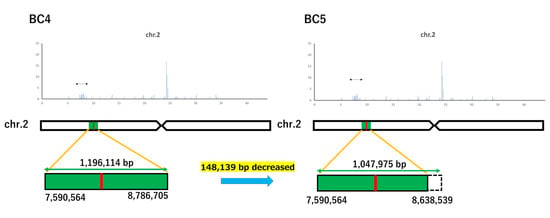
Figure 7.
Transitional changes in the size of DNA fragments integrated with GW2 via backcrossing. The size of the DNA fragment integrated with GW2 was determined as the distance between both ends of the SNP cluster. However, through the backcrossing from BC4 to BC6, the size of the DNA fragment integrated with GW2 decreased by 148,139 bp.

Table 1.
Mutant genes integrated with GW2 via backcrossing.
3.3. Trait Expression of “Koshihikari sd1GW2”, a Large-Grain/Semidwarf Type Isogenic Line That Integrates sd1 and GW2 in the Genetic Background of Koshihikari
Compared with Koshihikari, the large-grain/semidwarf isogenic line Koshihikari sd1GW2 was fixed to the sd1GW2 homozygous genotype in BC5F3 and was 12.6 cm shorter (Figure 8). For the sd1GW2 homozygous plants identified in BC5F2 and BC5F3 progeny, we measured the length, width, and surface area of grains on the tip of the main stem and compared these values with those obtained in Koshihikari, Inochinoichi, and Koshihikari GW2 (BC6F3) (Table 2). We found that the grain width of Koshihikari sd1GW2 (BC5F2, BC5F3) (3.87 mm) was approximately 1.11 times that of Koshihikari; however, it was almost the same as that of InochinoIchi (3.84 mm) and Koshihikari GW2 (BC6F3) (3.93 mm), which were 1.10 and 1.13 times that of Koshihikari, respectively. The grain surface area of Koshihikari sd1GW2 (23.5 mm2) was 17% larger than that of Koshihikari (20.1 cm2) but was slightly smaller than that of InochinoIchi (25.7 mm2) and Koshihikari GW2 (BC6F3) (24.3 mm2), which were 1.28 and 1.21 times that of Koshihikari (20.1 mm2) (Table 2, Figure 8). The thousand-grain weight of Koshihikari sd1GW2 (27.8 g) was 18% greater than that of Koshihikari (23.6 g), and the grain yield of Koshihikari sd1GW2 (42.6 kg/a) was 5% higher than that of Koshihikari (42.4 kg/a) (Table 3).

Figure 8.
Phenotype of Koshihikari sd1GW2 (Koshihikari sd1 × //Koshihikari × 3/((Koshihikari × Inochinoichi) F3) BC5F3. Compared with Koshihikari, the large-grain/semidwarf isogenic line Koshihikari sd1GW2 was fixed to sd1GW2 homozygous genotype in BC5F3 and was 12.6 cm shorter. The grain surface area of Koshihikari GW2sd1 (23.5 mm2) was 17% larger than that of Koshihikari (20.1 cm2).

Table 2.
Grain size of Koshihikari sd1GW2.

Table 3.
Comparison of agronomic characteristics of Koshihikari, Koshihikari GW2, Koshihikari sd1, and Koshihikari sd1GW2.
4. Discussion
Crops in Japan and worldwide are damaged by climate change caused by global warming. There has been increased damage from lodging caused by severe weather events such as the Western Japan floods and multiple typhoons under the recently intensified climate change. The Japanese government introduced an innovation policy to contribute to the world by developing high-yield crops for a “New Green Revolution”. The rice variety “Inochinoichi” is highly rated at both the consumer and producer levels. However, the genes that control its large grain size, approximately 1.5 times that of Koshihikari, have not been elucidated; hence, the production of “Inochinoichi” is limited to the area around Gifu Prefecture. In a previous study, we identified the gene GW2 responsible for the large grain size of Inochinoichi, an unused and buried genetic resource, and then applied the gene to develop a large-grain isogenic line of Koshihikari (Koshihikari GW2) [27]. Furthermore, in this study, we developed a large-grain/semidwarf isogenic line (Koshihikari sd1GW2) that incorporates both the large-grain gene GW2 and semidwarf gene sd1, which we believe is a large-grain rice cultivar with enhanced resistance to lodging. Through backcrossing, sd1 (chromosome 1) and GW2 (chromosome 2) were inherited independently, and we finally acquired a two-gene homozygous isogenic line, Koshihikari sd1GW2.
There was no public database on the consensus sequence of Koshihikari; thus, we constructed a consensus sequence of Koshihikari via a high-coverage whole-genome analysis. We conducted resequencing analyses using a consensus sequence of Koshihikari as a reference. In our study, we clarified that one deletion of adenine was detected in the GW2 gene at 8,147,416 bp on chromosome 2, and a single SNP from thymine to guanine was detected in the sd1 gene at 38,267,510 bp on chromosome 1 in an isogenic large-grain Koshihikari integrated with GW2 and sd1. The size of the DNA fragment integrated with GW2 was determined as the distance between both ends of the SNP cluster. Through the backcrossing from BC4 to BC6, the size of the DNA fragment integrated with GW2 decreased by 148,139 bp.
GW2 was reported as the causative gene responsible for grain width in large-grain Japonica rice WY3. This allele (Os02g024410) encodes a RING protein with E3 ubiquitin ligase activity [33], which acts as a breakdown in the ubiquitin proteasome pathway [34]. The RING-type E3 ubiquitin ligase can control seed development by catalyzing the ubiquitination of expansion-like 1 (EXPLA1), a cell wall-loosening protein that increases cell growth [35] through the GW2-WG1-OsbZIP47 pathway [36]. In contrast, GW2 derived from WY3 lost function due to a frameshift caused by a single nucleotide deletion. In a near-isogenic line FAZ1 with GW2 derived from WY3, the width of the awn was extended by 26.2% because of the increased cell number [33], and the silencing of RING protein with E3 ubiquitin ligase also increases grain width and weight in indica rice [37]. In the present study, GW2 from Inochinoichi was also identified as a loss of function due to the single nucleotide deletion, increasing grain weight by 34% in the genetic background of Koshihikari. The grain surface area of Koshihikari GW2sd1 (29.7 mm2) was 14% larger than that of Koshihikari (26.1 cm2). In addition, compared with Koshihikari, the large-grain/semidwarf isogenic line Koshihikari sd1GW2, which was fixed to the sd1GW2 homozygous genotype in BC5F3, was 12.6 cm shorter. The semidwarf gene sd1 was well known as a contribution to the “miracle rice” variety IR8 released by the International Rice Research Institute (IRRI), which responds well to fertilizer inputs and produces increased yields without culm elongation [6]. The widespread adoption of IR8 brought about a “Green Revolution” in the monsoonal regions of Asia, where typhoons frequently occur during the yielding season. Since semidwarfness brings benefits such as erecting leaf angles, reducing photoinhibition, and planting at higher densities, rice yields worldwide have doubled due to the breeding of semidwarf varieties. We have developed a new variety by combining a large-grain gene and semidwarf gene. We believe that this new germplasm, which compatibly realizes both plant robustness and enlarged grain production, will significantly contribute to the “New Green Revolution”. It will be helpful in meeting the imminent challenges of population growth, climate change, and increasing globalization. A high-yield breakthrough could be brought about by integrating/adding genes related to high yield, including large grains and increased biomass, on the foundation of conventional semidwarfism. In this study, we combined the semidwarfing gene sd1 and the large-grain gene GW2 in the isogenic background of Koshihikari.
However, market liberalization through the Comprehensive and Progressive Agreement for Trans-Pacific Partnership (CPTPP) and TAG negotiations may cause international competition in the rice market, resulting in the need for low-cost and high-yielding rice. The taste score of Koshihikari sd1GW2 (80.0) is comparable to that of Niigata Koshihikari (81.0). Grain-size-enlarged Koshihikari has the potential to become advantageously discriminated against US-made Koshihikari. The Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) has registered the large-grain isogenic Koshihikari, which was integrated with GW2, designated as a new plant variety “Koshihikari Suruga Gg” [38], under Japanese varietal protection. Furthermore, Koshihikari sd1GW2 (BC5F3), integrated with sd1 and GW2 in an isogenic genetic background of Koshihikari, is characterized by larger grains and shorter height compared to those of Koshihikari. Consequently, this semidwarf Koshihikari sd1GW2 will be less susceptible to lodging by typhoons, cyclones, and heavy rainfall, ordinarily a concern in heavier panicle weight cultivars. We have submitted a Japanese varietal protection application for the large-grain and semidwarf isogenic Koshihikari, designated as a new plant variety “Koshihikari Suruga sd1Gw” [39].
5. Conclusions
The “Green Revolution” brought about by a single semidwarf gene sd1 has undoubtedly contributed to the stability of rice productivity. However, rice productivity is currently reaching a plateau, and furthermore, intensified climate change calls for high-yielding robust rice cultivars. In this study, we successfully integrated the large-grain gene GW2 with sd1 for the first time, specifically in the genome of the globally produced leading Japonica cultivar Koshihikari. This isogenic Koshihikari which has both sd1 and GW2 was designated as Koshihikari sd1GW2. Koshihikari sd1GW2 is a lodging-resistant panicle weight cultivar, which will correspond with sustainable agriculture.
Author Contributions
Conceptualization, M.T.; methodology, M.T.; investigation, M.T., H.E. and K.N.; resources—M.T.; writing—original draft preparation, M.T.; writing—review and editing, M.T.; project administration, M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Adaptable and Seamless Technology Transfer Program (A-STEP), Industry-Academia Joint Promotion Stage, High-Risk Challenge Type grant by Japan Science and Technology Agency (JST) to Motonori Tomita, project ID14529973, titled “Development of super high yield/large grain/early and late ripening rice suitable to the era of globalization and global warming by Next-Generation Sequencer/genome-wide analysis”, from 2014 to 2018 and by the Program for Creating Start-ups from Advanced Research and Technology (START), Project Promotion Type grant (Supporting Small Business Innovation Research (SBIR) Phase 1) by Japan Science and Technology Agency (JST) to Motonori Tomita, project ID21482194, titled “Process innovation through development of robust and high-yielding plants suitable for climate crisis and automated agriculture based on smart genome breeding”, from 2021 to 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated in this study are present in the main manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Japan Meteorological Agency. Heisei 30 July 7 Heavy Rain and Floods. Available online: https://www.data.jma.go.jp/obd/stats/data/bosai/report/2018/20180713/20180713.html (accessed on 12 August 2022).
- Japan Meteorological Agency. 2018 (Heisei 30) Typhoons (Quick Report). Available online: https://www.jma.go.jp/jma/press/1812/21f/typhoon2018.pdf (accessed on 12 August 2022).
- Japan Meteorological Agency. Monthly Values from Observation Commencement. Available online: http://www.data.jma.go.jp/obd/stats/etrn/view/monthly_s3.php?prec_no=44&block_no=47662/ (accessed on 12 August 2022).
- Japan Ministry of Agriculture, Forestry and Fisheries. Damage Situation by Heisei 30 July 7 Heavy Rain and Floods. Available online: https://www.maff.go.jp/j/saigai/ooame/20180628.html/ (accessed on 12 August 2022).
- Japan Meteorological Agency. Long-Term Changes in the Number and Frequency of Short-Term Heavy Rains Observed at AMeDAS. Available online: http://www.jma.go.jp/jma/kishou/info/heavyraintrend.html (accessed on 12 August 2022).
- Hergrove, T.; Coffman, W.R. Breeding History. In Rice That Changed the World: Cerebrating 50 Years of IR8; Rice Today: Laguna, Philippines, 2016; pp. 6–10. [Google Scholar]
- Khush, G.S. Green revolution: Preparing for the 21st century. Genome 1999, 42, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Hu, Y.G.; Qian, Q.; Ren, D.Y. Progress and prospect of breeding utilization of green revolution gene SD1 in rice. Agriculture 2021, 11, 611. [Google Scholar] [CrossRef]
- Gaur, V.S.; Channappa, G.; Chakraborti, M.; Sharma, T.R.; Mondal, T.K. ‘Green revolution’ dwarf gene sd1 of rice has gigantic impact. Brief. Funct. Genom. 2020, 19, 390–409. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef]
- Monna, L.; Kitazawa, N.; Yoshino, R.; Suzuki, J.; Masuda, H.; Maehara, Y.; Tanji, M.; Sato, M.; Nasu, S.; Minobe, Y. Positional cloning of rice semidwarfing gene, sd-1: Rice “Green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 2002, 9, 11–17. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Cho, Y.G.; Eun, M.Y.; Kim, Y.K.; Chung, T.Y.; Chae, Y.A. The semidwarf gene, sd-1, of rice (Oryza sativa L.). 1. Linkage with the esterase locus, EstI-2. Theor. Appl. Genet. 1994, 89, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.G.; Eun, M.Y.; McCouch, S.R.; Chae, Y.A. The semidwarf gene, sd-1, of rice (Oryza sativa L.) 2 Molecular mapping and marker-assisted selection. Theor. Appl. Genet. 1994, 89, 54–59. [Google Scholar] [CrossRef]
- Maeda, H.; Ishii, T.; Mori, H.; Kuroda, J.; Horimoto, M.; Takamure, I.; Kinoshita, T.; Kamijima, O. High density molecular map of semidwarfing gene, sd-1, in rice (Oryza sativa L.). Jpn. J. Breed. 1997, 47, 317–320. [Google Scholar] [CrossRef]
- Hedden, P. Constructing dwarf rice. Nat. Biotechnol. 2003, 21, 873–874. [Google Scholar] [CrossRef]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Tomita, M.; Ishii, K. Genetic performance of the semidwarfing allele sd1 derived from a Japonica rice cultivar and minimum requirements to detect its single-nucleotide polymorphism by MiSeq whole-genome sequencing. BioMed Res. Int. 2018, 2018, 4241725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bian, Z.; Liu, Q.Q. Exploration and selection of elite Sd1 alleles for rice design breeding. Mol. Breed. 2020, 40, 79. [Google Scholar] [CrossRef]
- San, N.S.; Suzuki, K.; Ookawa, T. Semi-dwarf 1 (sd1) gene enhances light penetration into the canopy through regulating leaf inclination angle in rice. Field Crops Res. 2020, 246, 107694. [Google Scholar] [CrossRef]
- Sha, H.J.; Liu, H.L.; Fang, J. Elite sd1 alleles in japonica rice and their breeding applications in northeast China. Crop J. 2022, 10, 224–233. [Google Scholar] [CrossRef]
- Tomita, M. Introgression of Green Revolution sd1 gene into isogenic genome of rice super cultivar Koshihikari to create novel semidwarf cultivar ‘Hikarishinseiki’ (Koshihikari-sd1). Field Crops Res. 2009, 114, 173–181. [Google Scholar] [CrossRef]
- Tomita, M.; Matsumoto, S. Transcription of rice green revolution gene sd1 is clarified by comparative RNA diagnosis using the isogenic background. Genom. Appl. Biol. 2011, 2, 29–35. [Google Scholar]
- MAFF. Hikarishinseiki varietal registration. In Official Gazette 8 November; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2004; Volume 8. [Google Scholar]
- MAFF. Hikarishinseiki brand rice description in Okayama and Tottori prefectures. In Official Gazette 28 March; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2007. [Google Scholar]
- Tomita, M. Hikarishinseiki. In Oryza sativa L. Plant Variety Protection Number: 201000072; USDA-AMS-ST-PVPO; USDA: Washington, DC, USA, 2013. Available online: http://www.ars-grin.gov/cgi-bin/npgs/pvp/showpvp.pl?pvpno=201000072 (accessed on 12 August 2022).
- Tomita, M.; Yazawa, S.; Uenishi, Y. Identification of rice large grain gene GW2 by whole-genome sequencing of a large grain-isogenic line integrated with Japonica native gene and its linkage relationship with the co-integrated semidwarf gene d60 on Chromosome 2. Int. J. Mol. Sci. 2019, 20, 5442. [Google Scholar] [CrossRef]
- Nemoto, M.; Hamasaki, T.; Matsuba, S.; Hayashi, S.; Yanagihara, S. Estimation of rice yield components with meteorological elements divided according to developmental stages. Nogyokishou 2016, 72, 128–141. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2014, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The sequence alignment/map (SAM) format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kemytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Hauksdóttir, H.; Troy, A.; Herschleb, J.; Kraft, E.; Callis, J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005, 137, 13–30. [Google Scholar] [CrossRef]
- Choi, B.S.; Kim, Y.J.; Markkandan, K.; Koo, Y.J.; Song, J.T.; Seo, H.S. GW2 functions as an E3 Ubiquitin ligase for rice Expansin-Like 1. Int J. Mol. Sci. 2018, 19, 1904. [Google Scholar] [CrossRef]
- Hao, J.; Wang, D.; Wu, Y.; Huang, K.; Duan, P.; Li, N.; Ran, X.; Dali, Z.; Guojun, D.; Baolan, Z.; et al. The GW2-WG1-OsbZIP47 pathway controls grain size and weight in rice. Mol. Plant 2021, 14, 1266–1280. [Google Scholar] [CrossRef]
- Verma, A.; Prakash, G.; Ranjan, R.; Tyagi, A.K.; Agarwal, P. Silencing of an ubiquitin ligase increases grain width and weight in indica rice. Front. Genet. 2021, 11, 600378. [Google Scholar] [CrossRef]
- Official Gazette No. 391, 18 March 2021, Ministry of Agriculture, Forestry and Fisheries. Plant Varietal Registration No. 28387, “Koshihaikri Suruga Gg”, Tokyo, Japan. Available online: http://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=28387&LANGUAGE=Japanese (accessed on 12 August 2022).
- Official Gazette No. 1321, 5 August 2021, Ministry of Agriculture, Forestry and Fisheries. Plant Varietal Registration Application No. 35370, “Koshihikari Suruga sd1Gw”, Tokyo, Japan. Available online: http://www.hinshu2.maff.go.jp/vips/cmm/apCMM111.aspx?SHUTSUGAN_NO=35370&LANGUAGE=Japanese (accessed on 12 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).