Moving towards Valorization of Biowastes Issued from Biotrickling Filtration of Contaminated Gaseous Streams: A Thermochemical Analysis-Based Perspective
Abstract
:1. Introduction
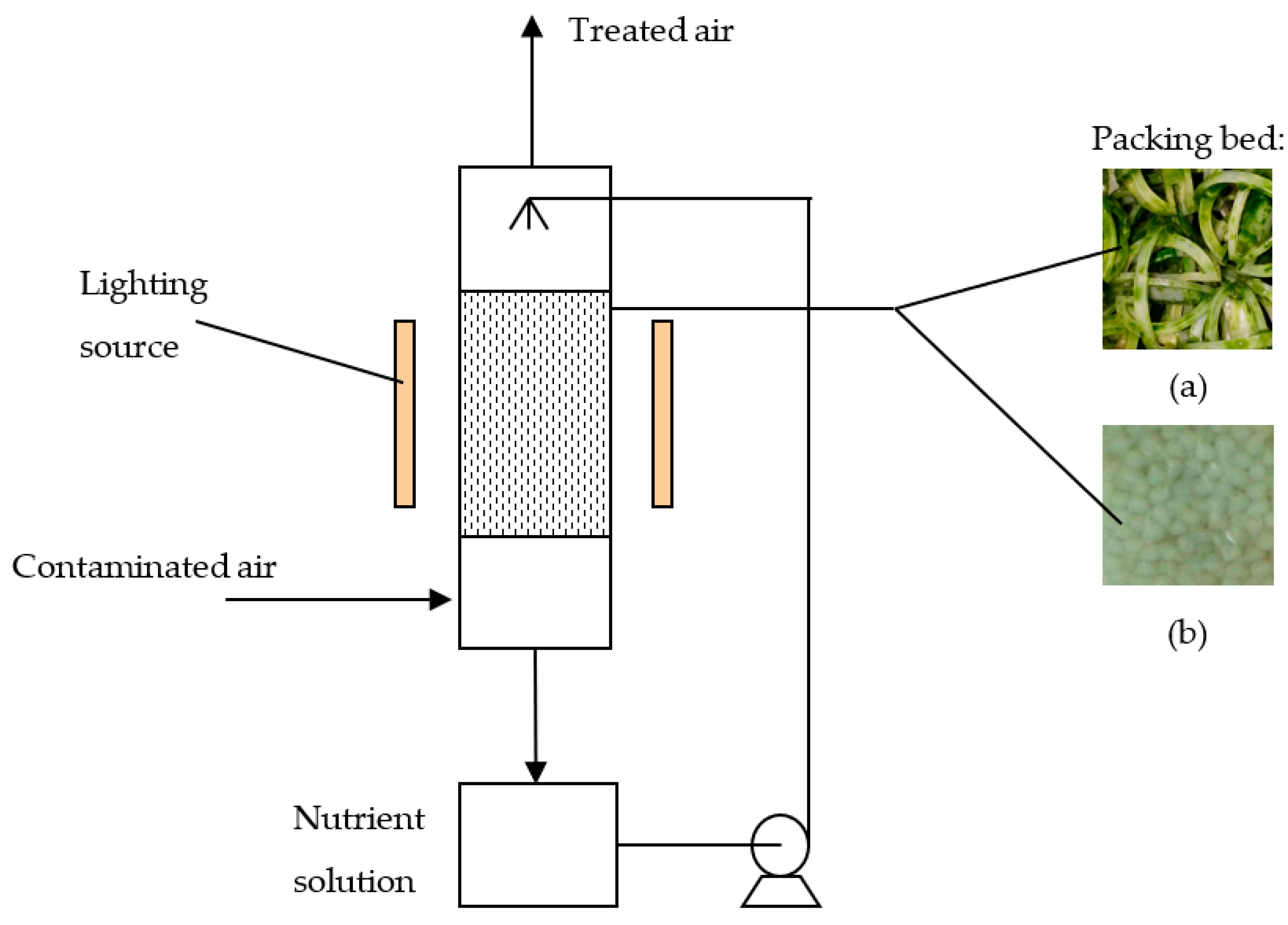
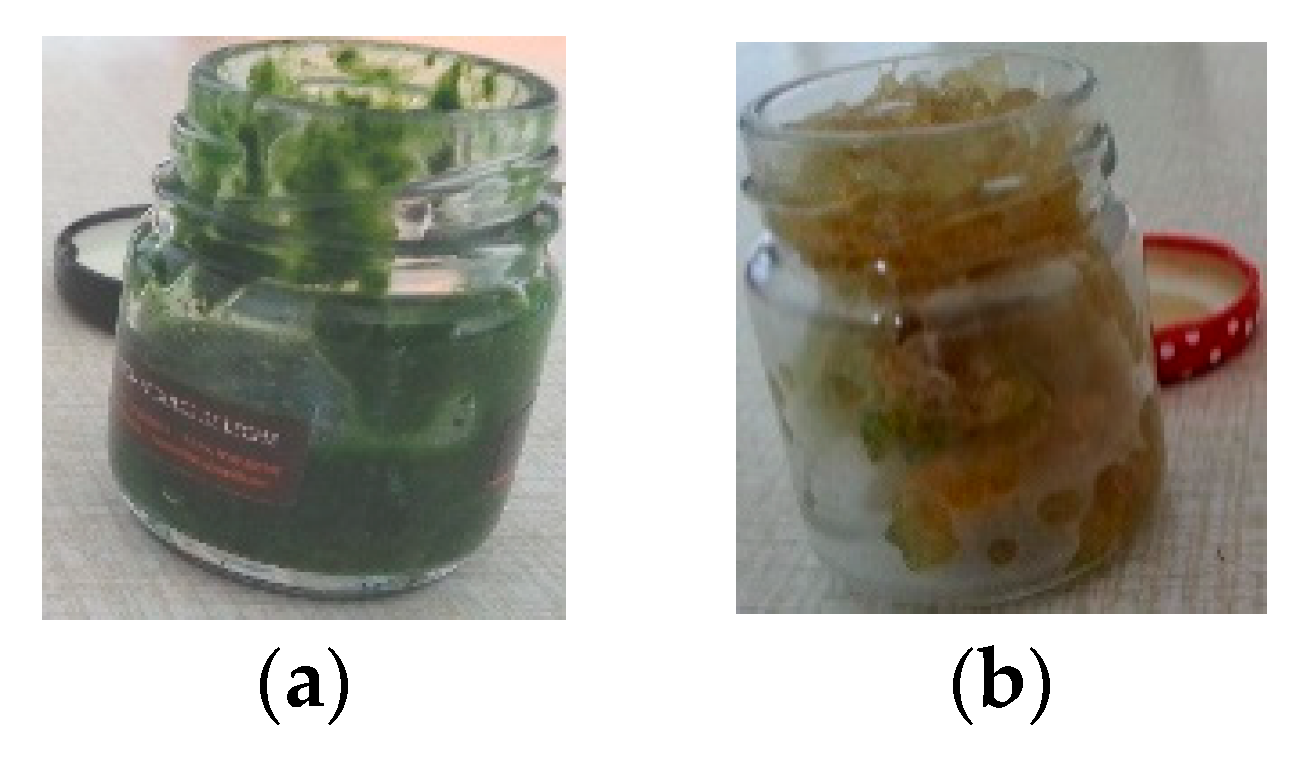
2. Materials and Methods
3. Results and Discussion
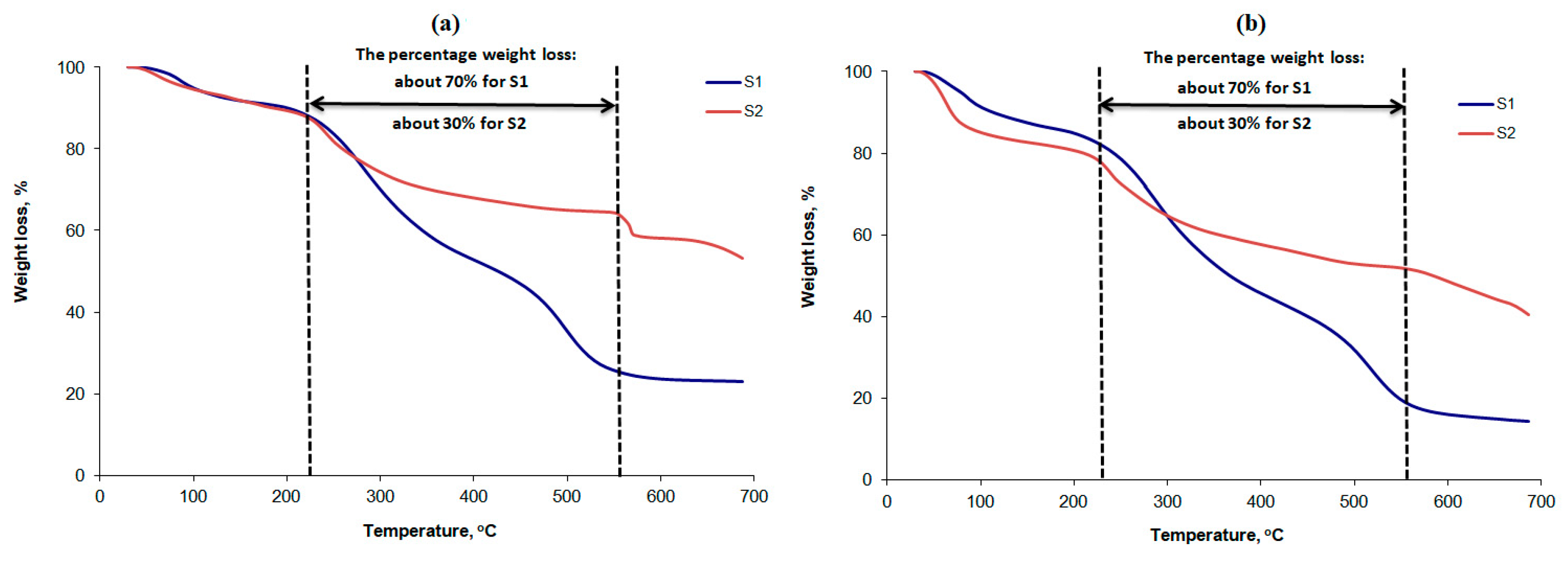
3.1. Assessment of Thermal Decomposition in Air and Nitrogen
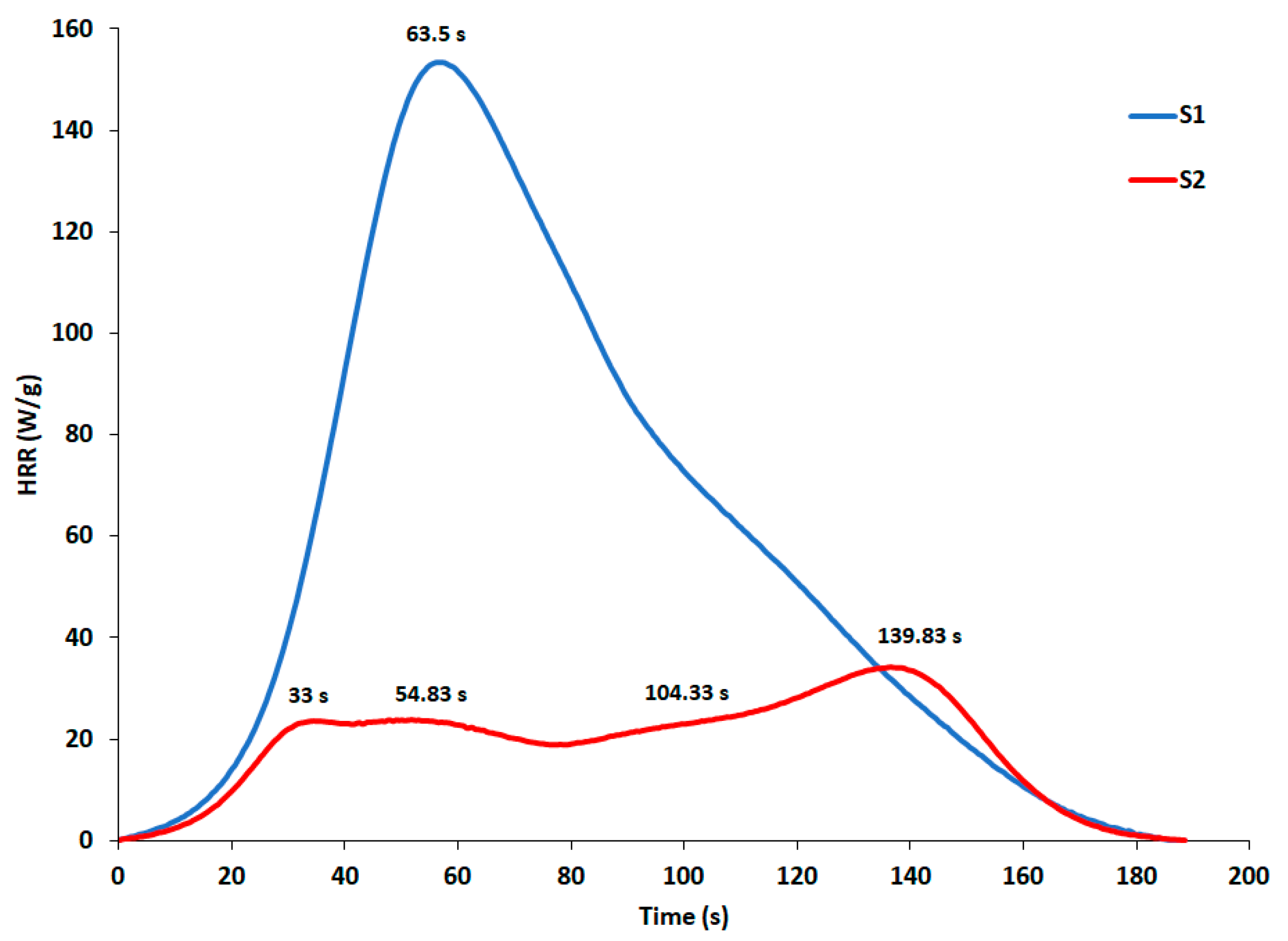
3.2. Assessment of Energy Characteristics by Microscale Combustion Calorimetry (MCC)
3.3. Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soreanu, G.; Diaconu, M.; Maier, S.S.; Volf, I.; Cretescu, I. Enhancing the environmental performance of biotrickling filters treating volatile organic compounds in air. IOP Conf. Ser. Earth Environ. Sci. 2021, 906, 012124. [Google Scholar] [CrossRef]
- Soreanu, G.; Diaconu, M.; Maier, S.S.; Volf, I.; Cretescu, I. Moving forward sustainable solutions for VOCs biotrickling filtration through co-immobilised microorganisms. Rom. J. Ecol. Environ. Chem. 2021, 3, 54–60. [Google Scholar] [CrossRef]
- Mata, T.M.; Oliveira, G.M.; Monteiro, H.; Silva, G.V.; Caetano, N.S.; Martins, A.A. Indoor Air Quality Improvement Using Nature-Based Solutions: Design Proposals to Greener Cities. Int. J. Environ. Res. Public Health 2021, 18, 8472. [Google Scholar] [CrossRef] [PubMed]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A Review: Microalgae and Their Applications in CO2 Capture and Re-newable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; Pimentel, T.C.; Sant’Ana, A.S.; Magnani, M. Microalgae in the meat processing chain: Feed for animal production or source of techno-functional ingredients. Curr. Opin. Food Sci. 2021, 37, 125–134. [Google Scholar] [CrossRef]
- Moon, M.; Park, W.-K.; Lee, S.Y.; Hwang, K.-R.; Lee, S.; Kim, M.-S.; Kim, B.; Oh, Y.-K.; Lee, J.-S. Utilization of whole microalgal biomass for advanced biofuel and biorefinery applications. Renew. Sustain. Energy Rev. 2022, 160, 112269. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.; Yap, Y.T.; Danquah, M.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Ravichandran, S.R.; Sengottian, M. Lignocellulosic and algal biomass for bio-crude production using hydrothermal liquefaction: Conversion techniques, mechanism and process conditions: A review. Environ. Eng. Res. 2021, 27, 200555. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; He, Y. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresour. Technol. 2012, 117, 264–273. [Google Scholar] [CrossRef]
- Baloyi, H.; Dugmore, G. Influences of microalgae biomass on the thermal behaviour of waste coal fines. J. Energy S. Afr. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Azizi, K.; Haghighi, A.M.; Moraveji, M.K.; Olazar, M.; Lopez, G. Co-pyrolysis of binary and ternary mixtures of microalgae, wood and waste tires through TGA. Renew. Energy 2019, 142, 264–271. [Google Scholar] [CrossRef]
- Sukarni, S. Thermogravimetric analysis of the combustion of marine microalgae Spirulina platensis and its blend with synthetic waste. Heliyon 2020, 6, e04902. [Google Scholar] [CrossRef] [PubMed]
- Dessì, F.; Mureddu, M.; Ferrara, F.; Fermoso, J.; Orsini, A.; Sanna, A.; Pettinau, A. Thermogravimetric characterisation and kinetic analysis of Nannochloropsis sp. and Tetraselmis sp. microalgae for pyrolysis, combustion and oxy-combustion. Energy 2020, 217, 119394. [Google Scholar] [CrossRef]
- López-González, D.; Fernandez-Lopez, M.; Valverde, J.; Sanchez-Silva, L. Kinetic analysis and thermal characterization of the microalgae combustion process by thermal analysis coupled to mass spectrometry. Appl. Energy 2014, 114, 227–237. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dąbrowska, A.; Muñoz, R.; Gębicki, J. A state of the art review on the use of fungi in biofiltration to remove volatile hydrophobic pollutants. Rev. Environ. Sci. Bio/Technol. 2022, 21, 225–246. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Estrada, A.; Morales, M. Carbon dioxide capture and utilization using microalgae. In Handbook of Microalgae-Based Processes and Products /Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Elsevier: London, UK, 2020; Chapter 8; pp. 185–206. [Google Scholar] [CrossRef]
- Soreanu, G.; Cretescu, I.; Diaconu, M.; Ignat, M.; Harabagiu, V.; Cojocaru, C.; Samoila, P. A model microalga for addresing air treatment in spacecrafts, Chapter 19. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment—Recent Develoments, New Trends, Advances and Opportunities; Soreanu, G., Dumont, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 397–417. [Google Scholar]
- da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt for-mulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef]
- Gouda, N.; Panda, A.K.; Meher, S.N. Kinetic study of thermal degradation of microalgae Arthrospira platensis by iso-conversational methods. Res. J. Chem. Environ. 2018, 22, 22–28. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Biochemical and Thermal Analysis of Spirulina Biomass through FTIR, TGA, CHN. Energy Eng. 2021, 118, 1045–1056. [Google Scholar] [CrossRef]
- Ananda, V.; Sunjeeva, V.; Vinu, R. Catalytic fast pyrolysis of Arthrospira platensis (spirulina) algae using zeolites. J. Anal. Appl. Pyrolysis 2016, 118, 298–307. [Google Scholar] [CrossRef]
- de Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Biofunctionalized Nanofibers Using Arthrospira(Spirulina) Biomass and Biopolymer. BioMed Res. Int. 2015, 2015, 967814. [Google Scholar] [CrossRef] [Green Version]
- Jamilatun, S.; Budhijanto, B.; Rochmadi, R.; Budiman, A. Thermal Decomposition and Kinetic Studies of Pyrolysis of Spirulina Platensis Residue. Int. J. Renew. Energy Dev. 2017, 6, 193–201. [Google Scholar] [CrossRef]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and Thermal Properties of Calcium Alginate/Reduced Graphene Oxide Composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.D.S.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal degradation of calcium and sodium alginate: A greener synthesis towards calcium oxide micro/nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Quim. J. 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Ayoola, B.; Balachandran, R.; Frank, J.; Mastorakos, E.; Kaminski, C. Spatially resolved heat release rate measurements in turbulent premixed flames. Combust. Flame 2006, 144, 1–16. [Google Scholar] [CrossRef]
- Gady, O.; Poirson, M.; Vincent, T.; Sonnier, R.; Guibal, E. Elaboration of light composite materials based on alginate and algal biomass for flame retardancy: Preliminary tests. J. Mater. Sci. 2016, 51, 10035–10047. [Google Scholar] [CrossRef]
- Lyon, R.E.; Speitel, L.; Walters, R.N.; Crowley, S. Fire-resistant elastomers. Fire Mater. 2003, 27, 195–208. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Z.; Lei, Y. Flame retardant and smoke-suppressant rigid polyurethane foam based on sodium alginate and aluminum diethylphosphite. Des. Monomers Polym. 2021, 24, 46–52. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Dumazert, L.; Otazaghine, B.; Sonnier, R.; Guibal, E. Fire behavior of innovative alginate foams. Carbohydr. Polym. 2020, 250, 116910. [Google Scholar] [CrossRef]
- Cogen, J.M.; Lin, T.S.; Lyon, R.E. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame retardant polyolefin compounds. Fire Mater. 2008, 33, 33–50. [Google Scholar] [CrossRef]
- Li, G.; Dong, R.-J.; Fu, N.; Zhou, Y.-G.; Li, D.; Chen, X.D. Temperature-Oriented Pyrolysis on the Decomposition Characteristics of Chlorella pyrenoidosa. Int. J. Food Eng. 2015, 12, 295–301. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef] [Green Version]


| Sample | Working Atmosphere | Stage | Tonset, °C | Tpeak, °C | Tendset, °C | W, % | Residue, % |
|---|---|---|---|---|---|---|---|
| S1 | air | I | 62 | 88 | 138 | 9.14 | 22.55 |
| II | 236 | 283 | 344 | 38.14 | |||
| III | 454 | 495 | 553 | 30.17 | |||
| nitrogen | I | 49 | 84 | 143 | 14.65 | 13.74 | |
| II | 252 | 285 | 344 | 42.11 | |||
| III | 473 | 515 | 559 | 29.50 | |||
| S2 | air | I | 48 | 62 | 114 | 6.55 | 58.03 |
| II | 114 | 142 | 226 | 6.39 | |||
| III | 226 | 242 | 332 | 15.85 | |||
| IV | 332 | 448 | 558 | 6.60 | |||
| V | 558 | 566 | 570 | 6.58 | |||
| VI | 656 | – | – | 5.54 | |||
| nitrogen | I | 44 | 61 | 81 | 17.88 | 39.60 | |
| II | 217 | 237 | 332 | 20.81 | |||
| III | 332 | 454 | 564 | 9.22 | |||
| IV | 564 | – | 12.49 |
| Sample | Mass (mg) | Waste (mg) | Char Yield (wt%) | Decomposition Rate (%) | THR (kJ/g) | HRC (J/(g·K)) |
|---|---|---|---|---|---|---|
| S1 | 20.19 ± 0.06 | 6.44 ± 0.04 | 31.89 ± 0.20 | 68.11 ± 0.20 | 11.03 ± 0.39 | 95.67 ± 2.09 |
| S2 | 20.17 ± 0.03 | 10.92 ± 0.21 | 54.15 ± 1.01 | 45.85 ± 1.01 | 3.64 ± 0.20 | 44.11 ± 4.05 |
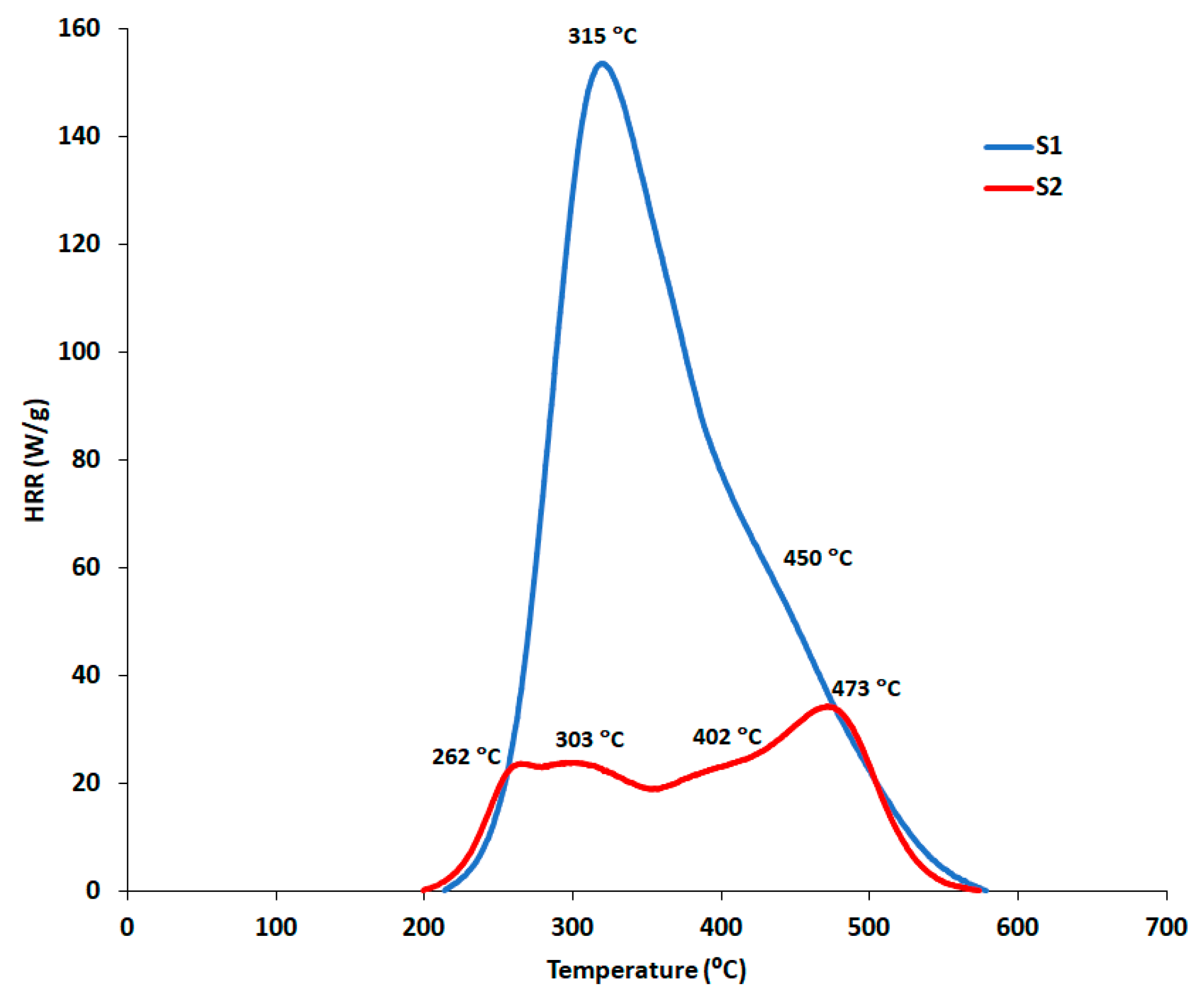
| Sample | Peak Number | PHRR (W/g) | TPHRR (°C) | Time (s) |
|---|---|---|---|---|
| S1 | 1 | 158.84 ± 5.03 | 314.72 ± 1.63 | 63.50 ± 8.41 |
| 2 | 51.16 ± 2.37 | 450.30 ± 4.86 | 127.00 ± 11.27 | |
| S2 | 1 | 21.41 ± 2.88 | 261.66 ± 3.35 | 33.00 ± 1.32 |
| 2 | 22.75 ± 1.89 | 303.32 ± 8.72 | 54.83 ± 5.48 | |
| 3 | 25.24 ± 1.77 | 401.60 ± 5.44 | 104.33 ± 3.69 | |
| 4 | 36.46 ± 1.82 | 472.76 ± 0.88 | 139.83 ± 2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisa, G.; Anghel, I.; Preda, D.-M.; Lisa, C.; Cretescu, I.; Buciscanu, I.I.; Diaconu, M.; Soreanu, G. Moving towards Valorization of Biowastes Issued from Biotrickling Filtration of Contaminated Gaseous Streams: A Thermochemical Analysis-Based Perspective. Sustainability 2022, 14, 10737. https://doi.org/10.3390/su141710737
Lisa G, Anghel I, Preda D-M, Lisa C, Cretescu I, Buciscanu II, Diaconu M, Soreanu G. Moving towards Valorization of Biowastes Issued from Biotrickling Filtration of Contaminated Gaseous Streams: A Thermochemical Analysis-Based Perspective. Sustainability. 2022; 14(17):10737. https://doi.org/10.3390/su141710737
Chicago/Turabian StyleLisa, Gabriela, Ion Anghel, Dana-Maria Preda, Catalin Lisa, Igor Cretescu, Ingrid Ioana Buciscanu, Mariana Diaconu, and Gabriela Soreanu. 2022. "Moving towards Valorization of Biowastes Issued from Biotrickling Filtration of Contaminated Gaseous Streams: A Thermochemical Analysis-Based Perspective" Sustainability 14, no. 17: 10737. https://doi.org/10.3390/su141710737
APA StyleLisa, G., Anghel, I., Preda, D.-M., Lisa, C., Cretescu, I., Buciscanu, I. I., Diaconu, M., & Soreanu, G. (2022). Moving towards Valorization of Biowastes Issued from Biotrickling Filtration of Contaminated Gaseous Streams: A Thermochemical Analysis-Based Perspective. Sustainability, 14(17), 10737. https://doi.org/10.3390/su141710737







