Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Reagents
2.2. Substrates Characterization
2.3. Extraction Procedure, HPLC-DAD Extract Analysis, Determination of Total Phenolic Content
2.4. Composting Tests
2.5. Seed Germination and Seedling Growth
2.6. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols Extraction
3.2. Evolution of the Composting Process and Quality of the Products
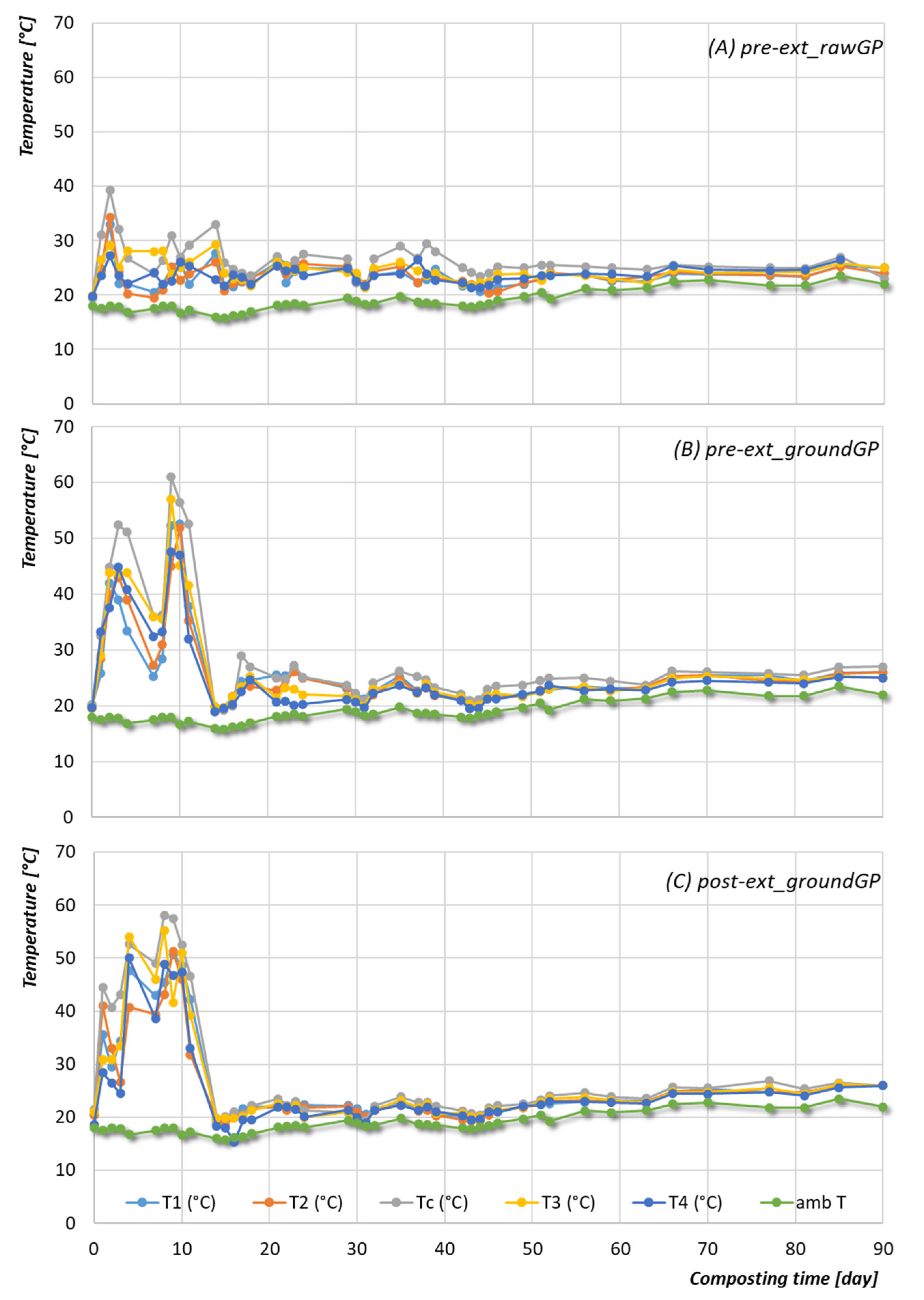
3.2.1. Temperature
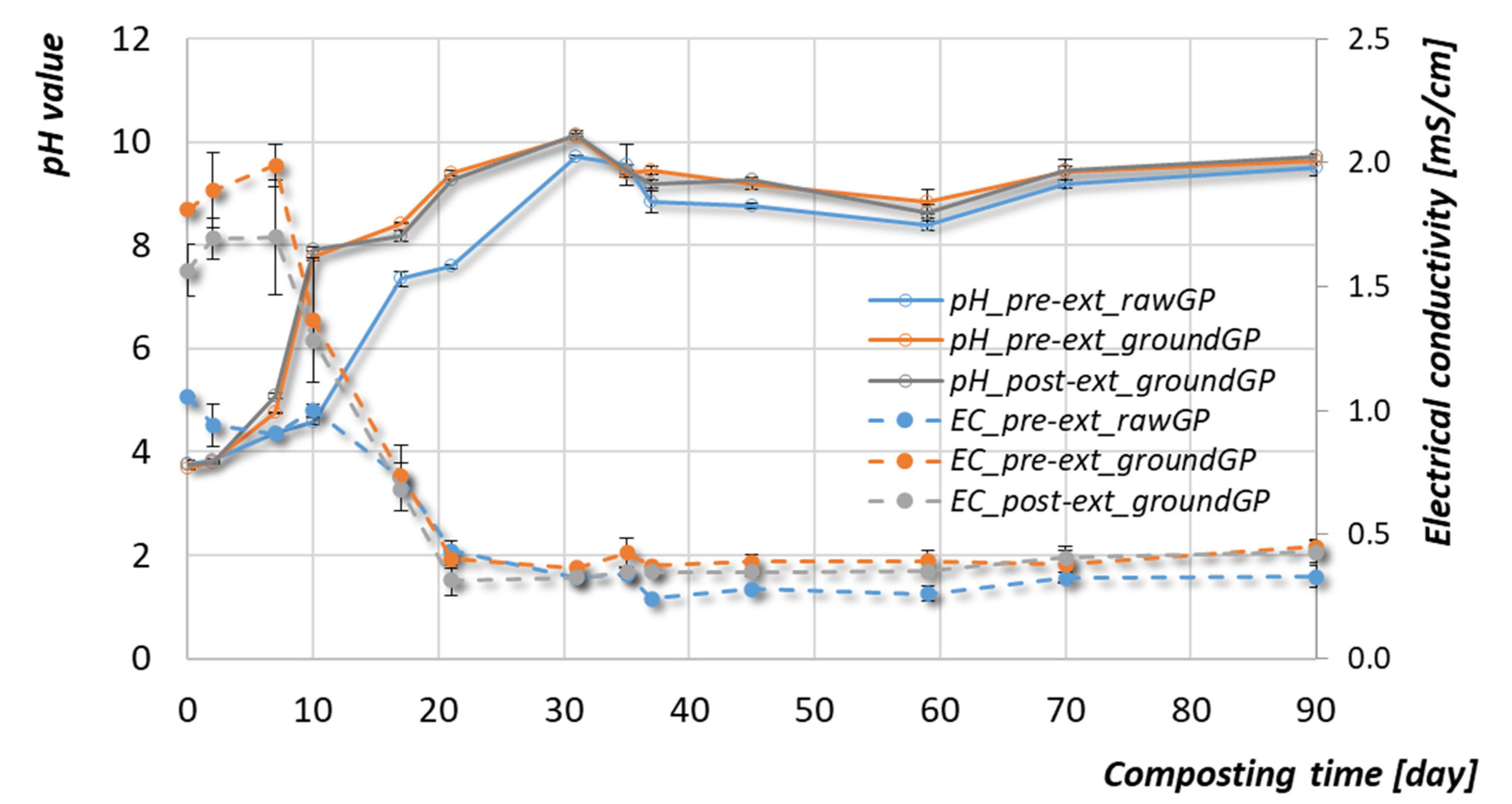
3.2.2. pH and Electrical Conductivity
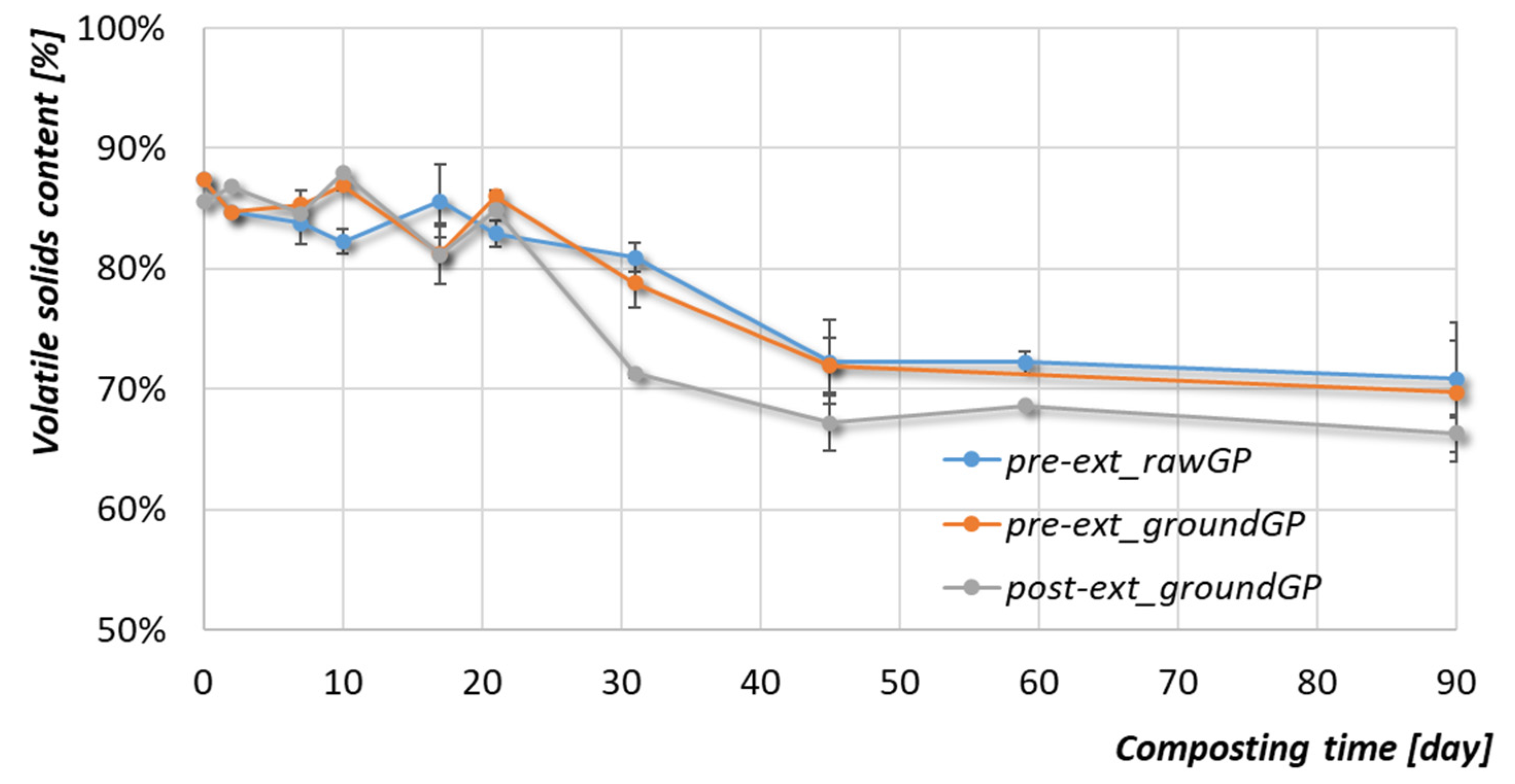
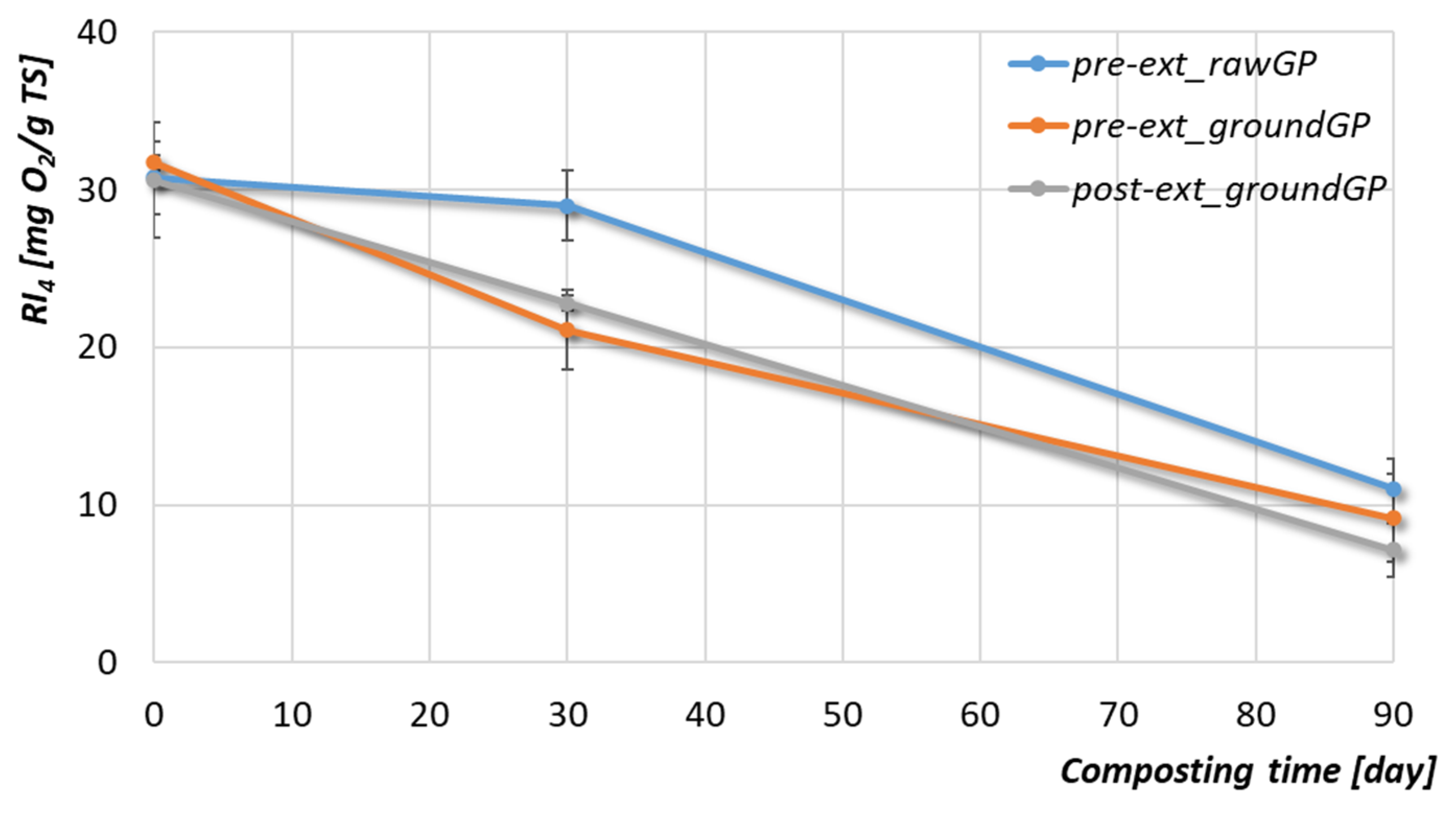
3.2.3. Organic Matter and Phenolic Compounds
3.2.4. Effects on Seed Germination and Seedling Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef]
- Zhang, N.; Hoadley, A.; Patel, J.; Lim, S.; Li, C. Sustainable options for the utilization of solid residues from wine production. Waste Manag. 2017, 60, 173–183. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E.D.A. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2014, 10, 168–176. [Google Scholar] [CrossRef]
- Semitela, S.; Pirra, A.; Braga, F.G. Impact of mesophilic co-composting conditions on the quality of substrates produced from winery waste activated sludge and grape stalks: Lab-scale and pilot-scale studies. Bioresour. Technol. 2019, 289, 121622. [Google Scholar] [CrossRef]
- Heidari, M.; Bahramsoltani, R.; Abdolghaffari, A.H.; Rahimi, R.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; Farzaei, M.H. Efficacy of topical application of standardized extract of Tragopogon graminifolius in the healing process of experimental burn wounds. J. Tradit. Complement. Med. 2018, 9, 54–59. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T.; Kefalas, P. Factorial design optimisation of grape (Vitis vinifera) seed polyphenol extraction. Eur. Food Res. Technol. 2009, 229, 731–742. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef]
- Yi, O.-S.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of grape extracts in a lecithin liposome system. J. Am. Oil Chem. Soc. 1997, 74, 1301–1307. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Sustainable Exploitation of By-Products of Vitivinicultural Origin in Winemaking. Proceedings 2020, 67, 5. [Google Scholar]
- Ertani, A.; Francioso, O.; Nardi, S. Mini review: Fruit residues as plant biostimulants for bio-based product recovery. AIMS Agric. Food 2017, 2, 251–257. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Bacchetta, G.; Muntoni, A.; de Gioannis, G.; Castangia, I.; Manca, M.L.; Manconi, M. An Outlook on Modern and Sustainable Approaches to the Management of Grape Pomace by Integrating Green Processes, Biotechnologies and Advanced Biomedical Approaches. Nanoscale Horiz. 2022; submmited. [Google Scholar]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From waste to health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional ingredient from avocado peel: Microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 2021, 352, 129300. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Impact of Eutectic Solvents Utilization in the Microwave Assisted Extraction of Proanthocyanidins from Grape Pomace. Molecules 2021, 27, 246. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.-J.; Manca, M.L.; Manconi, M.; Caddeo, C.; Vázquez, J.A.; Carbone, C.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A. Development of advanced phospholipid vesicles loaded with Lippia citriodora pressurized liquid extract for the treatment of gastrointestinal disorders. Food Chem. 2020, 337, 127746. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: Box–Behnken design optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Optimized Extraction of Phenylpropanoids and Flavonoids from Lemon Verbena Leaves by Supercritical Fluid System Using Response Surface Methodology. Foods 2020, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Pazir, F.; Koçak, E.; Turan, F.; Ova, G. Extraction of anthocyanins from grape pomace by using supercritical carbon dioxide. J. Food Process. Preserv. 2020, 45, e14950. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; De La Ossa, E.J.M. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686. [Google Scholar] [CrossRef] [PubMed]
- Pinter, I.F.; Fernández, A.S.; Martínez, L.E.; Riera, N.; Fernández, M.; Aguado, G.D.; Uliarte, E.M. Exhausted grape marc and organic residues composting with polyethylene cover: Process and quality evaluation as plant substrate. J. Environ. Manag. 2019, 246, 695–705. [Google Scholar] [CrossRef]
- Salgado, M.M.M.; Blu, R.O.; Janssens, M.; Fincheira, P. Grape pomace compost as a source of organic matter: Evolution of quality parameters to evaluate maturity and stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Carmona, E.; Moreno, M.; Avilés, M.; Ordovás, J. Use of grape marc compost as substrate for vegetable seedlings. Sci. Hortic. 2012, 137, 69–74. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Waste Management for the Food Industries; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Fincheira-Robles, P.; Martínez-Salgado, M.M.; Ortega-Blu, R.; Janssens, M. Compost and humic substance effects on soil parameters of Vitis vinifera L. cv. Thompson seedless. Sci. Agropecu. 2016, 7, 291–296. [Google Scholar] [CrossRef][Green Version]
- Ferrer, J. Agronomic use of biotechnologically processed grape wastes. Bioresour. Technol. 2001, 76, 39–44. [Google Scholar] [CrossRef]
- Gaiotti, F.; Marcuzzo, P.; Battista, F.; Belfiore, N.; Petoumenou, D.; Tomasi, D. Compost amendment effects on grapevine root density and distribution. Acta Hortic. 2016, 1136, 115–120. [Google Scholar] [CrossRef]
- Hungría, J.; Gutiérrez, M.; Siles, J.; Martín, M. Advantages and drawbacks of OFMSW and winery waste co-composting at pilot scale. J. Clean. Prod. 2017, 164, 1050–1057. [Google Scholar] [CrossRef]
- Muntoni, A. Waste Biorefineries: Opportunities and Perspectives. Detritus 2019, 5, 1–2. [Google Scholar] [CrossRef]
- Eaton, A.D.; APHA; AWWA. WEF: Standard Methods for the Examination of Water and Wastewater; WEF: Washington, DC, USA, 2005.
- Corte-Real, J.; Archaimbault, A.; Schleeh, T.; Cocco, E.; Herrmann, M.; Guignard, C.; Hausman, J.; Iken, M.; Legay, S. Handling wine pomace: The importance of drying to preserve phenolic profile and antioxidant capacity for product valorization. J. Food Sci. 2021, 86, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.-J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the antioxidant phytocomplex from wine-making by-products and sustainable loading in phospholipid vesicles specifically tailored for skin protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef]
- Montoro, P.; Serreli, G.; Gil, K.A.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. Evaluation of bioactive compounds and antioxidant capacity of edible feijoa (Acca sellowiana (O. Berg) Burret) flower extracts. J. Food Sci. Technol. 2020, 57, 2051–2060. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M.A. Characterization, phenolic profile, nitrogen compounds and antioxidant activity of Carignano wines. J. Food Compos. Anal. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef]
- Asquer, C.; Cappai, G.; De Gioannis, G.; Muntoni, A.; Piredda, M.; Spiga, D. Biomass ash reutilisation as an additive in the composting process of organic fraction of municipal solid waste. Waste Manag. 2017, 69, 127–135. [Google Scholar] [CrossRef]
- Mohee, R.; Mudhoo, A. Analysis of the physical properties of an in-vessel composting matrix. Powder Technol. 2005, 155, 92–99. [Google Scholar] [CrossRef]
- European Union. Working Document: Biological Treatment on Biowaste; 2nd Draft Brussels; European Union: Brussels, Belgium, 2001. [Google Scholar]
- Binner, E.; Böhm, K.; Lechner, P. Large scale study on measurement of respiration activity (AT4) by Sapromat and OxiTop. Waste Manag. 2012, 32, 1752–1759. [Google Scholar] [CrossRef]
- Arias, V.S.; Fernández, F.; Rodríguez, L.; Villaseñor, J. Respiration indices and stability measurements of compost through electrolytic respirometry. J. Environ. Manag. 2012, 95, S134–S138. [Google Scholar] [CrossRef] [PubMed]
- Roletto, E.; Consiglio, M.; Jodice, R.; Barberis, R. Chemical Parameters for Evaluating Compost Maturity. Biocycle 1985, 26, 46–47. [Google Scholar]
- Bernal, M.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.; Casas, L.; Mantell, C.; de la Ossa, E.M.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef]
- Fontana, A.; Antoniolli, A.; Fernández, M.A.D.; Bottini, R. Phenolics profiling of pomace extracts from different grape varieties cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Hossain, S.; Karim, T.U.; Onik, M.H.; Kumar, D.; Rahman, A.; Abu Yousuf, A.; Uddin, M.R. Impact of temperature, inoculum flow pattern, inoculum type, and their ratio on dry anaerobic digestion for biogas production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef]
- Manpreet, S.; Sawraj, S.; Sachin, D.; Pankaj, S.; Banerjee, U.C. Influence of Process Parameters on the Production of Metabolites in Solid-State Fermentation. Malays. J. Microbiol. 2005, 2, 1–9. [Google Scholar] [CrossRef]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Soares, M.A.; Quina, M.J.; Quinta-Ferreira, R. Prediction of free air space in initial composting mixtures by a statistical design approach. J. Environ. Manag. 2013, 128, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Qasim, W.; Moon, B.E.; Okyere, F.G.; Khan, F.; Nafees, M.; Kim, H.T. Influence of aeration rate and reactor shape on the composting of poultry manure and sawdust. J. Air Waste Manag. Assoc. 2019, 69, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Goufo, P.; Fonseca, J.; Pereira, J.L.; Ferreira, L.; Coutinho, J.; Trindade, H. Effect of lignocellulosic and phenolic compounds on ammonia, nitric oxide and greenhouse gas emissions during composting. J. Clean. Prod. 2018, 171, 548–556. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, Z.; Zhang, Y.; Wu, H.; Zhou, L.; Zhang, H. Impact of wine grape pomace on humification performance and microbial dynamics during pig manure composting. Bioresour. Technol. 2022, 358, 127380. [Google Scholar] [CrossRef]
- Mishra, S.K.; Yadav, K.D. Assessment of the effect of particle size and selected physico-chemical and biological parameters on the efficiency and quality of composting of garden waste. J. Environ. Chem. Eng. 2022, 10, 107925. [Google Scholar] [CrossRef]
- Ho, T.T.K.; Tra, V.T.; Le, T.H.; Nguyen, N.-K.; Tran, C.-S.; Nguyen, P.-T.; Vo, T.-D.; Thai, V.-N.; Bui, X.-T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Fernández, F.; Sánchez-Arias, V.; Villaseñor, J.; Rodríguez, L. Evaluation of carbon degradation during co-composting of exhausted grape marc with different biowastes. Chemosphere 2008, 73, 670–677. [Google Scholar] [CrossRef]
- Bustamante, M.; Paredes, C.; Marhuenda-Egea, F.C.; Espinosa, A.P.; Bernal, M.P.; Moral, R. Co-composting of distillery wastes with animal manures: Carbon and nitrogen transformations in the evaluation of compost stability. Chemosphere 2008, 72, 551–557. [Google Scholar] [CrossRef]
- Gil, M.; Carballo, M.; Calvo, L. Fertilization of maize with compost from cattle manure supplemented with additional mineral nutrients. Waste Manag. 2008, 28, 1432–1440. [Google Scholar] [CrossRef]
- Golueke, C.G. Principles of Biological Resource Recovery. Biocycle 1981, 22, 36–40. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.B.; Ruslan, M.S.H. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Makanjuola, S.A. Influence of particle size and extraction solvent on antioxidant properties of extracts of tea, ginger, and tea-ginger blend. Food Sci. Nutr. 2017, 5, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Stevenson, F. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994; Volume 72. [Google Scholar]
- Bertran, E.; Sort, X.; Soliva, M.; Trillas, M.I. Composting winery waste: Sludges and grape stalks. Bioresour. Technol. 2004, 95, 203–208. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.-C.; Popa, V.I. Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- Ignat, I.; Radu, D.G.; Volf, I.; Pag, A.I.; Popa, V.I. Antioxidant and Antibacterial Activities of Some Natural Polyphenols. Cellul. Chem. Technol. 2013, 47, 387–399. [Google Scholar]
- Vyvyan, J.R. Allelochemicals as Leads for New Herbicides and Agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
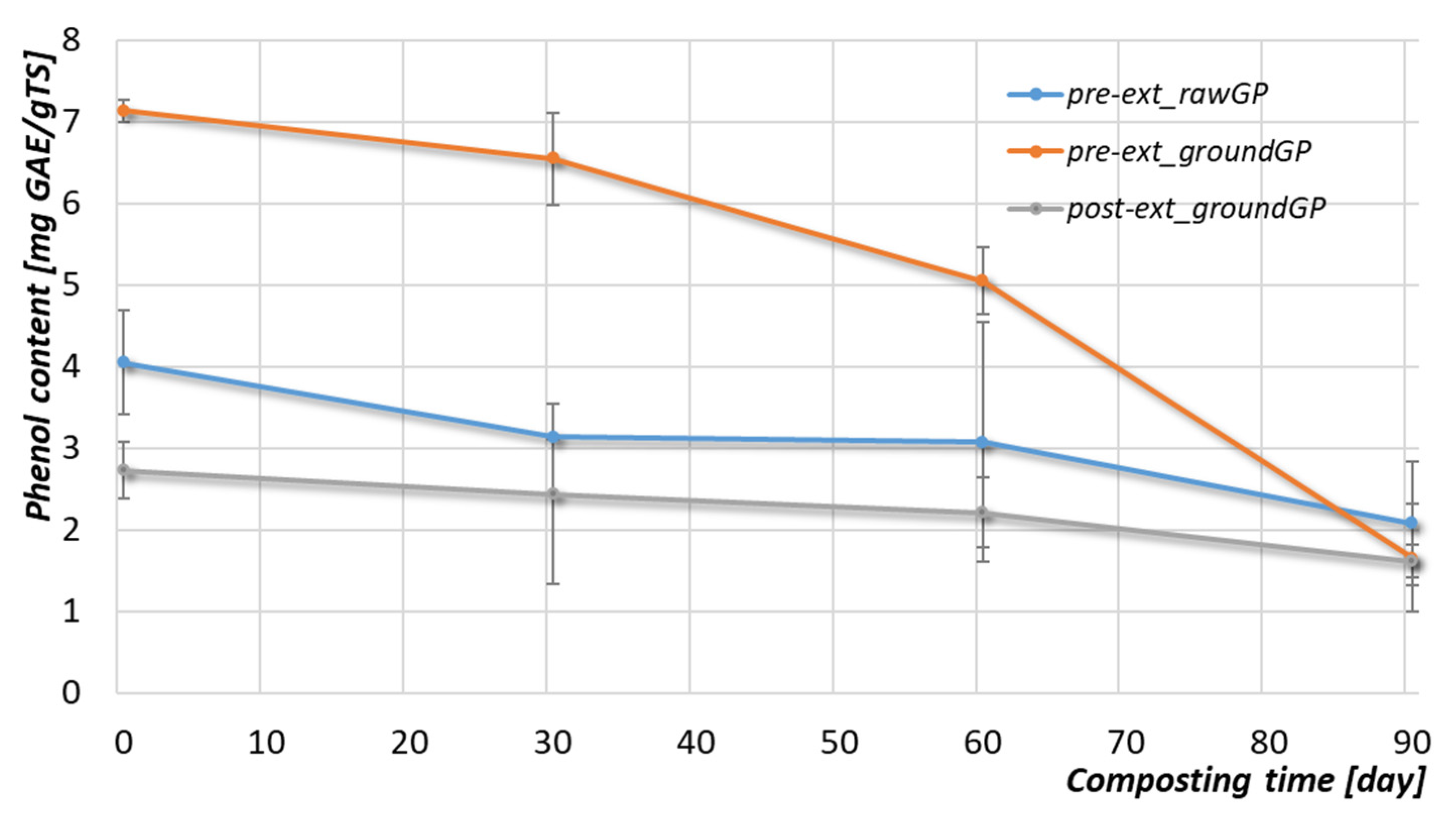
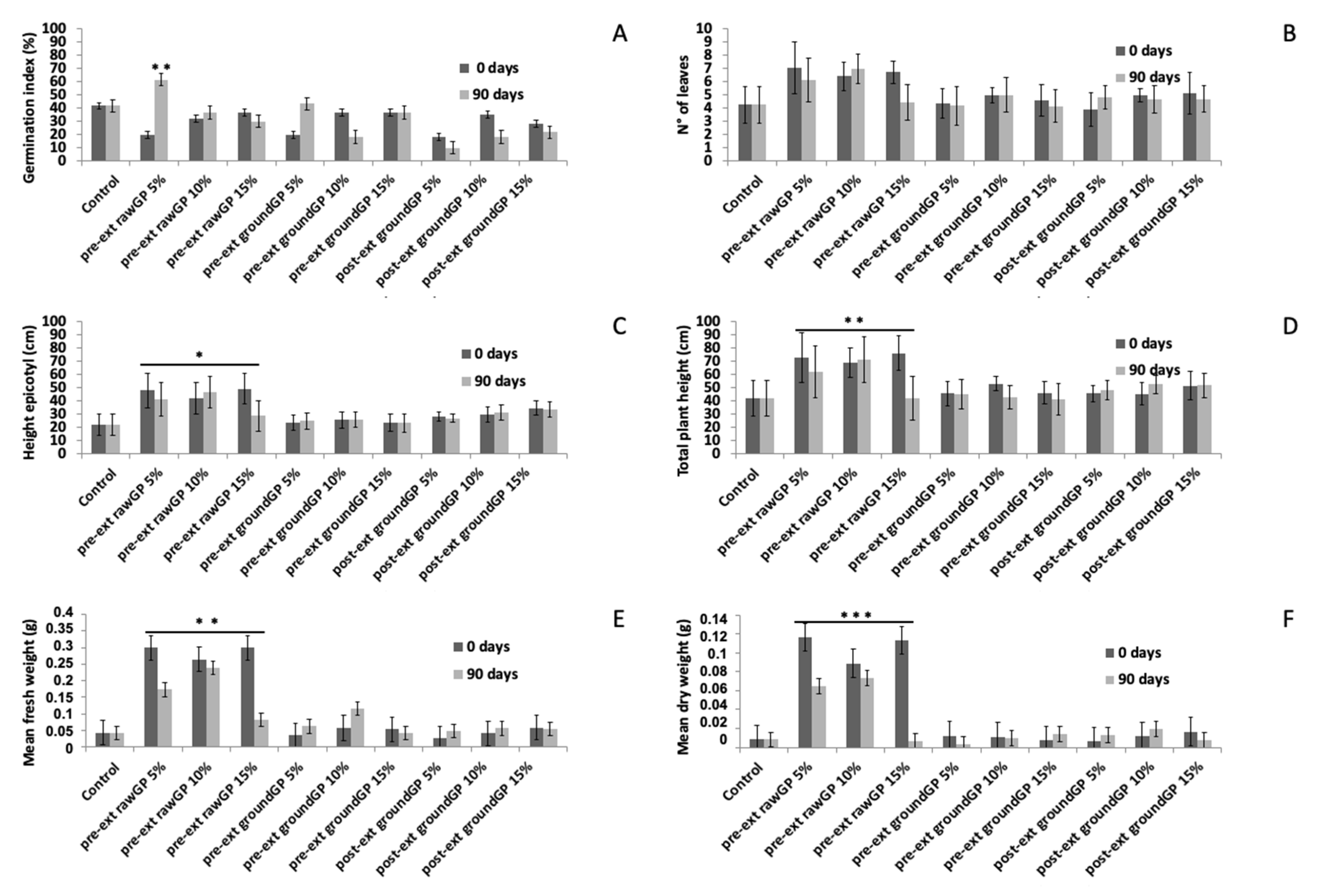
| Parameter | Unit | Pre-ext_rawGP 1 | Pre-ext_groundGP 2 | Post-ext_groundGP 3 |
|---|---|---|---|---|
| Total solids (TS) | [% w/w] | 61.20 ± 1.62 | 63.30 ± 0.02 | 63.50 ± 3.52 |
| Volatile solids (VS) | [% TS] | 86.20 ± 0.36 | 89.30 ± 0.08 | 85.30 ± 1.20 |
| pH | - | 3.75 ± 0.09 | 3.69 ± 0.00 | 3.76 ± 0.00 |
| Electrical conductivity (EC) | [mS/cm] | 1.05 ± 0.00 | 1.81 ± 0.15 | 1.56 ± 0.08 |
| Carbon | [% TS] | 47.52 ± 0.14 | 47.52 ± 0.14 | 48.12 ± 0.15 |
| Nitrogen | [% TS] | 1.68 ± 0.12 | 1.68 ± 0.12 | 1.72 ± 0.14 |
| Carbon/Nitrogen (C/N) | - | 28.20 | 28.20 | 27.98 |
| Bulk density (BD) | [kg/m3] | 213 | 273 | 265 |
| Particle density (PD) | [kg/m3] | 1214 | 1208 | 1198 |
| Free air space (FAS) | [%] | 81.0 | 75.7 | 76.3 |
| Total phenolic content | [mgGAE/gTS] | 4.05 ± 0.14 | 7.13 ± 0.64 | 2.73 ± 0.34 |
| Retention Time [min] | Compound | Id a | Content [mg/gTS] |
|---|---|---|---|
| Total Anthocyanins | 6.12 ± 0.05 | ||
| 18.66 | Malvidin-3-O-glucoside | Rt | 1.19 ± 0.00 |
| 28.40 | Malvidin-3-O-(p-coumaroyl)glucoside b | UV-Vis | 2.26 ± 0.04 |
| Other Anthocyanins b | UV-Vis | 2.67 ± 0.03 | |
| Total Flavonols | 1.46 ± 0.03 | ||
| 21.95 | Quercetin-3-O-galactoside | Rt | 0.06 ± 0.00 |
| 22.08 | Quercetin-3-O-glucoside | Rt | 0.18 ± 0.00 |
| 22.25 | Quercetin derivative c | UV-Vis | 0.03 ± 0.00 |
| 24.56 | Quercetin-3-O-glucuronide | Rt | 0.37 ± 0.00 |
| 29.46 | Quercetin | Rt | 0.45 ± 0.01 |
| Other flavonols c | UV-Vis | 0.36 ± 0.02 | |
| Total Hydroxycinnamic acids | 1.30 ± 0.00 | ||
| 11.23 | Caftaric acid | Rt | 0.17 ± 0.00 |
| 13.20 | Hydroxycinnamic derivative d | UV-Vis | 0.14 ± 0.00 |
| 14.40 | p-Coumaric acid derivative d | UV-Vis | 0.11 ± 0.00 |
| 15.03 | Caffeic acid derivative d | UV-Vis | 0.14 ± 0.00 |
| 16.26 | p-Coumaric acid | Rt | 0.07 ± 0.00 |
| 19.31 | Hydroxycinnamic derivative d | UV-Vis | 0.08 ± 0.00 |
| Other Hydroxycinnamic acids d | UV-Vis | 0.59 ± 0.03 | |
| Total Hydroxybenzoic acids | 3.30 ± 0.03 | ||
| 4.91 | Gallic acid | Rt | 0.61 ± 0.01 |
| 7.24 | Protocatechuic acid | Rt | 0.09 ± 0.00 |
| 12.01 | Vanillic acid | Rt | 1.18 ± 0.01 |
| 13.63 | Syringic acid | Rt | 0.61 ± 0.00 |
| 21.49 | Ellagic acid | Rt | 0.14 ± 0.00 |
| Other Hydroxybenzoic acids e | UV-Vis | 0.67 ± 0.01 | |
| Total Flavan 3-ols | 11.16 ± 0.37 | ||
| 12.18 | Procyanidin B1 | Rt | 1.22 ± 0.16 |
| 12.40 | (+)-Catechin | Rt | 2.98 ± 0.03 |
| 14.67 | Procyanidin B2 f | UV-Vis | 1.56 ± 0.11 |
| 15.10 | (-)-Epicatechin | Rt | 1.77 ± 0.04 |
| 17.47 | Procyanidin B3 f | UV-Vis | 1.19 ± 0.05 |
| Other Flavan-3-ols f | UV-Vis | 2.45 ± 0.19 | |
| Total polyphenols | 23.33 ± 0.48 | ||
| Other compounds | |||
| 3.17 | Xanthine | Rt | 0.49 ± 0.00 |
| 4.41 | Tyrosine I | Rt | 1.20 ± 0.02 |
| 5.64 | Phenylalanine | Rt | 1.19 ± 0.03 |
| 9.07 | Tyrosine II | Rt | 0.10 ± 0.00 |
| 9.69 | Tryptophan I | Rt | 0.76 ± 0.00 |
| 13.18 | Tryptophan II | Rt | 0.33 ± 0.02 |
| 15.50 | Tryptophan III | Rt | 0.08 ± 0.00 |
| TOTAL | 27.49 ± 0.55 |
| Parameter | Unit | Pre-ext_rawGP 1 | Pre-ext_groundGP 2 | Post-ext_groundGP 3 |
|---|---|---|---|---|
| Total solids (TS) | [% w/w] | 51.20 ± 1.72 | 49.70 ± 1.22 | 50.60 ± 1.32 |
| Volatile solids (VS) | [%TS] | 70.86 ± 1.06 | 69.74 ± 1.38 | 66.32 ± 1.23 |
| pH | - | 9.51 ± 0.09 | 9.65 ± 0.00 | 9.71 ± 0.00 |
| Electrical conductivity (EC) | [mS/cm] | 0.33 ± 0.03 | 0.45 ± 0.17 | 0.43 ± 0.05 |
| Carbon | [%TS] | 45.08 ± 1.03 | 44.68 ± 0.31 | 44.69 ± 0.06 |
| Nitrogen | [%TS] | 2.89 ± 0.28 | 2.63 ± 0.11 | 2.64 ± 0.16 |
| Carbon/Nitrogen (C/N) | - | 15.59 | 16.98 | 16.94 |
| Total phenolic content | [mg GAE/gTS] | 2.08 ± 0.66 | 1.65 ± 0.76 | 1.61 ± 0.20 |
| Respirometric index (RI4) | [mg O2/gTS] | 11.03 ± 3.64 | 9.15 ± 0.51 | 7.13 ± 1.71 |
| Humic acids (HA) | [%TS] | <D.L. | 1.50 ± 0.10 | 1.89 ± 0.20 |
| Fulvic acids (FA) | [%TS] | 5.01 ± 0.10 | 3.86 ± 0.18 | 2.37 ± 0.19 |
| Humification ratio (HR) | [%TOC] | 40.88 | 50.28 | 38.96 |
| Humification index (HI) | [%] | - | 3.35 | 4.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.G.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. https://doi.org/10.3390/su141710690
Perra M, Cuena-Lombraña A, Bacchetta G, Manca ML, Manconi M, Maroun RG, Muntoni A, Tuberoso CIG, Gil KA, De Gioannis G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability. 2022; 14(17):10690. https://doi.org/10.3390/su141710690
Chicago/Turabian StylePerra, Matteo, Alba Cuena-Lombraña, Gianluigi Bacchetta, Maria Letizia Manca, Maria Manconi, Richard G. Maroun, Aldo Muntoni, Carlo Ignazio Giovanni Tuberoso, Katarzyna A. Gil, and Giorgia De Gioannis. 2022. "Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass" Sustainability 14, no. 17: 10690. https://doi.org/10.3390/su141710690
APA StylePerra, M., Cuena-Lombraña, A., Bacchetta, G., Manca, M. L., Manconi, M., Maroun, R. G., Muntoni, A., Tuberoso, C. I. G., Gil, K. A., & De Gioannis, G. (2022). Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability, 14(17), 10690. https://doi.org/10.3390/su141710690
















