Effect of Time since Afforestation on Soil Organic Carbon Stock and Turnover Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Details
2.2. Historical Land Use of Sampling Plots and Sample Collection
2.3. Sample Analysis
2.4. Data Calculation
2.5. Statistical Analysis
3. Results
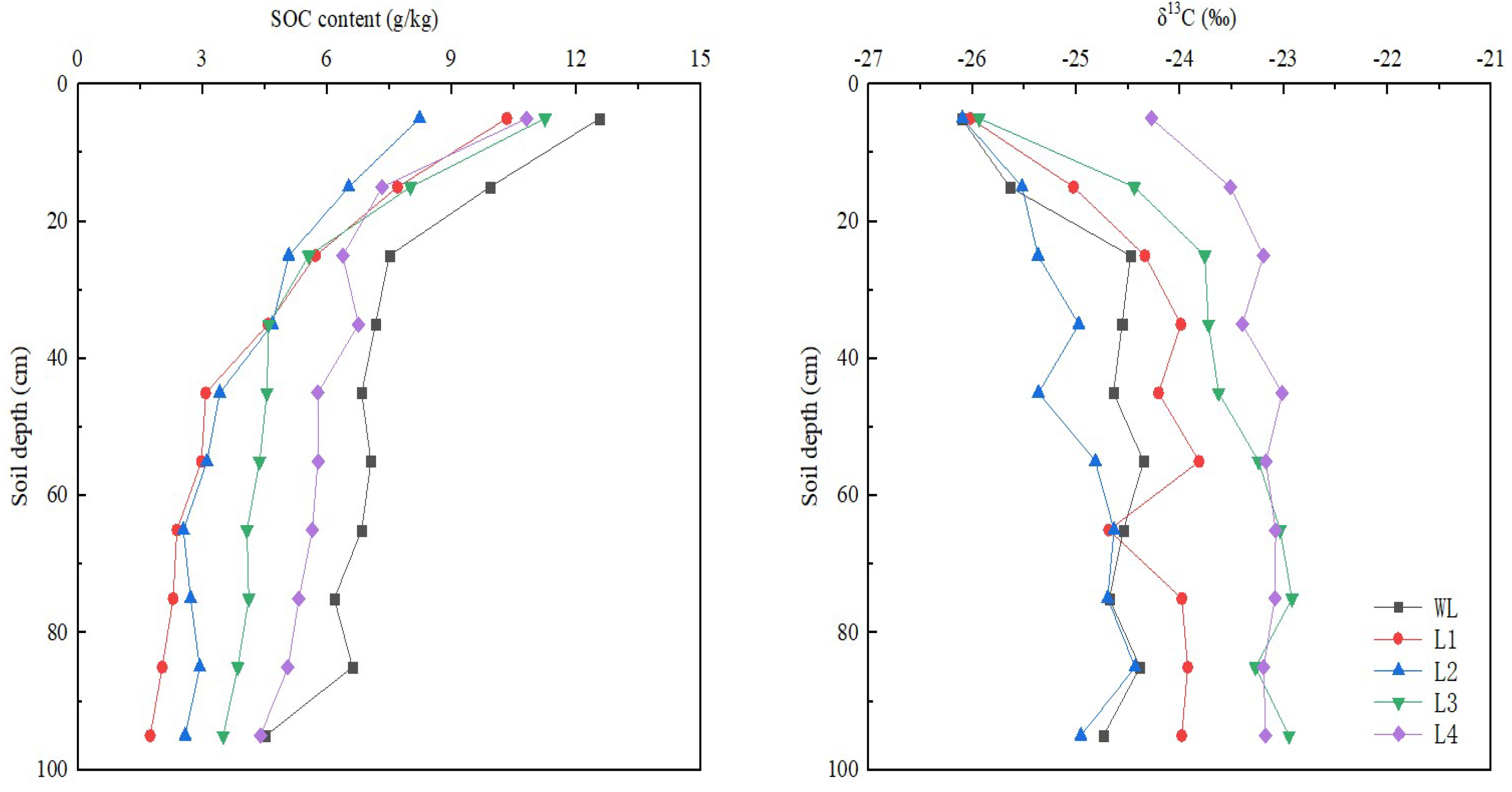
3.1. Vertical Distribution of δ13C and SOC in Soil Profile
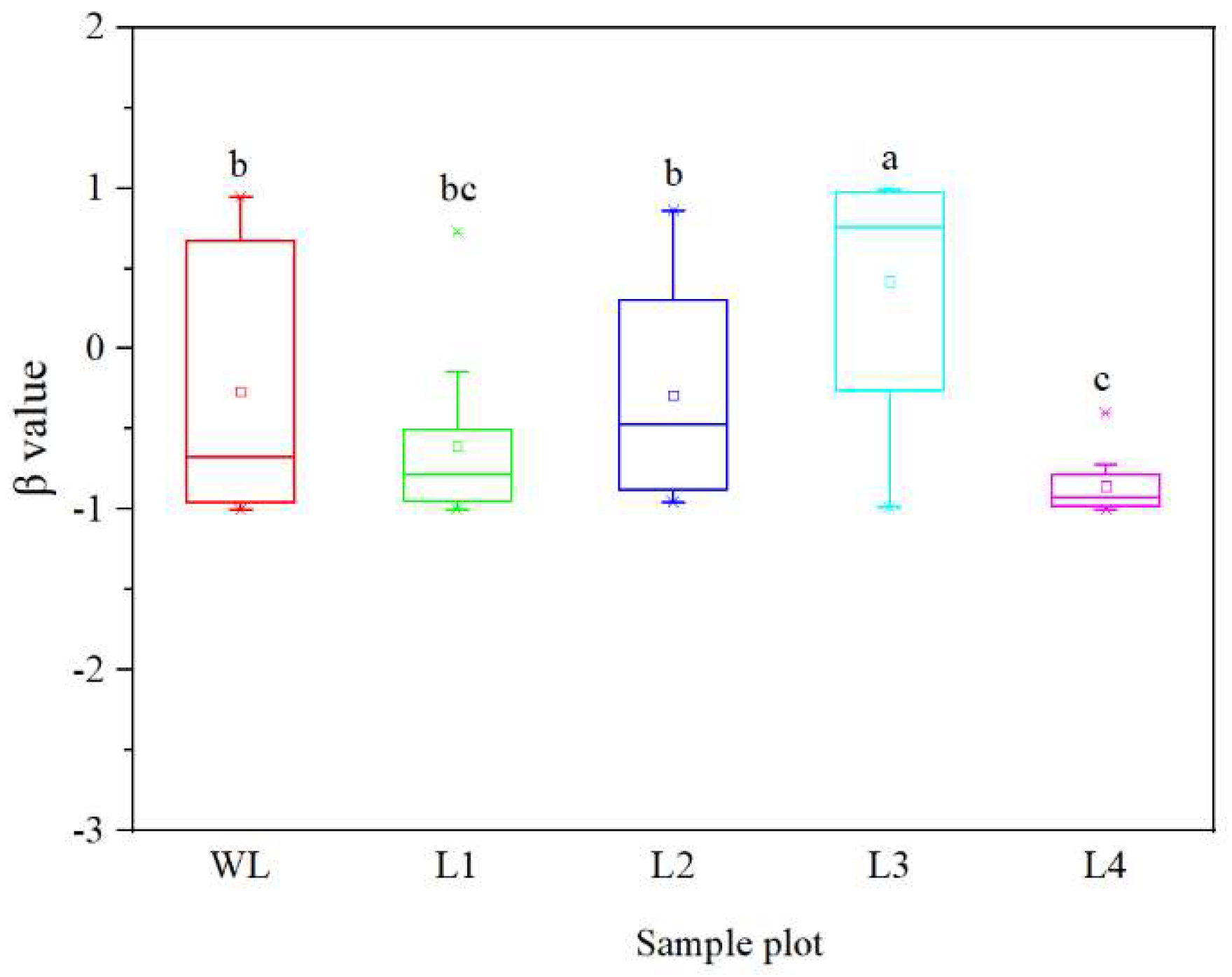
3.2. Variations in SOC Turnover Rate
3.3. SOC Stock
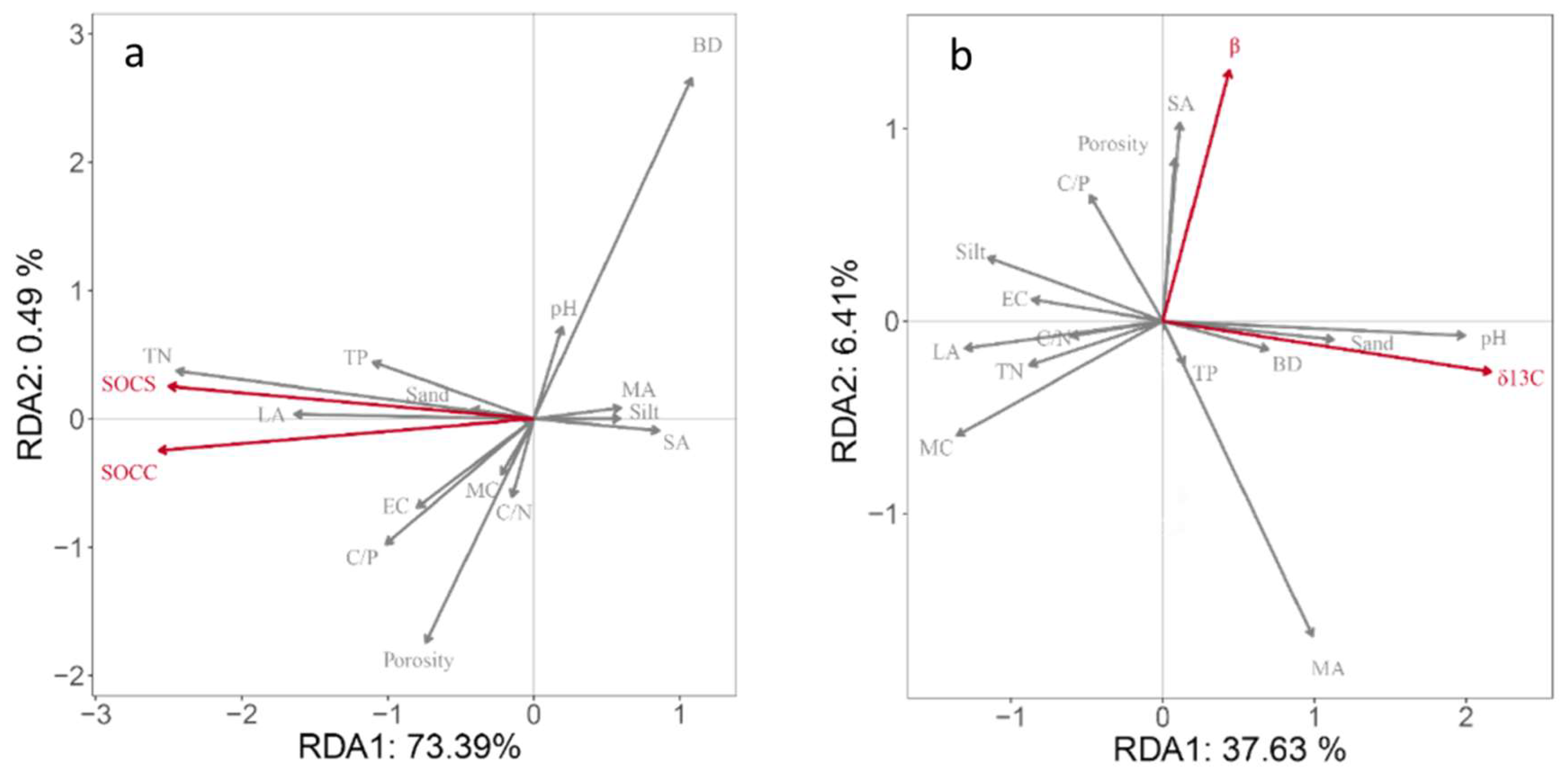
3.4. Relationship between Soil Physicochemical Properties and SOC, SOC Stocks and β, and δ13C Value
4. Discussion
4.1. SOC Turnover
4.2. Effects of Afforestation on SOC Sequestration
4.3. Factors Affecting SOC Sequestration and Turnover
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Gautam, M.K.; Lee, K.-S.; Song, B.-Y.; Bong, Y.-S. Site related δ13C of vegetation and soil organic carbon in a cool temperate region. Plant Soil 2017, 418, 293–306. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Liu, G.; Yamanaka, N. Soil properties in natural grassland, Caragana korshinskii planted shrubland, and Robinia pseudoacacia planted forest in gullies on the hilly Loess Plateau, China. Catena 2014, 119, 116–124. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Yue, F.; Li, N. Dynamics of carbon and nitrogen storage in two typical plantation ecosystems of different stand ages on the Loess Plateau of China. PeerJ 2019, 7, e7708. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Z.; Li, W.; Liu, W.; Zhang, X.; Wu, S.; Ren, Z.; Wu, Q.; Shen, Z.; Ren, C.; et al. Effects of Robinia pseudoacacia afforestation on aggregate size distribution and organic C dynamics in the central Loess Plateau of China: A chronosequence approach. J. Environ. Manag. 2020, 268, 110558. [Google Scholar] [CrossRef]
- Paul, K.; Polglase, P.; Nyakuengama, J.; Khanna, P. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Lynch, L.; Xie, H.; Bao, X. Reforestation accelerates soil organic carbon accumulation: Evidence from micro-bial biomarkers. Soil Biol. Biochem. 2019, 131, 182–190. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Chen, X.; He, K.; Zhu, B. Changes in soil organic matter stability with depth in two alpine ecosystems on the Tibetan Plateau. Geoderma 2019, 351, 153–162. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Ou, Y.; Jia, H.; Li, J.; Shi, C.; Liu, Y. Variations in soil δ13C with alpine meadow degradation on the eastern Qinghai–Tibet Plateau. Geoderma 2018, 338, 178–186. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Zhang, Q.; Cheng, X. Soil carbon dynamics following land-use change varied with temperature and pre-cipitation gradients: Evidence from stable isotopes. Glob Chang. Biol. 2015, 21, 2762–2772. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Tang, Z.; Shangguan, Z. Soil organic carbon dynamics following natural vegetation restoration: Evidence from stable carbon isotopes (δ13C). Agric. Ecosyst. Environ. 2016, 221, 235–244. [Google Scholar] [CrossRef]
- Powers, J.S.; Marín-Spiotta, E. Ecosystem Processes and Biogeochemical Cycles in Secondary Tropical Forest Succession. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 497–519. [Google Scholar] [CrossRef] [Green Version]
- Häring, V.; Fischer, H.; Cadisch, G.; Stahr, K. Implication of erosion on the assessment of decomposition and humification of soil organic carbon after land use change in tropical agricultural systems. Soil Biol. Biochem. 2013, 65, 158–167. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Chadwick, D.; Jones, D.; Evans, C.; Jones, M.; Rees, R.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2017, 253, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ai, Z.; Wu, Y.; Xu, H.; Li, Q.; Xue, S.; Liu, G. 16-Year fertilization changes the dynamics of soil oxidizable or-ganic carbon fractions and the stability of soil organic carbon in soybean-corn agroecosystem. Agric. Ecosyst. Environ. 2018, 265, 320–330. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Funes, I.; Savé, R.; Rovira, P.; Molowny-Horas, R.; Alcañiz, J.M.; Ascaso, E.; Herms, I.; Herrero, C.; Boixadera, J.; Vayreda, J. Agricultural soil organic carbon stocks in the north-eastern Iberian Peninsula: Drivers and spatial variability. Sci. Total Environ. 2019, 668, 283–294. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Dang, H.; Ye, C.; Zhang, Y.; Zhang, Q. Linking litter production, quality and decomposition to vegeta-tion succession following agricultural abandonment. Soil Biol. Biochem. 2013, 57, 803–813. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Flint, A.L.; Flint, L.E. Physical Methods. In Methods of Soil Analysis; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 229–240. [Google Scholar]
- Leonard, G. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, L.-Y.; Peng, Y.-F.; Ding, J.-Z.; Li, F.; Yang, G.B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.B.; et al. Linking microbial C: N: P stoichiometry to microbial community and abiotic factors along a 3500-km grassland transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- Lu, R.K. Analysis Methods of Soil and Agricultural Chemistry; Chinese Agriculture and Technology Press Publisher: Beijing, China, 1999. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbonand Organic Matter. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9, ASA and SSSA: Madison, WI, USA, 1982; pp. 534–580. [Google Scholar]
- Harris, D.; Horwáth, W.R.; Kessel, C.V. Acid fumigation of soils to remove car bonates prior to total carbon or carbon-13 iso-topic analysis. Soil Sci. Soc. Am. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-Term Effect of Manure and Fertilizer on Soil Organic Carbon Pools in Dryland Farming in Northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef] [Green Version]
- Garten, C.T. Relationships among forest soil C isotopic composition, partitioning, and turnover times. Can. J. For. Res. 2006, 36, 2157–2167. [Google Scholar] [CrossRef]
- Di, D.; Huang, G. Isotope analysis reveals differential impacts of artificial and natural afforestation on soil organic carbon dynamics in abandoned farmland. Plant Soil 2021, 471, 329–342. [Google Scholar] [CrossRef]
- De Clercq, T.; Heiling, M.; Dercon, G.; Resch, C.; Aigner, M.; Mayer, L.; Mao, Y.; Elsen, A.; Steier, P.; Leifeld, J.; et al. Predicting soil organic matter stability in agricultural fields through carbon and nitrogen stable isotopes. Soil Biol. Biochem. 2015, 88, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Fan, J.; Song, M.; Yu, G.; Zhou, L.; Liu, J.; Zhong, H.; Gao, L.; Hu, Z.; Wu, W.; et al. Patterns of SOC and soil 13C and their relations to climatic factors and soil characteristics on the Qinghai–Tibetan Plateau. Plant Soil 2012, 363, 243–255. [Google Scholar] [CrossRef]
- Huang, Z.; Davis, M.R.; Condron, L.M.; Clinton, P. Soil carbon pools, plant biomarkers and mean carbon residence time after afforestation of grassland with three tree species. Soil Biol. Biochem. 2011, 43, 1341–1349. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Z.; Wu, G.; Yang, Y.; Lin, G. The relationship between soil CO2 efflux and its carbon isotopic composition under non-steady-state conditions. Agric. For. Meteorol. 2018, 256–257, 492–500. [Google Scholar] [CrossRef]
- Acton, P.; Fox, J.; Campbell, E.; Rowe, H.; Wilkinson, M. Carbon isotopes for estimating soil decomposition and physical mix-ing in well-drained forest soils. J. Geophys. Res.-Biogeosciences 2013, 118, 1532–1545. [Google Scholar] [CrossRef]
- Marin-Spiotta, E.; Silver, W.L.; Swanston, C.; Ostertag, R. Soil organic matter dynamics during 80 years of reforestation of tropical pastures. Glob. Chang. Biol. 2009, 15, 1584–1597. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of soil carbon by converting tropical forest to plantations: Erosion and de-composition estimated by delta (13) C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Don, A.; Fiedler, S.; Bechtold, M.; Amelung, W.; Freibauer, A. Drained organic soils under agriculture—The more degraded the soil the higher the specific basal respiration. Geoderma 2019, 355, 113911. [Google Scholar] [CrossRef]
- Helfrich, M.; Ludwig, B.; Buurman, P.; Flessa, H. Effect of land use on the composition of soil organic matter in density and aggregate fractions as revealed by solid-state 13C NMR spectroscopy. Geoderma 2006, 136, 331–341. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Sande, B.; Omil, B.; Rovira, P.; Martin-Pastor, M.; Barros, N.; Salgado, J.; Merino, A. Organic matter proper-ties in soils afforested with Pinus radiata. Plant Soil 2014, 374, 381–398. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Zhang, T.; Zhao, X.; Chen, S.; Wang, Q. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: A meta-analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, R.; Cao, H.; Tan, W. Factor contribution to soil organic and inorganic carbon accumulation in the Loess Plat-eau: Structural equation modeling. Geoderma 2019, 352, 116–125. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Caviglia, O.P.; Agnusdei, M.G. Vertical distribution of root biomass and soil carbon stocks in forage cropping sys-tems. Plant Soil 2017, 423, 175–191. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.-M.; Huang, Z.; Zhao, Y.-F.; Ou, Y.-S.; Jia, H.-X.; Li, J. Biomechanical properties of plant root systems and their ability to stabilize slopes in geohazard-prone regions. Soil Tillage Res. 2019, 189, 148–157. [Google Scholar] [CrossRef]
- Chang, R.; Fu, B.; Liu, G.; Wang, S.; Yao, X. The effects of afforestation on soil organic and inorganic carbon: A case study of the Loess Plateau of China. CATENA 2012, 95, 145–152. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2016, 166, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Sun, Q.; Li, N.; Jiang, J.; Wang, R.; Zhang, Y.; Liu, Q.; Wu, D.; Li, R.; et al. Soil organic carbon sequestration potential of artificial and natural vegetation in the hilly regions of Loess Plateau. Ecol. Eng. 2015, 82, 547–554. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, W.; Gao, D.; Dai, Y.; Deng, J.; Yang, G.; Han, X.; Ren, G. Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. CATENA 2018, 161, 85–95. [Google Scholar] [CrossRef]
- Sayer, E. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. Camb. Philos. Soc. 2005, 81, 1–31. [Google Scholar] [CrossRef]
- Frouz, J.; Špaldoňová, A.; Fričová, K.; Bartuška, M. The effect of earthworms (Lumbricus rubellus) and simulated tillage on soil organic carbon in a long-term microcosm experiment. Soil Biol. Biochem. 2014, 78, 58–64. [Google Scholar] [CrossRef]
- Józefowska, A.; Pietrzykowski, M.; Woś, B.; Cajthaml, T.; Frouz, J. The effects of tree species and substrate on carbon seques-tration and chemical and biological properties in reforested post-mining soils. Geoderma 2017, 292, 9–16. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Kurganova, I.; Merino, A.; Lopes de Gerenyu, V.; Barros, N.; Kalinina, O.; Giani, L.; Kuzyakov, Y. Mechanisms of carbon se-questration and stabilization by restoration of arable soils after abandonment: A chronosequence study on Phaeozems and Chernozems. Geoderma 2019, 354, 113882. [Google Scholar] [CrossRef]
- DeMarco, J.; Filley, T.; Throop, H.L. Patterns of woody plant-derived soil carbon losses and persistence after brush manage-ment in a semi-arid grassland. Plant Soil 2016, 406, 277–293. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biol. Biochem. 2016, 101, 96–103. [Google Scholar] [CrossRef]
- Gu, X.; Fang, X.; Xiang, W.; Zeng, Y.; Zhang, S.; Lei, P.; Peng, C.; Kuzyakov, Y. Vegetation restoration stimulates soil carbon sequestration and stabilization in a subtropical area of southern China. CATENA 2019, 181, 104098. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B.; McCulley, R. Soil carbon response to woody plant encroachment: Importance of spatial heterogeneity and deep soil storage. J. Ecol. 2017, 105, 1738–1749. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.; Liu, Y.; Fang, Y.; Ma, R.; Yu, Q.; An, S. Using soil aggregate stability and erodibility to evaluate the sustaina-bility of large-scale afforestation of Robinia pseudoacacia and Caragana korshinskii in the Loess Plateau. Forest Ecol. Manag. 2019, 450, 117491. [Google Scholar] [CrossRef]
- Adhikari, D.; Dunham-Cheatham, S.M.; Wordofa, D.N.; Verburg, P.; Poulson, S.R.; Yang, Y. Aerobic respiration of miner-al-bound organic carbon in a soil. Sci. Total Environ. 2019, 651, 1253–1260. [Google Scholar] [CrossRef]
- Novelli, L.E.; Caviglia, O.P.; Piñeiro, G. Increased cropping intensity improves crop residue inputs to the soil and aggregate-associated soil organic carbon stocks. Soil Till Res. 2017, 165, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Michalzik, B.; Kalbitz, K.; Park, J.H.; Solinger, S.; Matzner, E. Fluxes and concentrations of dissolved organic carbon and nitro-gen—A synthesis for temperate forests. Biogeochemistry 2001, 52, 173–205. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Haney, R.; Hons, F.; Zuberer, D. Active fractions of organic matter in soils with different texture. Soil Biol. Biochem. 1996, 28, 1367–1372. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wang, J.; Liu, G.; Wang, G.; Guo, L.; Peng, S. Decreased temporary turnover of bacterial communities along soil depth gradient during a 35-year grazing exclusion period in a semiarid grassland. Geoderma 2019, 351, 49–58. [Google Scholar] [CrossRef]
- Brombin, V.; Mistri, E.; Feudis, M.D.; Forti, C.; Salani, G.M.; Natali, C. Soil carbon investigation in three pedoclimatic and agro-nomic settings of northern italy. Sustainability 2020, 12, 10539. [Google Scholar] [CrossRef]



| Site | Stand Age (Years) | Latitude–Longitude | Altitude (m) | MTH (m) | DBH (cm) | Species Records (in a 10 × 10 m Quadrat) |
|---|---|---|---|---|---|---|
| WL | 0 | N: 34°32′44.93″–E: 105°44′03.03″ | 1536 | --- | --- | 16 species of herbaceous plants |
| L1 | 5 | N: 34°32′40.66″–E: 105°44′05.95″ | 1532 | 3 | 4.32 ± 2.02 | 11 Robinia pseudoacacia; 9 species of herbaceous plants |
| L2 | 20 | N: 34°32′10.33″–E: 105°44′07.75″ | 1515 | 8 | 6.70 ± 0.49 | 10 Robinia pseudoacacia; 6 species of herbaceous plants |
| L3 | 40 | N: 34°32′42.86″–E: 105°44′06.45″ | 1527 | 12 | 9.36 ± 1.11 | 12 Robinia pseudoacacia; 5 species of herbaceous plants |
| L4 | 56 | N: 34°32′11.63″–E: 105°44′02.66″ | 1494 | 15 | 17.20 ± 1.69 | 14 Robinia pseudoacacia; 3 species of herbaceous plants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, J.; Zhao, Y.; Jiang, S.; Liu, H.; Wang, X. Effect of Time since Afforestation on Soil Organic Carbon Stock and Turnover Rate. Sustainability 2022, 14, 10403. https://doi.org/10.3390/su141610403
Zhou X, Li J, Zhao Y, Jiang S, Liu H, Wang X. Effect of Time since Afforestation on Soil Organic Carbon Stock and Turnover Rate. Sustainability. 2022; 14(16):10403. https://doi.org/10.3390/su141610403
Chicago/Turabian StyleZhou, Xiaohe, Jia Li, Yunfei Zhao, Silong Jiang, Huiying Liu, and Xia Wang. 2022. "Effect of Time since Afforestation on Soil Organic Carbon Stock and Turnover Rate" Sustainability 14, no. 16: 10403. https://doi.org/10.3390/su141610403
APA StyleZhou, X., Li, J., Zhao, Y., Jiang, S., Liu, H., & Wang, X. (2022). Effect of Time since Afforestation on Soil Organic Carbon Stock and Turnover Rate. Sustainability, 14(16), 10403. https://doi.org/10.3390/su141610403





