Is COVID-19 Herd Immunity Influenced by Population Densities of Cities?
Abstract
:1. Introduction
- (1)
- Many studies stress the lack of knowledge and the limited understanding of the factors influencing herd immunity (e.g., [5,6,7,8,9,10,11]). Neagu (2020) [9], for example, states that: “information on the long-term immune response against SARS-CoV-2 is yet scarce.” (Abstract). Avoidance of further spread of the pandemic and investigation of the efficiency of COVID-19 vaccination might prove to be especially important from a public policy perspective.
- (2)
- We attempt to address an unexplored question, as to whether instead of a country level, COVID-19 herd immunity can evolve at a city level.
- (3)
- We propose and apply a new methodology to estimate herd immunity in cities, which makes use of population density. Based on the median population density in the sample, we classify cities according to sparsely vs. densely populated municipalities, and examine the impact of population density on the formation of herd immunity.
2. Materials and Methods
2.1. Descriptive Statistics
2.2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, D.M.; Weisfuse, I.B.; Hernandez-Avila, M.; Del Rio, C.; Bustamante, X.; Rodier, G. Pandemic Influenza as 21st Century Urban Public Health Crisis. Emerg. Infect. Dis. 2009, 15, 1963–1969. [Google Scholar] [CrossRef]
- Dye, C. Health and Urban Living. Science 2008, 319, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Reid, E.; Sabouri, S. Longitudinal Analyses of the Relationship between Development Density and the COVID-19 Morbidity and Mortality Rates: Early Evidence from 1165 Metropolitan Counties in the United States. Health Place 2020, 64, 102378. [Google Scholar] [CrossRef]
- Wong, D.W.S.; Li, Y. Spreading of COVID-19: Density matters. PLoS ONE 2020, 15, e0242398. [Google Scholar] [CrossRef] [PubMed]
- García-García, D.; Morales, E.; Fonfría, E.S.; Vigo, I.; Bordehore, C. Caveats on COVID-19 herd immunity threshold: The Spain case. Sci. Rep. 2002, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Giurgea, L.T.; Morens, D.M. Great Expectations of COVID-19 Herd Immunity. MBio 2022, 13, e03495-21. [Google Scholar] [CrossRef] [PubMed]
- Khalife, J.; VanGennep, D. COVID-19 herd immunity in the absence of a vaccine: An irresponsible approach. Epidemiol. Health 2021, 43, e2021012. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The Concept of Classical Herd Immunity May Not Apply to COVID-19. J. Infect. Dis. 2022, jiac109. [Google Scholar] [CrossRef]
- Neagu, M. The bumpy road to achieve herd immunity in COVID-19. J. Immunoass. Immunochem. 2020, 41, 928–945. [Google Scholar] [CrossRef]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Sisay, T.; Tolessa, T. COVID-19 Pandemic: A Compressive Review on Gender, Herd Immunity, and Physiological Mechanisms. Risk Manag. Healthc. Policy 2020, 13, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Israeli Ministry of Health: COVID-19 Database (Hebrew). Available online: https://data.gov.il/dataset/covid-19 (accessed on 18 December 2021).
- Israeli Central Bureau of Statistics (ICBS): Table 2.24: Population and Density Per Sq. Km. of Land in Localities with 5000 or more Residents on 31.12.2017. Available online: https://www.cbs.gov.il/he/publications/doclib/2018/2.%20shnatonpopulation/st02_24.pdf (accessed on 18 December 2021).
- Ramanathan, R. Introductory Econometrics with Applications, 5th ed; Harcourt College Publishers: San Diego, CA, USA, 2002. [Google Scholar]
- Greene, W.H. Econometric Analysis, 7th ed.; Pearson Education Limited: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Cochrane, D.; Orcutt, G.H. Application of Least Squares Regression to Relationships Containing Auto- Correlated Error Terms. J. Am. Stat. Assoc. 1949, 44, 32–61. [Google Scholar] [CrossRef]
- Johnston, J.; John, D. Econometric Methods, 4th ed.; McGraw Hill International Edition: New York, NY, USA, 1997. [Google Scholar]
- Prais, S.J.; Winsten, C.B. Trend Estimation and Serial Correlation; Cowles Comission Discussion Paper. 1954, p. 383. Available online: https://cowles.yale.edu/sites/default/files/files/pub/cdp/s-0383.pdf (accessed on 13 June 2022).
- Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.; Hartley, D.; Beck, A.F.; Oliver, G.H.; Sampath, B.; Roderick, T.; Miff, S. Rethinking Herd Immunity: Managing the Covid-19 Pandemic in a Dynamic Biological and Behavioral Environment. NEJM Catal. Innov. Care Deliv. 2021, 2, 5. [Google Scholar] [CrossRef]
- Kalish, H.; Klumpp-Thomas, C.; Hunsberger, S. Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci. Transl. Med. 2020, 13, eabh3826. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Yamana, T.K.; Kandula, S.; Galanti, M.; Shaman, J. Burden and characteristics of COVID-19 in the United States during 2020. Nature 2021, 598, 338–341. [Google Scholar] [CrossRef]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0)”. Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R0) of measles: A systematic review. Lancet. Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- Ireland’s Health Services. Health Care Worker Information. Available online: https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter23.pdf (accessed on 11 January 2022).
- Australian Department of Health Mumps Laboratory Case Definition (LCD). Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-mumps.htm (accessed on 13 June 2022).
- Fine, P.E.M. Herd Immunity: History, Theory, Practice. Epidemiol. Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef]
- Jiles, R.B.; Fuchs, C.; Klevens, R.M. Vaccination coverage among children enrolled in Head Start programs or day care facilities or entering school. Morb. Mortal. Wkly. Rep. 2000, 49, 27–38. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss4909a2.htm (accessed on 13 June 2022).
- Luman, E.T.; Barker, L.E.; Simpson, D.M.; Rodewald, L.E.; Szilagyi, P.G.; Zhao, Z. National, state, and urban-area vaccination-coverage levels among children aged 19–35 months, United States, 1999. Am. J. Prev. Med. 2021, 20, 88–153. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Teunis, P.F.; Pebody, R.G. Incidence and reproduction numbers of pertussis: Estimates from serological and social contact data in five European countries. PLOS Med. 2010, 7, e1000291. [Google Scholar] [CrossRef] [PubMed]
- National Emerging Special Pathogen Training and Education Center. Playing the Numbers Game: R0. May 2020. Available online: https://netec.org/2020/01/30/playing-the-numbers-game-r0/ (accessed on 5 January 2022).
- Liu, Y.; Rocklöv, J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021, 28, taab124. [Google Scholar] [CrossRef]
- Prather, K.A.; Marr, L.C.; Schooley, R.T.; McDiarmid, M.A.; Wilson, M.E.; Milton, D.K. Airborne transmission of SARS-CoV-2. Science 2020, 370, 303–304. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; World Health Organization. History and Epidemiology of Global Smallpox Eradication. Smallpox: Disease, Prevention, and Intervention (Training Course). 2001. Available online: https://stacks.cdc.gov/view/cdc/27929 (accessed on 11 January 2022).
- Gani, R.; Leach, S. Transmission potential of smallpox in contemporary populations. Nature 2001, 414, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J. Covid: Is There a Limit to How Much Worse Variants Can Get? BBC News, 12 June 2021. Available online: https://www.bbc.com/news/health-57431420 (accessed on 5 January 2022).
- Martínez, V.P.; Di Paola, N.; Alonso, D.O.; Pérez-Sautu, U.; Bellomo, C.M.; Iglesias, A.A. ‘Super-Spreaders’ and Person-to-Person Transmission of Andes Virus in Argentina. N. Engl. J. Med. 2020, 383, 2230–2241. [Google Scholar] [CrossRef]
- Arif, B.; Mamun, M.; Nuruzzaman, K. Reproductive number of coronavirus: A systematic review and meta-analysis based on global level evidence. PLoS ONE 2020, 15, e0242128. [Google Scholar] [CrossRef]
- World Health Organization. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS); Technical Report; Department of Communicable Disease Surveillance and Response: Geneva, Switzerland, 2003; p. 26. [Google Scholar]
- Truelove, S.A.; Keegan, L.T.; Moss, W.J.; Chaisson, L.H.; Macher, E.; Azman, A.S.; Lessler, J. Clinical and Epidemiological Aspects of Diphtheria: A Systematic Review and Pooled Analysis. Clin. Infect. Dis. 2020, 71, 89–97. [Google Scholar] [CrossRef]
- Freeman, C. Magic Formula That Will Determine Whether Ebola is Beaten; Telegraph. Co.: London, UK, 2020; Available online: https://www.telegraph.co.uk/news/worldnews/ebola/11213280/Magic-formula-that-will-determine-whether-Ebola-is-beaten.html (accessed on 13 June 2022).
- Wong, Z.S.; Bui, C.M.; Chughtai, A.A.; Macintyre, C.R. A systematic review of early modelling studies of Ebola virus disease in West Africa. Epidemiol. Infect. 2017, 145, 1069–1094. [Google Scholar] [CrossRef]
- Fraser, C.; Donnelly, C.A.; Cauchemez, S.; Hanage, W.P.; Van Kerkhove, M.D.; Hollingsworth, T.D. Pandemic potential of a strain of influenza A (H1N1): Early findings. Science 2009, 324, 1557–1561. [Google Scholar] [CrossRef]
- Chowell, G.; Miller, M.A.; Viboud, C. Seasonal influenza in the United States, France, and Australia: Transmission and prospects for control. Epidemiol. Infect. 2008, 136, 852–864. [Google Scholar] [CrossRef] [PubMed]

| (a) Description of Variables | ||||||
| Variable | Description | |||||
| COVID-19 active cases divided by the population of the city and multiplied by 10,000. | ||||||
| Population density measured as . | ||||||
| 1 = Above or equal to the median population density; 0 = otherwise. | ||||||
| Percent of vaccinated persons in the city multiplied by 100. | ||||||
| (b) Active Cases ≥ 0. | ||||||
| Variable | Obs | Mean | Median | Std. Dev. | Min | Max |
| 91 | 10.54396 | 5.0 | 15.43522 | 0 | 85.4 | |
| 91 | 3711.9780 | 2388 | 4037.0320 | 67 | 26,512 | |
| 91 | 0.4945 | 0.0000 | 0.5027 | 0 | 1 | |
| 91 | 57.3662 | 61.76 | 15.4098 | 18.22 | 80.21 | |
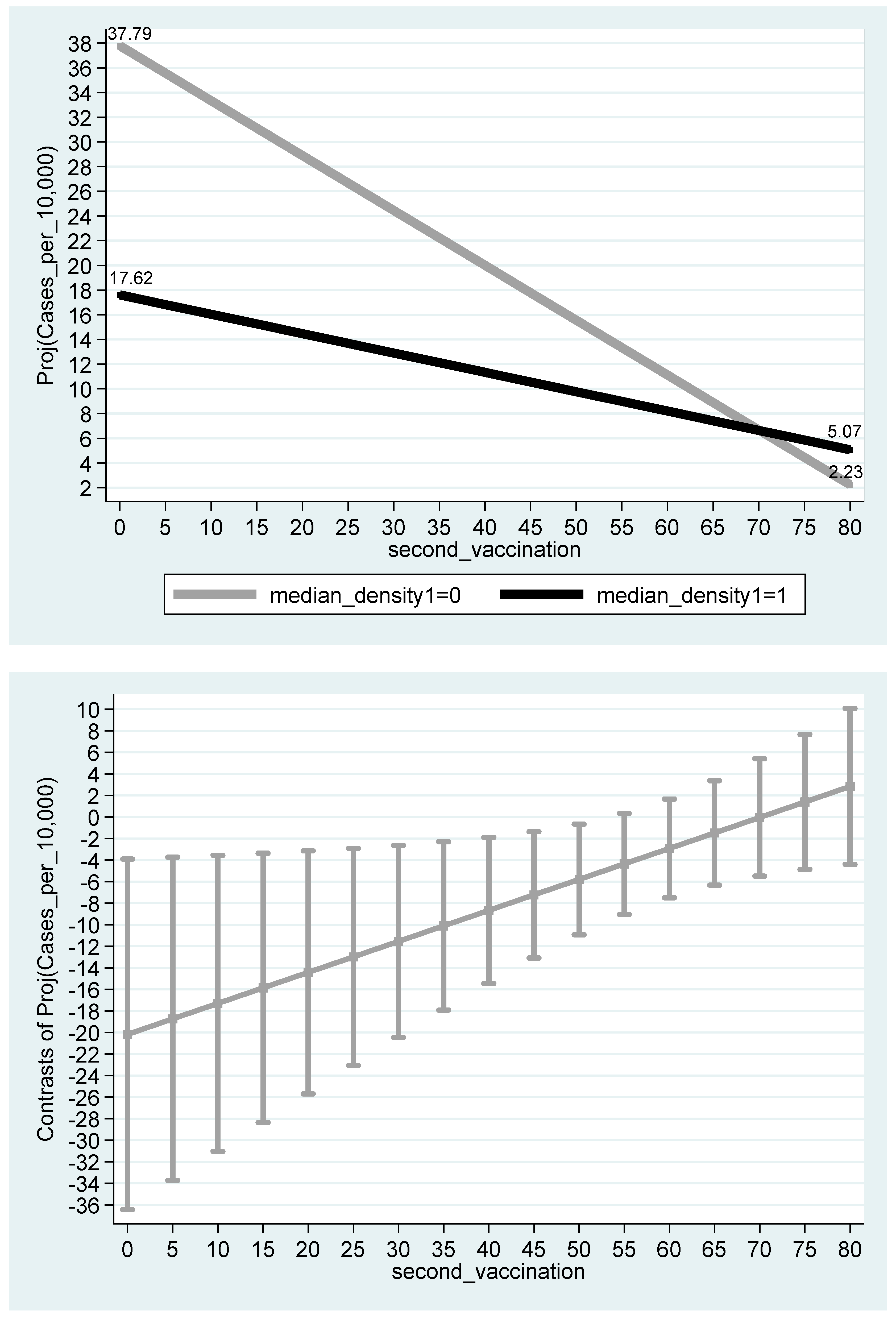
| (1) | |
|---|---|
| Variables | |
| Constant | 37.79 *** |
| (8.96 × 10−9) | |
| −20.17 ** | |
| (0.0171) | |
| −0.444 *** | |
| (1.90 × 10−5) | |
| 0.288 ** | |
| (0.0364) | |
| Observations | 91 |
| R-squared | 0.219 |
| Disease | Transmission | HIT | Reference Number |
|---|---|---|---|
| Measles | Aerosol | 92–94% | [23,24] |
| Chickenpox (varicella) | Aerosol | 90–92% | [25] |
| Mumps | Respiratory droplets | 90–92% | [26] |
| Rubella | Respiratory droplets | 83–86% | [27,28,29] |
| Polio | Fecal–oral route | 80–86% | [27,28,29] |
| Pertussis | Respiratory droplets | 82% | [30,31] |
| COVID-19 (Delta variant) | Respiratory droplets and aerosol | 80% | [22,32,33] |
| Smallpox | Respiratory droplets | 71–83% | [34,35] |
| COVID-19 (Alpha variant) | Respiratory droplets and aerosol | 75–80% | [36] |
| HIV/AIDS | Body fluids | 50–80% | [31] |
| COVID-19 (ancestral strain) | Respiratory droplets and aerosol [37] | 65% (58–71%) | [38] |
| SARS | Respiratory droplets | 50–75% | [39] |
| Diphtheria | Saliva | 62% (41–77%) | [40] |
| Ebola (2014 outbreak) | Body fluids | 44% (31–44%) | [41,42] |
| Influenza (2009 pandemic strain) | Respiratory droplets | 37% (25–51%) | [43] |
| Influenza (seasonal strains) | Respiratory droplets | 23% (17–29%) | [44] |
| Andes hantavirus | Respiratory droplets and body fluids | 16% (0–36%) | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbel, Y.; Arbel, Y.; Kerner, A.; Kerner, M. Is COVID-19 Herd Immunity Influenced by Population Densities of Cities? Sustainability 2022, 14, 10286. https://doi.org/10.3390/su141610286
Arbel Y, Arbel Y, Kerner A, Kerner M. Is COVID-19 Herd Immunity Influenced by Population Densities of Cities? Sustainability. 2022; 14(16):10286. https://doi.org/10.3390/su141610286
Chicago/Turabian StyleArbel, Yuval, Yifat Arbel, Amichai Kerner, and Miryam Kerner. 2022. "Is COVID-19 Herd Immunity Influenced by Population Densities of Cities?" Sustainability 14, no. 16: 10286. https://doi.org/10.3390/su141610286
APA StyleArbel, Y., Arbel, Y., Kerner, A., & Kerner, M. (2022). Is COVID-19 Herd Immunity Influenced by Population Densities of Cities? Sustainability, 14(16), 10286. https://doi.org/10.3390/su141610286






