Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature
Abstract
:1. Introduction
2. Methodology
3. History
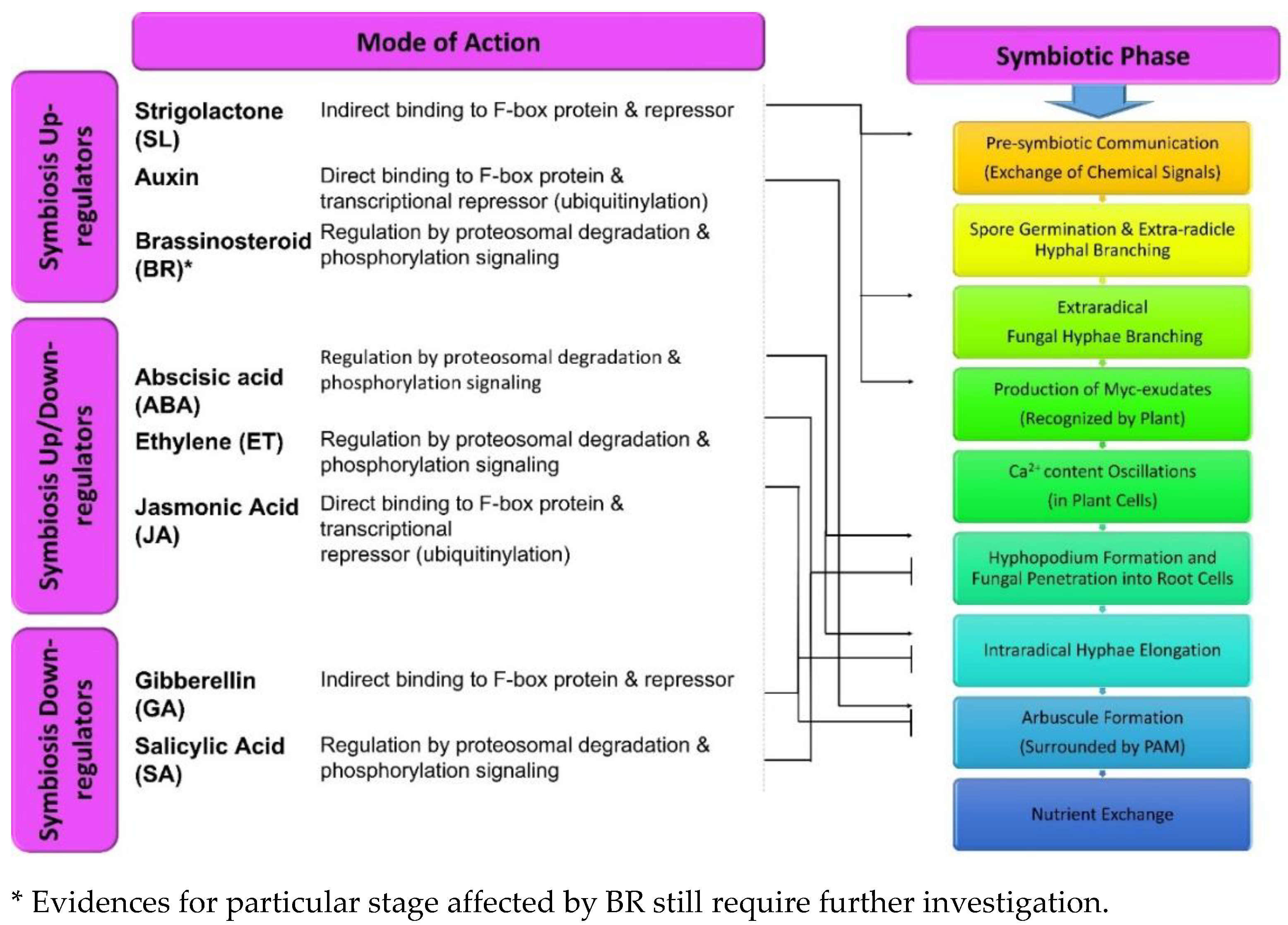
4. Role of Phytohormones in Regulating the Development of Plant-Fungus Symbiotic Association
4.1. Auxin
4.2. Strigolactone
4.3. Gibberellin
4.4. Abscisic Acid (ABA)
4.5. Jasmonate (JA)
4.6. Brassinosteroid
4.7. Ethylene
4.8. Salicylic Acid (SA)
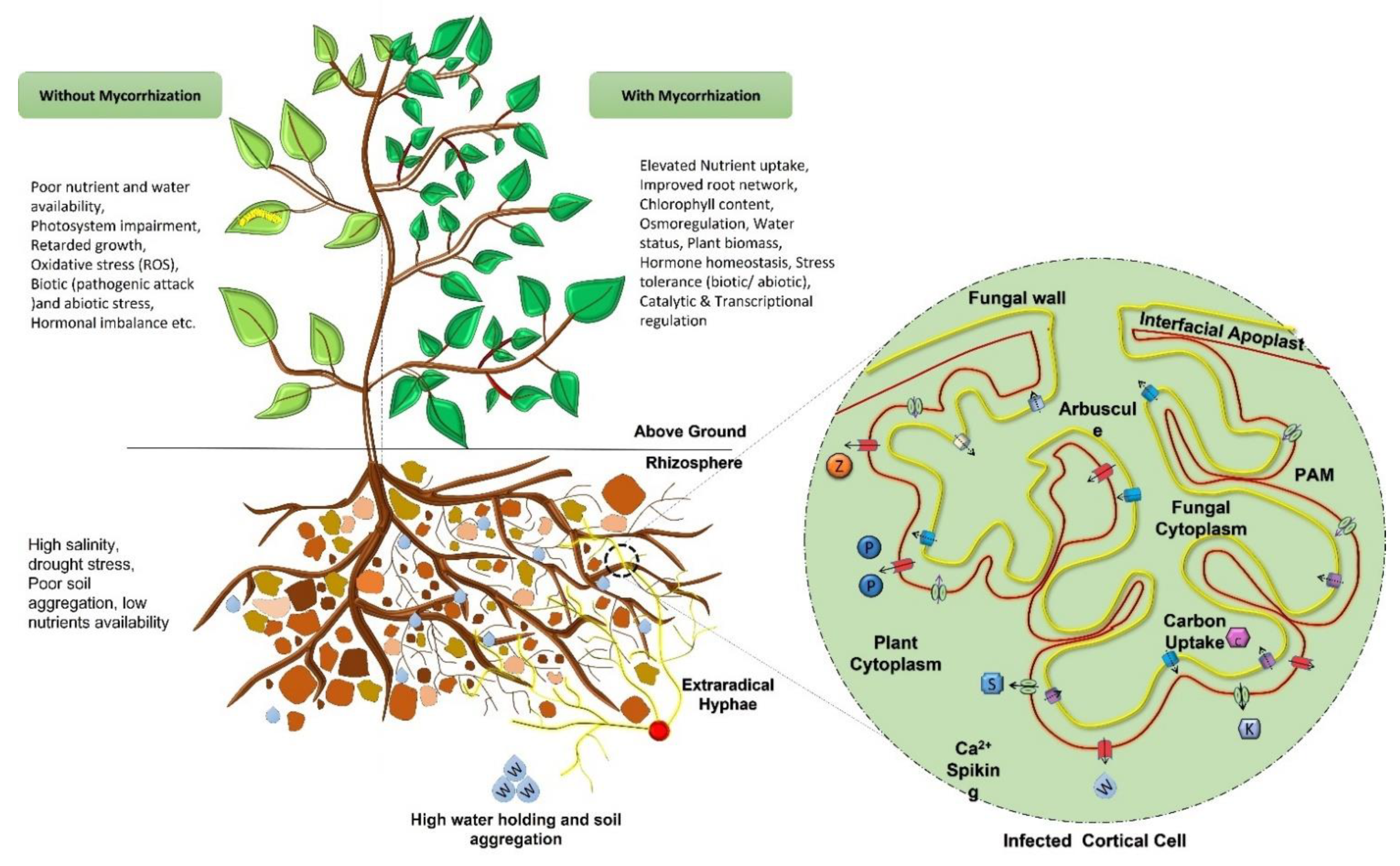
5. Applications of Mycorrhizal Symbiosis to the Ecosystem
5.1. Positive Impacts on Plant Growth and Nutritional Requirements
5.2. AMF and Mineral Nutrition
5.3. AMF as Bio-Fertilizer
5.4. Mitigation of Biotic & Abiotic Stress
5.5. Potential Applications in Phytoremediation
5.6. Enhanced Biological Produce and Agricultural Profitability
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
References
- Schweitzer, J.A.; Bailey, J.K.; Fischer, D.G.; LeRoy, C.J.; Lonsdorf, E.V.; Whitham, T.G.; Hart, S.C. Plant-soil-microorganism interactions: Heritable relationship between plant genotype and associated soil microorganisms. Ecology 2008, 89, 773–781. [Google Scholar] [CrossRef]
- Frank, B. Über die auf Wurzelsymbiose Beruhende Ernährung Gewisser Bäume Durch Unterirdische Pilze; Berichte der Deutschen Botanischen Gesellschaft: Berlin, Germany, 1885. [Google Scholar]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.E.; Metwally, R.A. Mitigation of salt stress by dual application of arbuscular mycor-rhizal fungi and salicylic acid. Agrochimica 2018, 62, 353–366. [Google Scholar] [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones regulate the development of Arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef]
- Rao, A.V.; Tak, R. Growth of different tree species and their nutrient uptake in limestone mine spoil as influenced by Arbuscular mycorrhizal (AM)-fungi in Indian arid zone. J. Arid Environ. 2002, 51, 113–119. [Google Scholar] [CrossRef]
- Remy, W.; Taylor, T.N.; Hass, H.; Kerp, H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl. Acad. Sci. USA 1994, 91, 11841–11843. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Ghosh, A.; Song, Y.; Chen, H.; Tang, M. Assessment of Arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead–zinc mine area: Potential applications for phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 13179–13193. [Google Scholar] [CrossRef]
- Gutjahr, C.; Parniske, M. Cell and Developmental Biology of Arbuscular Mycorrhiza Symbiosis. Annu. Rev. Cell Dev. Biol. 2013, 29, 593–617. [Google Scholar] [CrossRef]
- Harrison, M.J. Signaling in the Arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Molecular phylogeny and systematics of Glomeromycota: Methods and limitations. Plant Arch. 2018, 18, 1091–1101. [Google Scholar]
- Hlbbett, D.S.; Gilbert, L.B.; Donoghue, M.J. Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature 2000, 407, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M. Diversity and classification of mycorrhizal associations. Biol. Rev. Camb. Philos. Soc. 2004, 79, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J. The Arbuscular mycorrhizal symbiosis. In Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 1997; pp. 1–34. [Google Scholar]
- Prasad, R.; Bhola, D.; Akdi, K.; Cruz, C.; Sairam, K.; Tuteja, N.; Varma, A. Introduction to mycorrhiza: Historical development. In Mycorrhiza-Function, Diversity, State of the Art; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–7. [Google Scholar]
- Chagnon, P.-L. Ecological and evolutionary implications of hyphal anastomosis in Arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2014, 88, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Varma, A. Symbiosis: The art of living. In Symbiotic Fungi; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–28. [Google Scholar]
- Kehri, H.K.; Akhtar, O.; Zoomi, I.; Pandey, D. Arbuscular mycorrhizal Fungi: Taxonomy and its Systematics. Int. J. Life Sci. Res. 2018, 6, 58–71. [Google Scholar]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and Arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Hajiboland, R.; Dashtebani, F.; Aliasgharzad, N. Physiological responses of halophytic C4 grass Aeluropus littoralis to salinity and Arbuscular mycorrhizal fungi colonization. Photosynthetica 2015, 53, 572–584. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of Arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Liu, X.; Shan, L.; Wu, Q.; Liu, Y.; Zhang, Z.; Ma, F.; Li, C. Dopamine and Arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ. Exp. Bot. 2020, 178, 104159. [Google Scholar] [CrossRef]
- Hadian-Deljou, M.; Esna-Ashari, M.; Mirzaie-asl, A. Alleviation of salt stress and expression of stress-responsive gene through the symbiosis of Arbuscular mycorrhizal fungi with sour orange seedlings. Sci. Hortic. 2020, 268, 109373. [Google Scholar] [CrossRef]
- Shahvali, R.; Shiran, B.; Ravash, R.; Fallahi, H.; Banović Đeri, B. Effect of symbiosis with Arbuscular mycorrhizal fungi on salt stress tolerance in GF677 (peach × almond) rootstock. Sci. Hortic. 2020, 272, 109535. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-um, S. Alleviation of Salt Stress in Upland Rice (Oryza sativa L. ssp. indica cv. Leum Pua) Using Arbuscular mycorrhizal Fungi Inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Cui, M.; Lu, W.; Sun, H. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association With Two Different Arbuscular mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Saia, S.; Amato, G.; Frenda, A.S.; Giambalvo, D.; Ruisi, P. Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS ONE 2014, 9, e90738. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of Arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the Arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays) plants with Arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolrá, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Guo, J.; Li, X.; Lin, J.; Zhu, X. Changes in photosynthesis and respiratory metabolism of maize seedlings growing under low temperature stress may be regulated by Arbuscular mycorrhizal fungi. Plant Physiol. Biochem. 2020, 154, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with Arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Mehralian, M. Arbuscular mycorrhizal Fungi Inoculation to Enhance Chilling Stress Tolerance of Watermelon. Gesunde Pflanz. 2020, 72, 171–179. [Google Scholar] [CrossRef]
- Formenti, L.; Rasmann, S. Mycorrhizal fungi enhance resistance to herbivores in tomato plants with reduced jasmonic acid production. Agronomy 2019, 9, 131. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R. Sen Priming of Anti-Herbivore Defense in Tomato by Arbuscular mycorrhizal Fungus and Involvement of the Jasmonate Pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Di Fossalunga, A.S.; Ciancio, A.; Pentimone, I. Transcriptomic responses to water deficit and nematode infection in mycorrhizal tomato roots. Front. Microbiol. 2019, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Marquez, N.; Giachero, M.L.; Gallou, A.; Debat, H.J.; Declerck, S.; Ducasse, D.A. Transcriptome analysis of mycorrhizal and nonmycorrhizal soybean plantlets upon infection with Fusarium virguliforme, one causal agent of sudden death syndrome. Plant Pathol. 2019, 68, 470–480. [Google Scholar] [CrossRef]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef]
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010; ISBN 0080559344. [Google Scholar]
- Balestrini, R.; Kottke, I. Structure and development of ectomycorrhizal roots. In Molecular Mycorrhizal Symbiosis; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118951446. [Google Scholar]
- Agerer, R. Exploration types of ectomycorrhizae: A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Berbee, M.L.; Taylor, J.W. Dating the evolutionary radiations of the true fungi. Can. J. Bot. 1993, 71, 1114–1127. [Google Scholar] [CrossRef]
- Lepage, B.A.; Currah, R.S.; Stockey, R.A.; Rothwell, G.W. Fossil ectomycorrhizae from the middle Eocene. Am. J. Bot. 1997, 84, 410–412. [Google Scholar] [CrossRef]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Shabek, N.; Zheng, N. Plant ubiquitin ligases as signaling hubs. Nat. Struct. Mol. Biol. 2014, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light Regulation of Plant Defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Q.; Yao, T.; Fu, X. Shedding light on integrative GA signaling. Curr. Opin. Plant Biol. 2014, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- MacDougal, D.T.; Dufrenoy, J. Mycorrhizal Symbiosis In Aplectrum, Corallorhiza and Pinus. Plant Physiol. 1944, 19, 440–465. [Google Scholar] [CrossRef]
- Sitrit, Y.; Roth-Bejerano, N.; Kagan-Zur, V.; Turgeman, T. Pre-symbiotic Interactions Between the Desert Truffle Terfezia boudieri and Its Host Plant Helianthemum sessiliflorum. In Desert Truffles Phylogeny, Physiology, Distribution and Domestication; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.P.; Palme, K.; Martin, F.; Ditengou, F.A.; Legué, V. The ectomycorrhizal fungus laccaria bicolor stimulates lateral root formation in poplar and arabidopsis through auxin transport and signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef]
- Zaretsky, M.; Kagan-Zur, V.; Mills, D.; Roth-Bejerano, N. Analysis of mycorrhizal associations formed by Cistus incanus transformed root clones with Terfezia boudieri isolates. Plant Cell Rep. 2006, 25, 62–70. [Google Scholar] [CrossRef]
- Gay, G.; Normand, L.; Marmeisse, R.; Sotta, B.; Debaud, J.C. Auxin overproducer mutants of Hebeloma cylindrosporum Romagnesi have increased mycorrhizal activity. New Phytol. 1994, 128, 645–657. [Google Scholar] [CrossRef]
- Herrmann, S.; Oelmüller, R.; Buscot, F. Manipulation of the onset of ectomycorrhiza formation by indole-3-acetic acid, activated charcoal or relative humidity in the association between oak microcuttings and Piloderma croceum: Influence on plant development and photosynthesis. J. Plant Physiol. 2004, 161, 509–517. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Multiple control levels of root system remodeling in Arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2013, 4, 204. [Google Scholar] [CrossRef]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Atarashi, T.; Kuse, M.; Sugimoto, Y.; Takikawa, H. Concise synthesis of heliolactone, a non-canonical strigolactone isolated from sunflower. Biosci. Biotechnol. Biochem. 2020, 84, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Ruyter-Spira, C.; Bouwmeester, H.J. Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp. Plant Sci. 2011, 180, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in Arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate Arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Taulera, Q.; Lauressergues, D.; Martin, K.; Cadoret, M.; Servajean, V.; Boyer, F.D.; Rochange, S. Initiation of Arbuscular mycorrhizal symbiosis involves a novel pathway independent from hyphal branching. Mycorrhiza 2020, 30, 491–501. [Google Scholar] [CrossRef]
- Genre, A.; Chabaud, M.; Balzergue, C.; Puech-Pagès, V.; Novero, M.; Rey, T.; Fournier, J.; Rochange, S.; Bécard, G.; Bonfante, P.; et al. Short-chain chitin oligomers from Arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol. 2013, 198, 190–202. [Google Scholar] [CrossRef]
- Das, D.; Gutjahr, C. Role of phytohormones in arbuscular mycorrhiza development. In The Model Legume Medicago Truncatula; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Pimprikar, P.; Gutjahr, C. Transcriptional Regulation of Arbuscular Mycorrhiza Development. Plant Cell Physiol. 2018, 59, 678–695. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- McGuiness, P.N.; Reid, J.B.; Foo, E. The role of gibberellins and brassinosteroids in nodulation and Arbuscular mycorrhizal associations. Front. Plant Sci. 2019, 10, 269. [Google Scholar] [CrossRef]
- Takeda, N.; Handa, Y.; Tsuzuki, S.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by Arbuscular Mycorrhizal fungi in Lotus Japonicus. Plant Physiol. 2015, 167, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Foo, E. Auxin influences strigolactones in pea mycorrhizal symbiosis. J. Plant Physiol. 2013, 170, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.S.; Levy, J.G.; Lévesque-Tremblay, V.; Pumplin, N.; Harrison, M.J. DELLA proteins regulate arbuscule formation in Arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, E5025–E5034. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Luo, D.; Zhang, X.; Liu, J.; Wang, W.; Jin, Y.; Dong, W.; Liu, J.; Liu, H.; Yang, W.; et al. A della protein complex controls the Arbuscular mycorrhizal symbiosis in plants. Cell Res. 2014, 24, 130–133. [Google Scholar] [CrossRef]
- Carbonnel, S.; Gutjahr, C. Control of arbuscular mycorrhiza development by nutrient signals. Front. Plant Sci. 2014, 5, 462. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. B. 2013, 11, e0166. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J.A.; Huertas, R.; Ho-Plágaro, T.; Ocampo, J.A.; Čková, V.T.; Tarkowská, D.; Ludwig-Müller, J.; García-Garrido, J.M. Gibberellin-abscisic acid balances during arbuscular mycorrhiza formation in tomato. Front. Plant Sci. 2016, 7, 1273. [Google Scholar] [CrossRef]
- Meixner, C.; Ludwig-Müller, J.; Miersch, O.; Gresshoff, P.; Staehelin, C.; Vierheilig, H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 2005, 222, 709–715. [Google Scholar] [CrossRef]
- Gutjahr, C.; Siegler, H.; Haga, K.; Iino, M.; Paszkowski, U. Full establishment of Arbuscular mycorrhizal symbiosis in rice occurs independently of enzymatic jasmonate biosynthesis. PLoS ONE 2015, 10, e0123422. [Google Scholar] [CrossRef]
- Isayenkov, S.; Mrosk, C.; Stenzel, I.; Strack, D.; Hause, B. Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol. 2005, 139, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R.; Schaarschmidt, S.; Hause, B. Repeated leaf wounding alters the colonization of Medicago truncatula roots by beneficial and pathogenic microorganisms. Plant, Cell Environ. 2012, 35, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. Interaction of brassinosteroid functions and sucrose transporter SlSUT2 regulate the formation of arbuscular mycorrhiza. Plant Signal. Behav. 2014, 9, e970426. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. 2014, 78, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; McAdam, E.L.; Weller, J.L.; Reid, J.B. Interactions between ethylene, gibberellins, and brassinosteroids in the development of rhizobial and mycorrhizal symbioses of pea. J. Exp. Bot. 2016, 67, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, C.; Khavari-Nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Responses of wheat plants to interactions of 24-epibrassinolide and Glomus mosseae in saline condition. Physiol. Mol. Biol. Plants 2017, 23, 557–564. [Google Scholar] [CrossRef]
- Hansch, F.; Jaspar, H.; von Sivers, L.; Bitterlich, M.; Franken, P.; Kühn, C. Brassinosteroids and sucrose transport in mycorrhizal tomato plants. Plant Signal. Behav. 2020, 15, 1714292. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Liu, J.; Wang, H.; Wei, J.; Sun, P.; Wu, L.; Zheng, H. Progress of ethylene action mechanism and its application on plant type formation in crops. Saudi J. Biol. Sci. 2020, 27, 1667–1673. [Google Scholar] [CrossRef]
- Varma Penmetsa, R.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef]
- Fracetto, G.G.M.; Peres, L.E.P.; Lambais, M.R. Gene expression analyses in tomato near isogenic lines provide evidence for ethylene and abscisic acid biosynthesis fine-tuning during arbuscular mycorrhiza development. Arch. Microbiol. 2017, 199, 787–798. [Google Scholar] [CrossRef]
- Geil, R.D.; Peterson, R.L.; Guinel, F.C. Morphological alterations of pea (Pisum sativum cv. Sparkle) arbuscular mycorrhizas as a result of exogenous ethylene treatment. Mycorrhiza 2001, 11, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Banba, M.; Croset, V.; An, K.; Miyao, A.; An, G.; Hirochika, H.; Imaizumi-Anraku, H.; Paszkowski, U. Arbuscular Mycorrhiza-Specific Signaling in Rice Transcends the Common Symbiosis Signaling Pathway. Plant Cell 2008, 20, 2989–3005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Y.; Wang, H.; Ma, X.; Yao, W.; Wang, H. Light and ethylene coordinately regulate the phosphate starvation response through transcriptional regulation of PHOSPHATE STARVATION RESPONSE1. Plant Cell 2017, 29, 2269–2284. [Google Scholar] [CrossRef] [PubMed]
- Herrera Medina, M.J.; Gagnon, H.; Piché, Y.; Ocampo, J.A.; García Garrido, J.M.; Vierheilig, H. Root colonization by Arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci. 2003, 164, 993–998. [Google Scholar] [CrossRef]
- Blilou, I.; Ocampo, J.A.; García-Garrido, J.M. Induction of LTP (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the Arbuscular mycorrhizal fungus Glomus mosseae. J. Exp. Bot. 2000, 51, 1969–1977. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Weights in the balance: Jasmonic acid and Salicylic acid signaling in root-biotroph interactions. Mol. Plant-Microbe Interact. 2009, 22, 763–772. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A. Why farmers should manage the Arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Ericoid mycorrhizas. In Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Dhiman, M.; Sharma, L.; Singh, A.; Sharma, M.M. Exsitu Conservation Using In vitro Methods of an Endangered Plant Sterculia urens Roxb.: A High Volume Trade Plant for Gum Karaya. Ind. Crops Prod. 2020, 158, 113015. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Liu, K.; Chen, Y.; Yuan, J.; Chang, Q. Rhizoglomus intraradices Improves Plant Growth, Root Morphology and Phytohormone Balance of Robinia pseudoacacia in Arsenic-Contaminated Soils. Front. Microbiol. 2020, 11, 1428. [Google Scholar] [CrossRef]
- Singh, M.; Beura, K.; Pradhan, A.K.; Rakshit, R.; Lal, M. Ability of arbuscular mycorrhiza to promote growth of maize plant and enzymatic activity of an alluvial soil. J. Appl. Nat. Sci. 2015, 7, 1029–1035. [Google Scholar] [CrossRef]
- Pritsch, K.; Garbaye, J. Enzyme secretion by ECM fungi and exploitation of mineral nutrients from soil organic matter. Ann. For. Sci. 2011, 68, 25–32. [Google Scholar] [CrossRef]
- Gianfreda, L. Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nutr. 2015, 15, 283–306. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of Arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Lü, L.H.; Wu, Q.S. Mitigation of replant disease by mycorrhization in horticultural plants: A review. Folia Hortic. 2018, 30, 269–282. [Google Scholar] [CrossRef]
- Boddington, C.L.; Dodd, J.C. Evidence that differences in phosphate metabolism in mycorrhizas formed by species of Glomus and Gigaspora might be related to their life-cycle strategies. New Phytol. 1999, 142, 531–538. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Correction: Phosphorus and nitrogen regulate Arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2015, 10, e0127472. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Odoh, C.K.; Eze, C.N.; Obi, C.J.; Anyah, F.; Egbe, K.; Unah, U.V.; Akpi, U.K.; Adobu, U.S. Fungal Biofertilizers for Sustainable Agricultural Productivity. In Agriculturally Important Fungi for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–225. [Google Scholar]
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by Arbuscular mycorrhizal fungi. Plant Cell 2002, 14, 2413–2429. [Google Scholar] [CrossRef]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in Arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef]
- Xie, X.; Huang, W.; Liu, F.; Tang, N.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the Arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Bapaume, L.; Reinhardt, D. How membranes shape plant symbioses: Signaling and transport in nodulation and arbuscular mycorrhiza. Front. Plant Sci. 2012, 3, 223. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.D.; Hu, X.C.; Wu, Q.S.; Yang, T.Y.; Srivastava, A.K.; Zhang, D.J.; Gao, X.B.; Kuča, K. Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. S. Afr. J. Bot. 2021, 137, 455–462. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Ludewig, U. Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Ann. Bot. 2021, 127, 155–166. [Google Scholar] [CrossRef]
- Volpe, V.; Giovannetti, M.; Sun, X.G.; Fiorilli, V.; Bonfante, P. The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots. Plant Cell Environ. 2016, 39, 660–671. [Google Scholar] [CrossRef]
- Chorianopoulou, S.N.; Sigalas, P.P.; Tsoutsoura, N.; Apodiakou, A.; Saridis, G.; Ventouris, Y.E.; Bouranis, D.L. Regulation of sulfur homeostasis in mycorrhizal maize plants grown in a fe-limited environment. Int. J. Mol. Sci. 2020, 21, 3249. [Google Scholar] [CrossRef]
- Senovilla, M.; Abreu, I.; Escudero, V.; Cano, C.; Bago, A.; Imperial, J.; González-Guerrero, M. MtCOPT2 is a Cu+ transporter specifically expressed in Medicago truncatula mycorrhizal roots. Mycorrhiza 2020, 30, 781–788. [Google Scholar] [CrossRef]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef]
- Drechsler, N.; Courty, P.-E.; Brulé, D.; Kunze, R. Identification of arbuscular mycorrhiza-inducible Nitrate Transporter 1/Peptide Transporter Family (NPF) genes in rice. Mycorrhiza 2018, 28, 93–100. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 14880. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci. 2018, 274, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The potassium transporter slhak10 is involved in mycorrhizal potassium uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef]
- Drew, E.A.; Murray, R.S.; Smith, S.E.; Jakobsen, I. Beyond the rhizosphere: Growth and function of Arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 2003, 251, 105–114. [Google Scholar] [CrossRef]
- Rooney, D.C.; Killham, K.; Bending, G.D.; Baggs, E.; Weih, M.; Hodge, A. Mycorrhizas and biomass crops: Opportunities for future sustainable development. Trends Plant Sci. 2009, 14, 542–549. [Google Scholar] [CrossRef]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of Arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ma, L.; He, X.; Liu, Z.; Wang, F.; Chu, G.; Tang, X. Accumulation of glomalin-related soil protein benefits soil carbon sequestration: Tropical coastal forest restoration experiences. Land Degrad. Dev. 2022, 33, 1541–1551. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of Arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Chen, B.D.; Sun, Y.Q.; Ren, B.H.; Zhang, X.; Wang, Y.S. Chromium resistance of dandelion (Taraxacum platypecidum Diels.) and bermudagrass (Cynodon dactylon [Linn.] Pers.) is enhanced by arbuscular mycorrhiza in Cr(VI)-contaminated soils. Environ. Toxicol. Chem. 2014, 33, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.A.; Peralta-Videa, J.R.; Ellzey, J.T.; Viveros, M.N.; Ren, M.; Mokgalaka-Matlala, N.S.; Castillo-Michel, H.; Gardea-Torresdey, J.L. Plant growth and metal distribution in tissues of Prosopis Juliflora-velutina grown on chromium contaminated soil in the presence of Glomus deserticola. Environ. Sci. Technol. 2010, 44, 7272–7279. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Wu, S.C.; Kuek, C.; Khan, A.G.; Wong, M.H. The role of mycorrhizae associated with vetiver grown in Pb-/ Zn-contaminated soils: Greenhouse study. Restor. Ecol. 2007, 15, 60–67. [Google Scholar] [CrossRef]
- Chen, B.D.; Li, X.L.; Tao, H.Q.; Christie, P.; Wong, M.H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 2003, 50, 839–846. [Google Scholar] [CrossRef]
- Arriagada, C.; Pereira, G.; García-Romera, I.; Ocampo, J.A. Improved zinc tolerance in Eucalyptus globulus inoculated with Glomus deserticola and Trametes versicolor or Coriolopsis rigida. Soil Biol. Biochem. 2010, 42, 118–124. [Google Scholar] [CrossRef]
- Arriagada, C.A.; Herrera, M.A.; Ocampo, J.A. Contribution of Arbuscular mycorrhizal and saprobe fungi to the tolerance of Eucalyptus globulus to Pb. Water. Air. Soil Pollut. 2005, 166, 31–47. [Google Scholar] [CrossRef]
- Maldaner, J.; Steffen, G.P.K.; Saldanha, C.W.; Steffen, R.B.; Tabaldi, L.A.; Missio, E.L.; De Morais, R.M.; Flores, R. Combining tolerant species and microorganisms for phytoremediation in aluminium-contaminated areas. Int. J. Environ. Stud. 2020, 77, 108–121. [Google Scholar] [CrossRef]
- Merlos, M.A.; Zitka, O.; Vojtech, A.; Azcón-Aguilar, C.; Ferrol, N. The Arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci. 2016, 253, 68–76. [Google Scholar] [CrossRef]
- Ruscitti, M.; Arango, M.; Beltrano, J. Improvement of copper stress tolerance in pepper plants (Capsicum annuum L.) by inoculation with Arbuscular mycorrhizal fungi. Theor. Exp. Plant Physiol. 2017, 29, 37–49. [Google Scholar] [CrossRef]
- Li, J.; Liang, H.; Yan, M.; Chen, L.; Zhang, H.; Liu, J.; Wang, S.; Jin, Z. Arbuscular mycorrhiza fungi facilitate rapid adaptation of Elsholtzia splendens to copper. Sci. Total Environ. 2017, 599–600, 1462–1468. [Google Scholar] [CrossRef]
- Meier, S.; Cornejo, P.; Cartes, P.; Borie, F.; Medina, J.; Azcón, R. Interactive effect between Cu-adapted Arbuscular mycorrhizal fungi and biotreated agrowaste residue to improve the nutritional status of Oenothera picensis growing in Cu-polluted soils. J. Plant Nutr. Soil Sci. 2015, 178, 126–135. [Google Scholar] [CrossRef]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef]
- Santana, N.A.; Ferreira, P.A.A.; Tarouco, C.P.; Schardong, I.S.; Antoniolli, Z.I.; Nicoloso, F.T.; Jacques, R.J.S. Earthworms and mycorrhization increase copper phytoextraction by Canavalia ensiformis in sandy soil. Ecotoxicol. Environ. Saf. 2019, 182, 109383. [Google Scholar] [CrossRef]
- Santana, N.A.; Rabuscke, C.M.; Soares, V.B.; Soriani, H.H.; Nicoloso, F.T.; Jacques, R.J.S. Vermicompost dose and mycorrhization determine the efficiency of copper phytoremediation by Canavalia ensiformis. Environ. Sci. Pollut. Res. 2018, 25, 12663–12677. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; García, S.; Ferrol, N.; Durán, P.; Borie, F.; Seguel, A. Contribution of inoculation with Arbuscular mycorrhizal fungi to the bioremediation of a copper contaminated soil using Oenothera picensis. J. Soil Sci. Plant Nutr. 2017, 17, 14–21. [Google Scholar] [CrossRef]
- Fiqri, A.; Utomo, W.H.; Handayanto, E. Effect of Arbuscular mycorrhizal fungi on the potential of three wild plant species for phytoextraction of mercury from small-scale gold mine tailings. J. Degrad. Min. Lands Manag. 2016, 3, 551–558. [Google Scholar] [CrossRef]
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M.J. Erato polymnioides—A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 2017, 188, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.D.P.; Saldarriaga, J.F. Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils. Sustainability 2020, 12, 3795. [Google Scholar] [CrossRef]
- Shabani, L.; Sabzalian, M.R.; Mostafavi pour, S. Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 2016, 26, 67–76. [Google Scholar] [CrossRef]
- Akib, M.A.; Mustari, K.; Kuswinanti, T.; Syaiful, S.A.; Syatrawati; Kumalawati, Z. Nickel (Ni) reduction in Sorowako post-mining soil through application of mycorrhiza Acaulospora sp. associated with Canavalia ensiformis L. J. Microb. Syst. Biotechnol. 2019, 1, 30–37. [Google Scholar] [CrossRef]
- Alam, M.Z.; Anamul Hoque, M.; Ahammed, G.J.; Carpenter-Boggs, L. Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris. PLoS ONE 2019, 14, e0211441. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, E.; Godzik, B.; Turnau, K. Effect of different Arbuscular mycorrhizal fungal isolates on growth and arsenic accumulation in Plantago lanceolata L. Environ. Pollut. 2012, 168, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Shahabivand, S.; Maivan, H.Z.; Mahmoudi, E.; Soltani, B.M.; Sharifi, M.; Aliloo, A.A. Antioxidant activity and gene expression associated with cadmium toxicity in wheat affected by mycorrhizal fungus. Zemdirbyste 2016, 103, 53–60. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of Arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by Arbuscular mycorrhizal symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ho, S.H.; Zhu, S.; Ma, F.; Wu, J.; Yang, J.; Wang, L. Adaptive response of Arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. J. Environ. Manag. 2017, 197, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential contribution of Arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 2006, 65, 1959–1965. [Google Scholar] [CrossRef]
- Garg, N.; Kaur, H. Response of Antioxidant Enzymes, Phytochelatins and Glutathione Production Towards Cd and Zn Stresses in Cajanus cajan (L.) Millsp. Genotypes Colonized by Arbuscular Mycorrhizal Fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Babadi, M.; Zalaghi, R.; Taghavi, M. A non-toxic polymer enhances sorghum-mycorrhiza symbiosis for bioremediation of Cd. Mycorrhiza 2019, 29, 375–387. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water. Air. Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [PubMed]
- Joner, E.J.; Leyval, C. Time-course of heavy metal uptake in maize and clover as affected by root density and different mycorrhizal inoculation regimes. Biol. Fertil. Soils 2001, 33, 351–357. [Google Scholar] [CrossRef]
- Ortega-Larrocea, M.P.; Siebe, C.; Estrada, A.; Webster, R. Mycorrhizal inoculum potential of Arbuscular mycorrhizal fungi in soils irrigated with wastewater for various lengths of time, as affected by heavy metals and available P. Appl. Soil Ecol. 2007, 37, 129–138. [Google Scholar] [CrossRef]
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.T.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47. [Google Scholar] [CrossRef]
- Mishra, A.; Bhattacharya, A.; Mishra, N. Mycorrhizal symbiosis: An effective tool for metal bioremediation. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbes in Soil, Crop and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128182581. [Google Scholar]
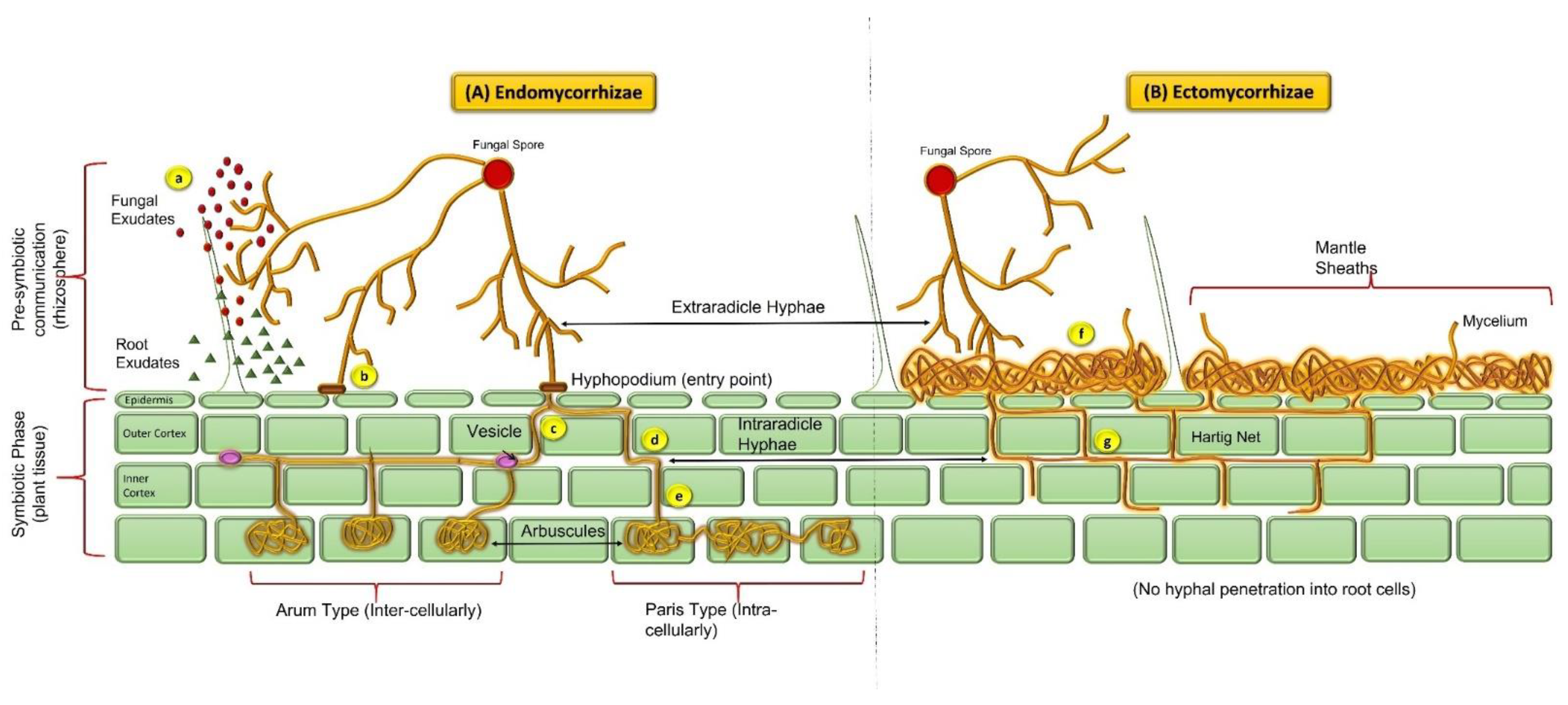
| Sr. No. | Stress Condition | Mycorrhizal sp. | Host Plant | Possible Mechanism | Reference |
|---|---|---|---|---|---|
| 1 | Salinity, 200 mM NaCl | Rhizophagus irregularis (Formerly Glomus intraradices) | R. pseudoacacia | Improved photosynthetic rate, PS-II photochemistry, water status, K+, Chloroplast (RppsbA, RppsbD, RprbcL)& transporter genes (RpSOS1, RpHKT1, RpSKOR) up-regulation, lower shoot:root Na+ content | [21] |
| 2 | 80 mM NaCl | Glomus intraradices | Lactuca sativa | Alleviates salt stress through improved stomata performance, photosystem (PS-II), Carotenoid deoxygenase gene (LsNCED2) induction, normalized ABA level and by altering the hormonal profiles (SLs induction) | [22] |
| 3 | 100 mM NaCl | Rhizophagus irregularis | Solanum lycopersicum | Elevated K+ and K+/Na+ ratio (prevention of metabolic processes disruption), regulated hormone synthesis & cross talk | [23] |
| 4 | 200 mM NaCl | Glomuse tunicatum, Glomus intraradices, Glomus mosseae | Cucumis sativus L. | Photosynthetic pigments regulation, enhanced antioxidant activities, osmolyte (proline& phenols) regulation, improved water status; regulated mineral uptake; reduced uptake of Na+. | [24] |
| 5 | 200 mM NaCl | Claroideoglomus etunicatum | Aeluropus littoralis | Overcome free radical formation by elevated antioxidant activity, high CO2 synthesis and nitrate assimilation | [25] |
| 6 | 120 mM NaCl | Funnelliformis mosseae, Acaulospora laevis, Gigaspora margarita | Oryza sativa L. | Rise in chlorophyll content, K+/Na+ ratio, photosynthesis, and dropped shoot/root Na+ ratio by limiting Na+ uptake and translocation. | [26] |
| 7 | 200 mM NaCl | Funneliformis mosseae | Malus domestica Borkh. | AMF in combination with dopamine help to maintain host cell membrane integrity, improves photosynthesis | [27] |
| 8 | 35 and 70 mM NaCl. | Glomus sp. mix (G. mosseae, G. intraradices, G. hoi) | Citrus aurantium L. | Elevation in plant growth, chlorophyll levels, improved water status, gas exchange capacities (increased photosynthetic rate, stomatal conductance and transpiration rate), enhanced oxidative stress defense system | [28] |
| 9 | 160 mM NaCl | R. intraradices and F. mosseae. | Prunusdulcis× Prunuspersica hybrid | Improved physiological parameters (chlorophyll, osmolytes that are soluble sugars and proline content to combat salt toxicity) and increased antioxidant enzymes activity compared to non-inoculated. F. mosseae elevated chlorophyll content more efficiently, whereas R. intraradices prevailed total sugars and proline content. | [29] |
| 10 | 150 mM NaCl | Glomus etunicatum, Glomusgeo sporum, and Glomus mosseae | Oryza sativa L. | Improved physiological parameters (chlorophyll, osmolytes that are soluble sugars and proline content to combat salt toxicity) and increased antioxidant enzymes activity | [30] |
| 11 | - | Funneliformis mosseae and Claroideoglomus etunicatum | Puccinellia tenuiflora | Increased P uptake, high antioxidant capacities, enhanced biomass to dilute the salt concentration, elevated K+/Na+ ratio, restricted Na+ translocation towards aerial parts. | [31] |
| 12 | 200 mM NaCl | Glomus monosporum, G. clarum, Gigasporanigra, and Acaulospora laevis | Vigna unguiculata L. | Elevated photosynthetic pigments, soluble sugar contents, ions accumulation and compartmentalization (maintained membrane integrity) and high enzymatic activities. | [5] |
| 13 | Drought Stress | Funneliformis mosseae (formerly Glomus mosseae) and Rhizophagus intraradices | Solanum lycopersicum | Stress tolerance varies depending upon the myc-species. F. mossaeae promoted volatile emission (VOC), high arbuscule formation in colonization regions R. intraradices is more efficient towards P uptake (upregulated P transporters; LePT4,5), high plant performance to lower water dispersal by adopting a compact structure (high internode/height ratio), high water utilization efficiency | [32] |
| 14 | Drought Stress | AMF | Glycine max L. | Increased water holding capacity, photosynthesics, osmoregulation | [33] |
| 15 | Drought Stress | Rhizophagus irregularis (formerly Glomus intraradices) and Funneliformis mosseae (formerly G. mosseae) | Trifolium alexandrinum L. | Enhanced nutrient uptake, increase in phosphorus acquisition, defense against oxidative stress, increased N2 fixation, sufficient availability of the photosynthates | [34] |
| 16 | Drought Stress | Funneliformis mosseae and Rhizophagus intraradices | Solanum lycopersicum | Aquaporin genes regulation; LeNIP3;1 (overexpressed), LeNIP3;1 & LeTIP2;3 (suppressed) by F. mosseae, and RiAQPF1 & 2 (overexpressed) by R. intraradices, elevated stomatal density, activation of LOX (lipoxygenase) genes, increased antioxidant activity, proline content (osmoregulation) | [35] |
| 17 | Drought Stress | Funneliformis mosseae | Triticum durum Desf., Triticum aestivum L. | Positive impact on root metabolome, high C fixation, high P sugar accumulation, osmoregulatory effects, anti-oxidative behavior, regulated phytohormone profile | [3] |
| 18 | Drought Stress | Rhizophagus irregularis | Zea mays | Efficiency of photosystem II, membrane stability, osmotic regulation via accumulation of soluble sugars and plant biomass production. Root hydraulic conduction via down-regulating aquaporin genes (ZmPIP1;6, ZmPIP2;2, and ZmTIP4;1) | [36] |
| 19 | Drought Stress | Myc-mix. (Rhizophagus intraradices + Funneliformis mosseae + F. geosporum) | Triticum aestivum | Elevation in photosynthetic pigments, high Mg uptake, C fixation (photosynthate) and biomass; improved water status; enhanced PSI & PSII photochemistry | [37] |
| 20 | - | Rhizophagus irregularis | Solanum lycopersicum | Promoted photosynthesis, improved C fixation, osmoregulation and root hydraulic conductivity via enhanced aquaporin. | [38] |
| 21 | Temperature stress (43–44 °C) | Funneliformis sp. AMF | Zea mays | Up-regulated water transport and transpiration, regulated PSII heterogeneity, stomatal conductance | [39] |
| 22 | (44 °C) | Rhizophagus intraradices, Funneliformis mosseae, F. geosporum | Zea mays | Enhanced PSI & PSII photochemistry, high Mg2+ uptake. | [40] |
| 23 | (35 °C) | Rhizophagus irregularis, Funneliformis mosseae, Funneliformis geosporum, Claroideoglomus claroideum | Triticum aestivum L. | Increased photosynthetic yield, nutrient distribution and nutrient composition in roots, lowered the K/Ca ratio | [41] |
| 24 | (3–5 °C) | Glomus versiforme and Rhizophagus irregularis | Hordeum vulgare L. | Enhanced membrane stability, antioxidative capacity & phenolics metabolism Glomus sp. imparted more alleviation against cold stress. Rhizophagus found more efficient towards survival rate. | [42] |
| 25 | (15 °C) | Rhizophagus irregularis | Zea mays L. | Down-regulated PS-I & PS-II genes and decreased oxidative stress, enhanced C assimilation by metabolic upregulation, high ATP production by increased P concentration | [43] |
| 26 | (5–25 °C) | Funneliformis mosseae, Claroideoglomus etunicatum, Rhizophagus irregularis, and Diversispora versiformis) | Solanum melongena L. | Promoted photochemical, antioxidant activities, and maintained membrane integrity, proline and phenolics accumulation (protection against stress) | [44] |
| 27 | (4 ± 0.5 °C) | Glomus intraradices | Citrullus lanatus | Improved photosynthesis, induced peroxidase (POX) activity, restoring photosynthesis efficiency, released oxidative stress | [45] |
| 28 | Biotic stress Aphids (M. euphorbiae) | Rhizophagus intraradices | Solanum lycopersicum L. | Indirect defense via enzymatic release of methyl salicylate to attract parasitoid A.ervi | [32] |
| 29 | Spodoptera littoralis | Rhizophagus irregularis | Solanum lycopersicum L. | Enhanced nutrient acquisition, N2 fixation, defense activation | [46] |
| 30 | Caterpillar, Helicoverpaarimigera | Glomus mosseae | Solanum lycopersicum Mill. | Activation of stress responsive genes (LOXD, AOC, PI-I & II) in leaves, regulated JA cascade | [47] |
| 31 | Meloidogyne incognita (severe yield losses in tomato) | Rhizophagus intraradices | Solanum lycopersicum | Improved plant peroxidases for ROS scavenging, Upregulated flavonoid enzymes, modulation of pathogen related genes (LTP), phytohormonal regulation, increased glutathione transferases | [48] |
| 32 | Fusarium virguliforme | Rhizophagus irregularis | Glycine max | Peroxidase genes regulation, decreased. Down-regulation of several genes coding for glutathione-S-transferase (GST) | [49] |
| 33 | Xiphynema index | Rhizophagus intraradices | Grapevine rootstock SO4 (Vitis berlandieri × V.riparia) | Decreased down-regulation of several genes coding for glutathione-S-transferase (GST) | [50] |
| Sr. No. | Mineral | Mycorrhizal sp. | Plant sp. | Host Plant Transporters | Effect of Mycorrhizal Symbiosis | Reference |
|---|---|---|---|---|---|---|
| 1. | Phosphate | Claroideoglomus etunicatum | Camellia sinensis | CsPT1 & CsPT4 | AMF up-regulated root CsPT1 expression, while down-regulated the CsPT4 expression. AMF inoculation significantly promoted P acquisition capacity of tea plants, especially in roots through improving root growth and enhancing soil acid phosphatase activity and root CsPT1 expression. | [124] |
| Rhizophagus irregularis | Zea mays | ZmPht1;6 & ZmPht1;11 | AMF improved plant growth and Pi assimilation, AMF colonization strongly improved the nutritional status of the plants and increased the internal P concentration. ZmPht1;6 over expression at a high level in AMF-colonized roots. While less expressed ZmPht1;11 also stimulated by AMF colonization. | [125] | ||
| 2. | Gigaspora margarita or Funnelliformis mosseae | Lotus japonicus | LjPT4 | LjPT4 affects proper arbuscule formation on the fungal side and for improved Pi uptake on the plant side. | [126] | |
| 3. | Sulfur | Rhizophagus irregularis | Zea mays | ZmSULTR1.2a, ZmSULTR2.1, ZmSULTR3.5 | Upregulation of ZmSULTR1.2a & ZmSULTR2.1 in sulfur deprived conditions while downregulation of ZmSULTR3.5 in mycorrhized plants. | [127] |
| 4. | Copper | Rhizophagus irregularis | Medicago truncatula | MtCOPT2 | Preferential expression of MtCOPT2 during mycorrhizal symbiosis. | [128] |
| Nitrate | Rhizophagus irregularis | Oryza sativa, Zea mays, Sorghum bicolor, Medicago truncatula | OsNPF4.5, ZmNPF4.5, SbNPF4.5, MtNPF4.5 | Myc-symbiosis resulted in efficient up-regulation of OsNPF4.5, ZmNPF4.5 and SbNPF4.5, while slight induction of MtNPF4.5. | [129] | |
| Rhizophagus irregularis | Oryza sativa | OsNPF genes: NPF2.2/ PTR2, NPF1.3, NPF6.4 and NPF4.12 | Enhanced expression of nitrate transporter genes in mycorrhizal roots in nutrient dependent manner. | [130] | ||
| 5. | Ammonium | Rhizophagus irregularis | Oryza sativa | OsAM1, OsAM10, OsAM20, OsAM25 | Significant upregulation in roots via AMF symbiosis. | [130] |
| Rhizophagus irregularis | Oryza sativa | OsAMT3.1 | Up-regulation of OsAMT3.1 in rice mycorrhizal roots | [129] | ||
| 6. | Zinc | Rhizophagus irregularis | Medicago truncatula | MtZIP5, MtZIP2 | AMF symbiosis caused higher expression of MtZIP5 in poor rhizospheric Zn condition and reduction in MtZIP2 at elevated soil Zn concentration. | [131] |
| Rhizophagus irregularis/mock-inoculated | Hordeum vulgare | HvZIP3, HvZIP7, HvZIP8, HvZIP10, HvZI13 | Out of five transporters, HvZI13 found most significantly upregulated, HvZI3 & 8 upregulated also in Zn deficient conditions, while HvZI7 & 10 downregulated. | [132] | ||
| 7. | Potassium | Rhizophagus irregularis | Lycium barbarum Solanum lycopersicum | LbKT1, LbSKOR SlHAK10 | Regulated expression of LbKT1 and LbSKOR for varying water & potassium availability | [133,134] |
| Pollutant | Mycorrhizal Species | Plant Species | Possible Mechanism | Literature Cited |
|---|---|---|---|---|
| Chromium (Cr) | Rhizophagus irregularis | Daucuscarota | Reduced translocation, and immobilization of Cr6+ through EPS (extracellular polymers) production. distribution of Cr in roots | [140] |
| Rhizophagus irregularis | bermudagrass [Cynodondactylon (Linn.) | Cr absorption and immobilization by AM roots, Reduction of Cr6+ to Cr3+ within fungal structures, inhibited Cr flow from roots to shoots, | [141] | |
| Rhizophagus irregularis | Taraxacum platypecidum | Cr absorption and immobilization by AM roots, inhibit Cr translocation from roots to shoots, promoted plant growth | [141] | |
| Glomus deserticola | Prosopisjuli flora-velutina | Accumulation of Cr in vascular tissue and decreased the translocation of Cr into shoots | [142] | |
| Zinc (Zn) | Glomus mosseae & G. intraradices | Vetiver grass | Increased P uptake by the plant and improved overall growth (G. intraradices showed more rehabilitation capacity) | [143] |
| Glomu smosseae | Trifolium pratense | Zn accumulation in roots which decreases in shoots as the Zn conc. rises to its maximum, improved P sustenance | [144] | |
| Glomus deserticola | Eucalyptus globulus | Increased root to shoot metal accumulation, high metabolic activity, symbiotic effect of saprophytic fungal sp. on mycoremediation process | [145] | |
| Lead (Pb) | Glomus mosseae& G. intraradices | Vetiver grass | Increased P uptake by the plant and improved overall growth (G. mosseae showed more rehabilitation capacity) | [143] |
| Glomus mosseae and G. deserticola | Eucalyptus globulus | Promoted overall growth, mineral nutrition, chlorophyll production and enzymatic performances (which further increased due to synergistic effect of G. deserticola and T. koningii), enhanced Pb accumulation | [146] | |
| Aluminium | Pisolithus sp. | Schinusmolle | Phytoextraction or phytostbilization, Glomalin production supported chelation, rise in photochemical efficacy | [147] |
| Copper (Cu) | R. irregularis | Zea mays | Increased accumulation of total phytochelating content in shoots | [148] |
| Funneliformis mosseae; R. intraradices | Capsicum annuum | Cu Higher total dry weight and the leaf | [149] | |
| Arbascular Mycorrhizal Fungi (AMF) | Elsholtzia splendens | Increase in germination rate and the germination index of the seeds as well as the fresh weights of hypocotyl and radicle | [150] | |
| Claroideoglomus claroideum | Oenothera picensis | Protect plant from metal toxicity, enhance both plant establishment and nutrition | [151] | |
| R. irregularis | Phragmites australis | Stress tolerance via up-regulating photo systems membrane complexes, improved plant growth. | [152] | |
| Rhizoglomus clarum | Canavalia ensiformis | Alleviated amounts of Cu due to phytoextraction in addition to earthworms | [153] | |
| Rhizophagus clarus | Canavalia ensiformis | Alleviated amounts of Cu due to phytoextraction & phytostabilization in addition to bovine | [154] | |
| Claroideo glomu sclaroideum and | Oenothera picensis | Cu chelation with AM-secreted glomalin protein | [155] | |
| Mercury (Hg) | Glomussp.,Gigaspora sp. &Skutelespora sp. | Cyperus kyllingia, Lindernia crustacea, Paspalum conjugatum | P. conjugatum resulted maximum phytoextraction, while C.kyllingia exhibited maximum (Hg) tolerance | [156] |
| Native AM fungal morphotypes | Axonopus compressus, and Erato polymnioides | A. compressus ensued phythoextracting; Eratopolymnioides–Hg phytostabilization | [157] | |
| AMF | Lolium perenne | Decreased shoot:root (St:Rt) (Hg conc.), increased metal assimilation in roots | [158] | |
| Nickel (Ni) | Funneliformis mosseae (also named as Glomus mosseae) | Festuca arundinacea | Enhance expression of ABC transporters and metallothione induced metal intoxication, decreased metal translocation | [159] |
| Acaulospora sp. (indigenous) | Canavalia ensiformis | [160] | ||
| Arsenic (As) | AMF mix | Lens culinaris | Alleviated uptake by roots and shoots as an effect of mycorrhizal association | [161] |
| Rhizophagus intraradices (formerly named G. intraradices) | Plantago lanceolata | Down-regulating phosphate/arsenate transporters could assist plants to enhance the As tolerance | [162] | |
| Rhizoglomus intraradices & Glomus etunicatum | Triticum aestivum | Regulated P/As ratio, enhanced antioxidant production, holding As into non-toxic forms via increased production of biopolymers | [108] | |
| Rhizoglomus intraradices | Robiniapseudoacacia | Induced changes in root morphology, increased shoot-root dry weights, controlled phyto-hormone concentration etc. | [108] | |
| Acaulospora scrobiculata | Anadenantheraperegrina | Promoted P uptake lead to higher growth rates, As concentrations in the roots and shoots. | [109] | |
| Cadmium (Cd) | Funelliformis mosseae and Piriformos poraindica | T. aestivum | Biomass uplift, imposed catalytic activities for G-SH transferase, catalase, peroxidase etc., and antioxidant genes upregulation | [163] |
| Glomus intraradices | Zea mays | Mycorrhizae in association with biochar resulted alleviation in Cd accumulation in plant and restricted mobilization, soil rehabiliation | [164] | |
| Glomus monosporum, G. clarum, Gigaspora nigra, and Acaulospora laevis | Trigonella foenum-graecum | Decreased St: Rt Cd ratio, enhanced antioxidant activities | [165] | |
| Rhizophagus irregularis | Phragmites australis | Immobilization of Cd in roots, increased mineral uptake (Mn& P mainly) to survive Cd-toxicity | [166] | |
| Glomus intraradices, Glomus mosseae, Glomus claroideum, and Glomus geosporum | Nicotiana tabacum | Phyto stabilization of lead via immobilization in extraradical mycelial network | [167] | |
| Glomusmosseae | Cajanus ajan | Diminished oxidative disturbances (free radicle formation), high non-protein thiols (-SH) production and high antioxidant activities | [168] | |
| Claroideoglomus etunicatum | Sorghum bicolor | Increased the glomalin content for improved soil, Cd stabilization in mycorrhizal roots &phytoextraction (by shoots), high nutrient uptake | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, M.; Sharma, L.; Kaushik, P.; Singh, A.; Sharma, M.M. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability 2022, 14, 10220. https://doi.org/10.3390/su141610220
Dhiman M, Sharma L, Kaushik P, Singh A, Sharma MM. Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability. 2022; 14(16):10220. https://doi.org/10.3390/su141610220
Chicago/Turabian StyleDhiman, Mamta, Lakshika Sharma, Prashant Kaushik, Abhijeet Singh, and Madan Mohan Sharma. 2022. "Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature" Sustainability 14, no. 16: 10220. https://doi.org/10.3390/su141610220
APA StyleDhiman, M., Sharma, L., Kaushik, P., Singh, A., & Sharma, M. M. (2022). Mycorrhiza: An Ecofriendly Bio-Tool for Better Survival of Plants in Nature. Sustainability, 14(16), 10220. https://doi.org/10.3390/su141610220







