Reduction and Degradation of Paraoxon in Water Using Zero-Valent Iron Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZVI NPs
2.2. Characterization of Zero Valent Iron Nanoparticles
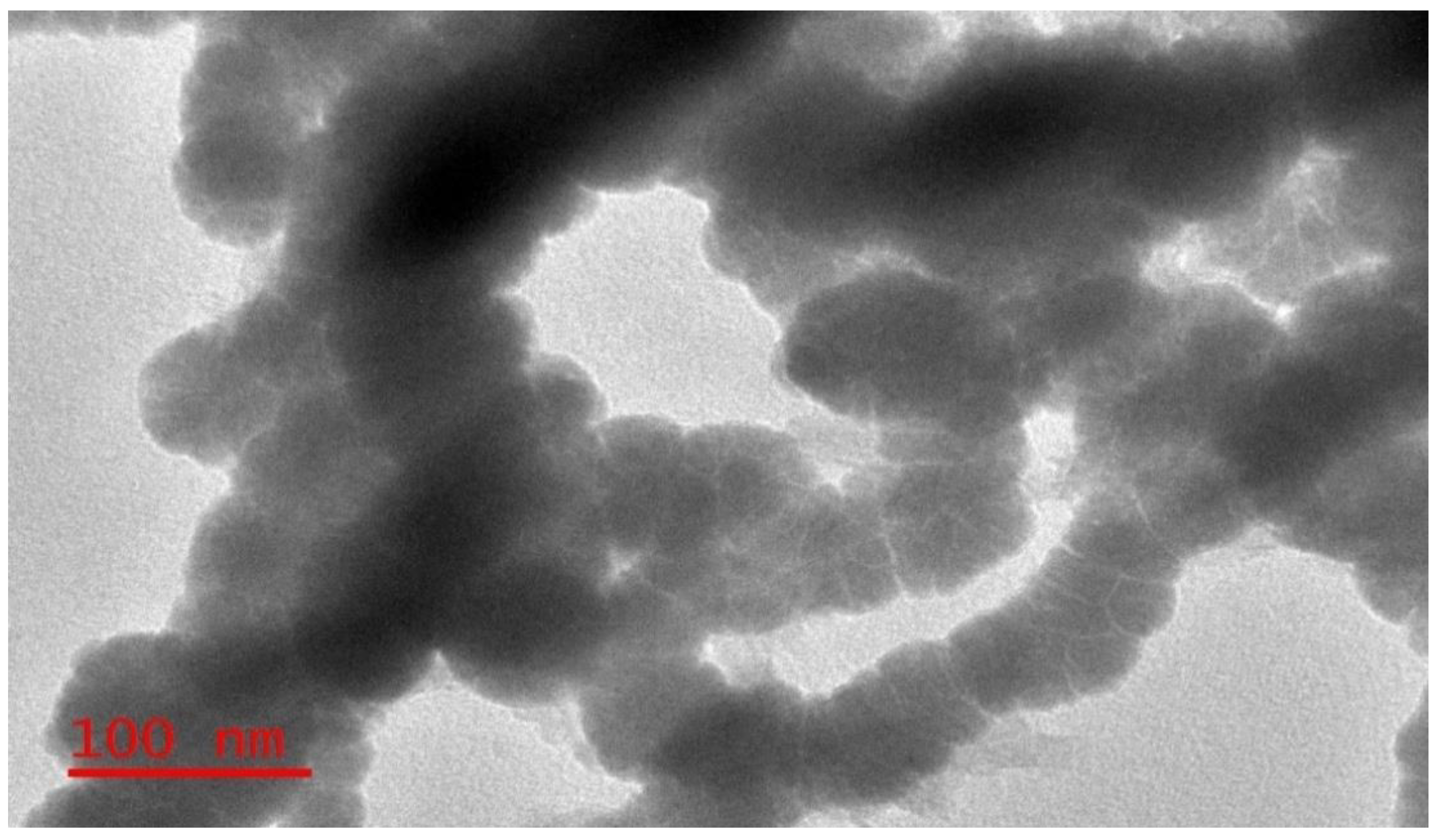
2.2.1. Transmission Electron Microscopy (TEM) Analysis
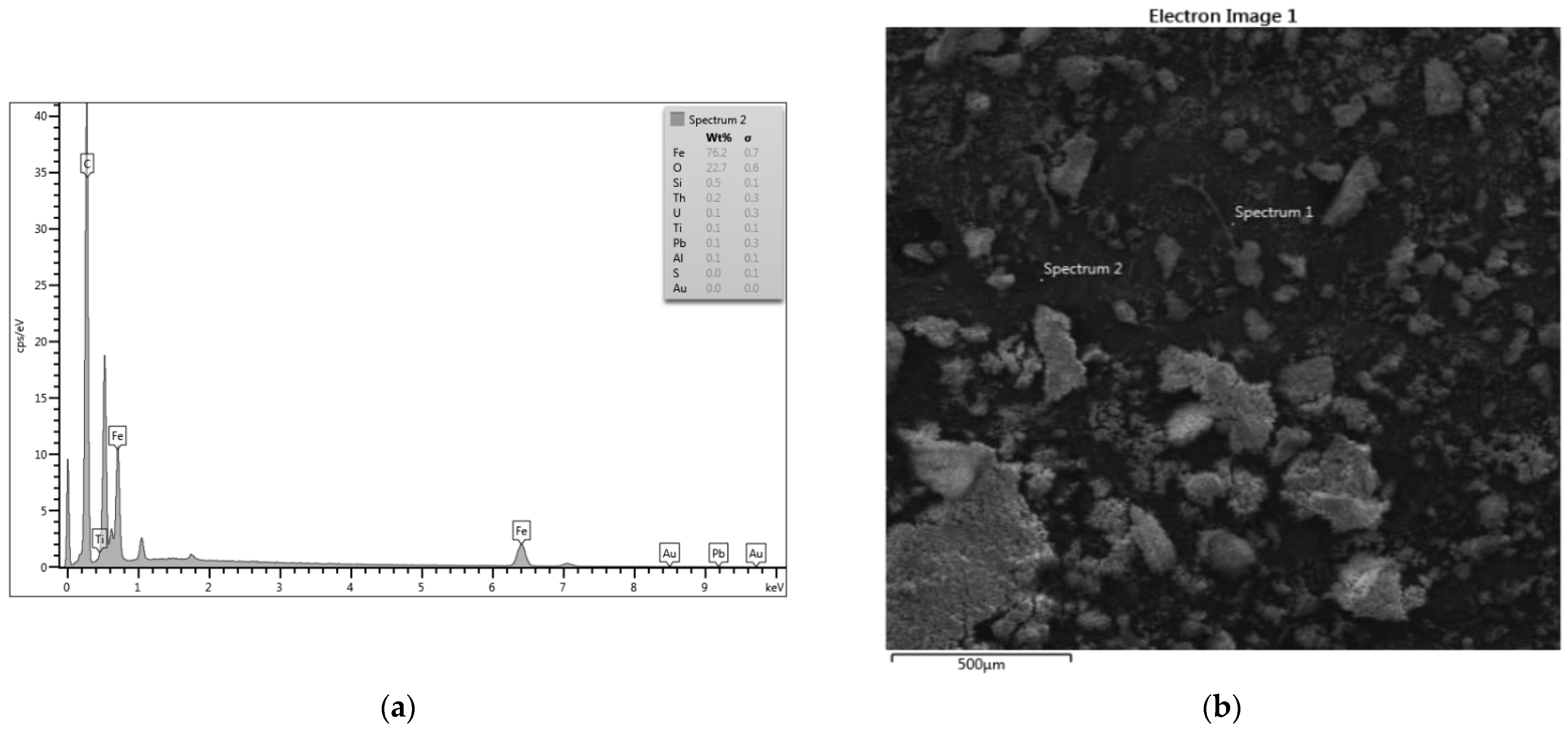
2.2.2. Scanning Electron Microscopy (SEM) Analysis
2.3. Batch Experiments
2.4. Analytical Methods
Quality Control
3. Results and Discussion
3.1. Characterization of Zero Valent Iron Nanoparticles
3.1.1. Transmission Electron Microscopy (TEM)
3.1.2. Scanning Electron Microscopy (SEM)
3.1.3. Energy Dispersive X-ray Analysis (EDXA)
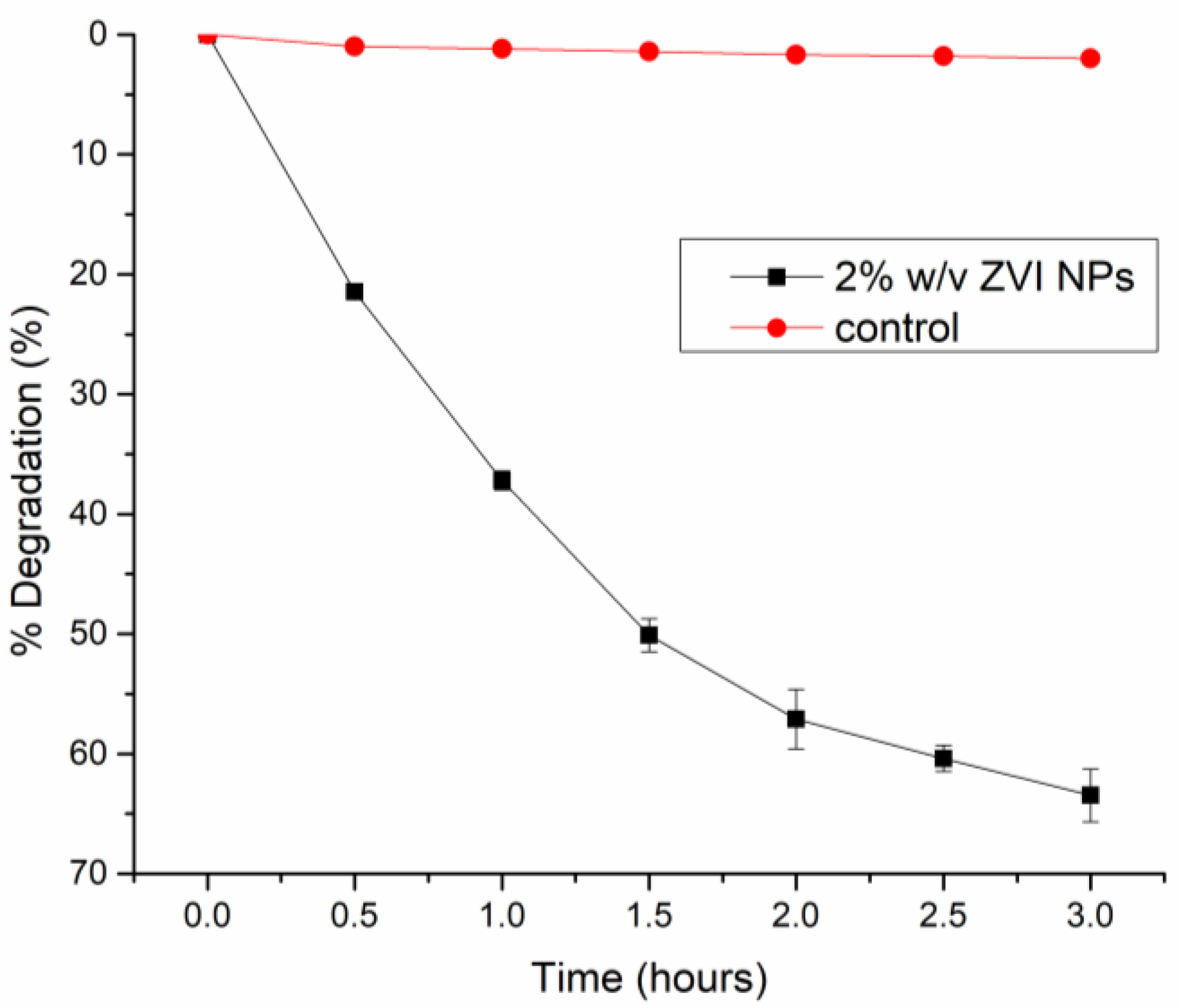
3.2. Degradation kinetics of Paraoxon
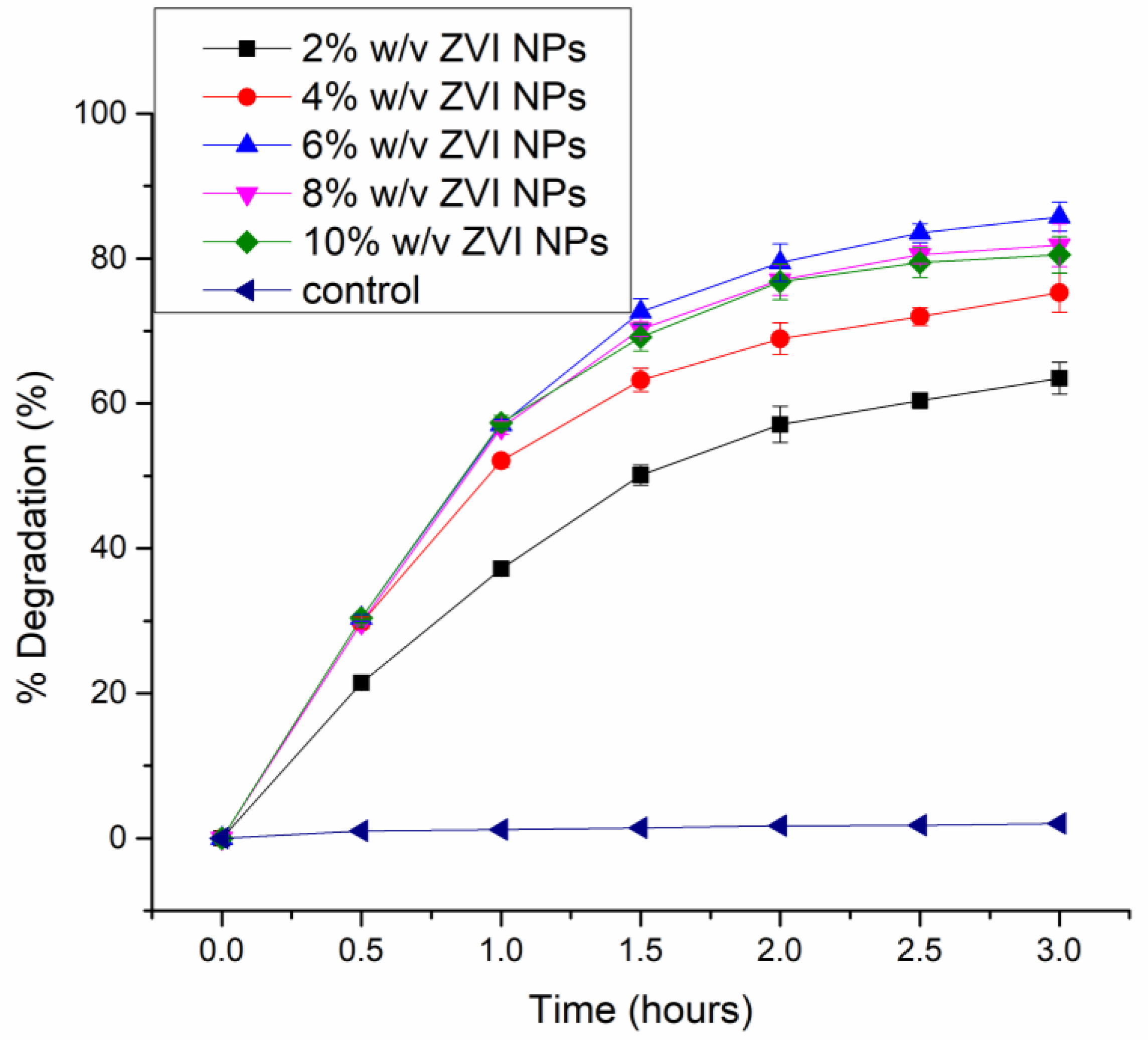
3.2.1. Effect of Time on the Degradation of Paraoxon
3.2.2. Effect of Nano Zero Valent Iron (Fe°) Dosage on Degradation Efficiency
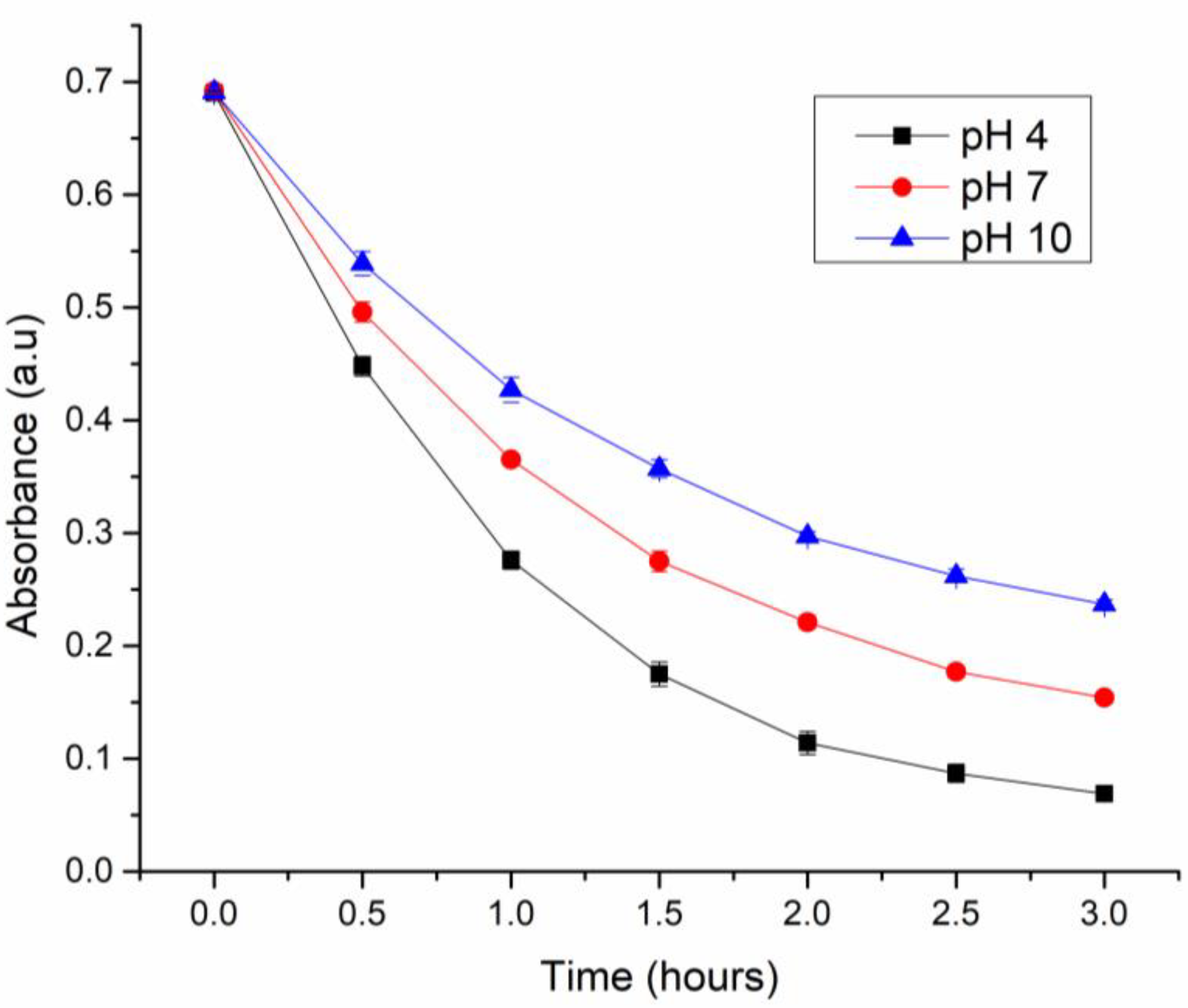
3.2.3. Effect of pH on Paraoxon degradation
3.2.4. Effect of Nano Zero Valent Iron (Fe°) Dosage on Degradation Kinetics
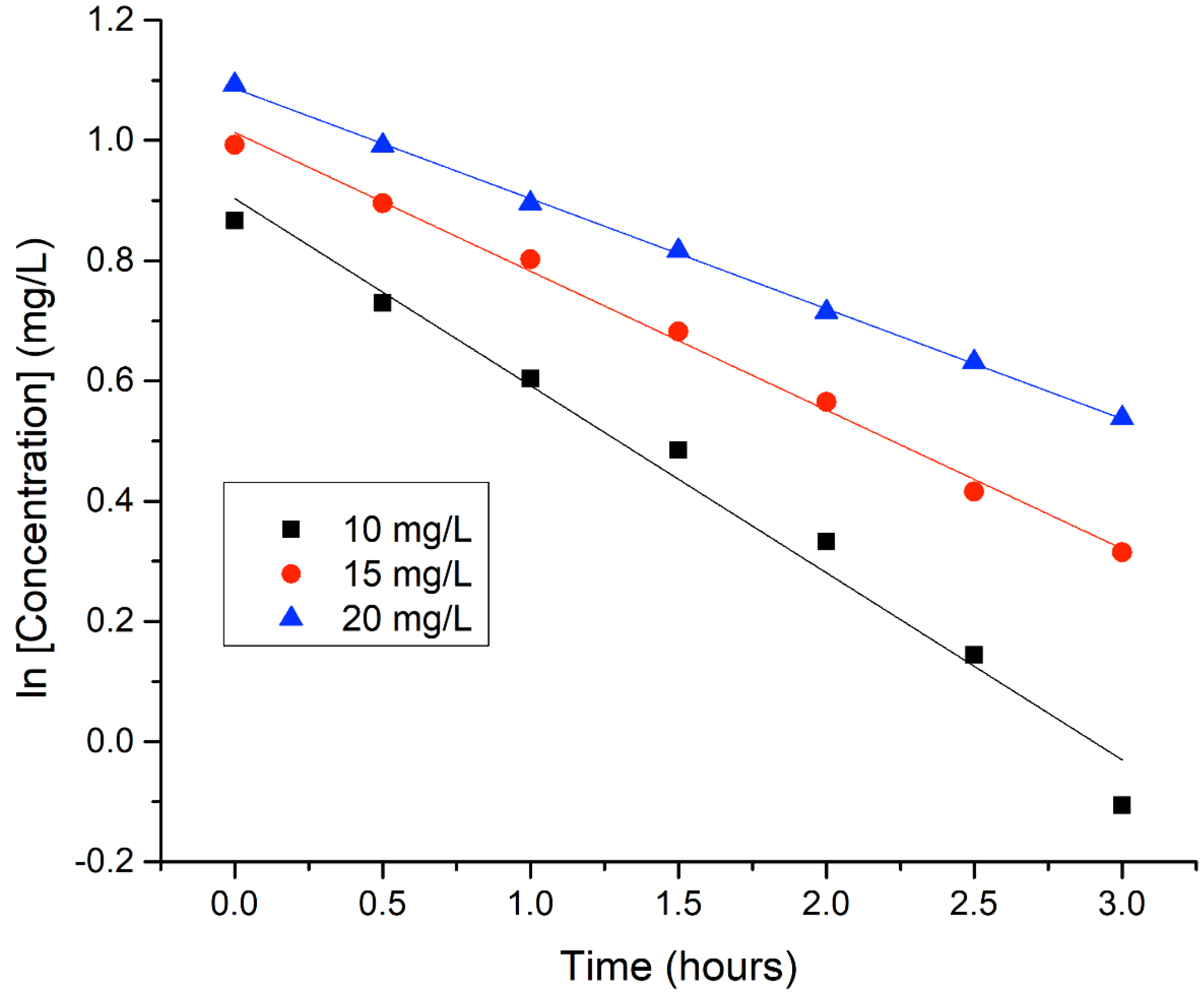
3.2.5. Effect of Paraoxon Initial Concentration on Degradation Kinetics
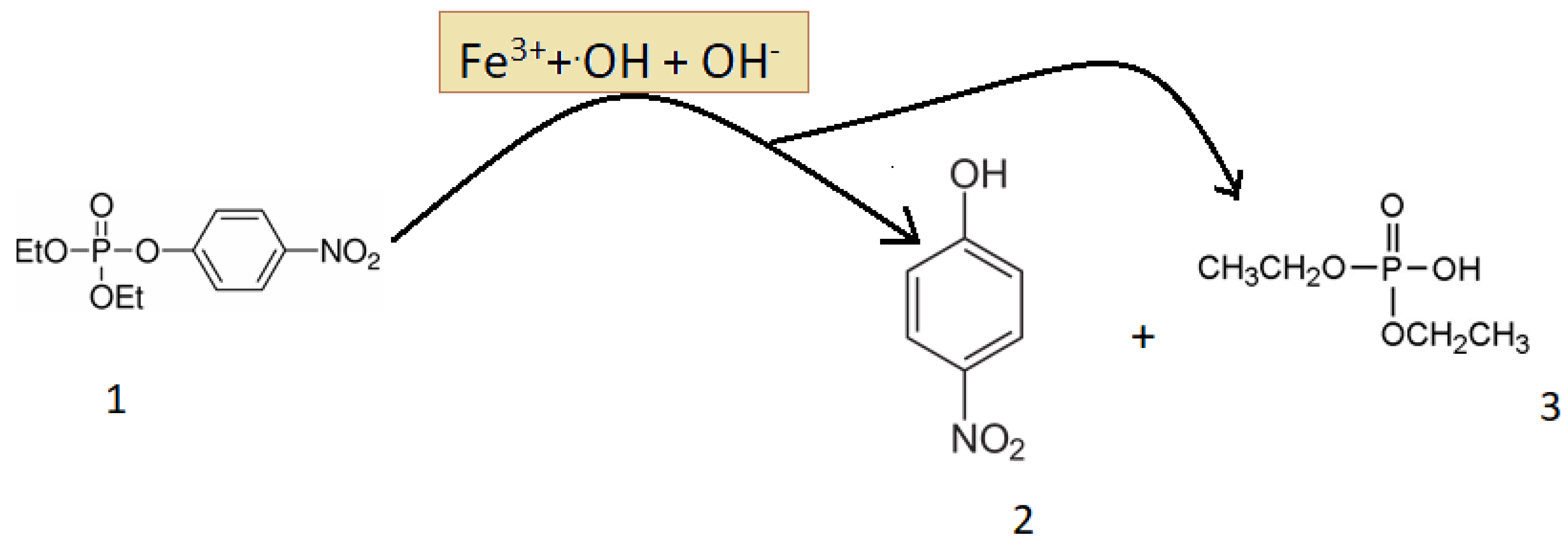
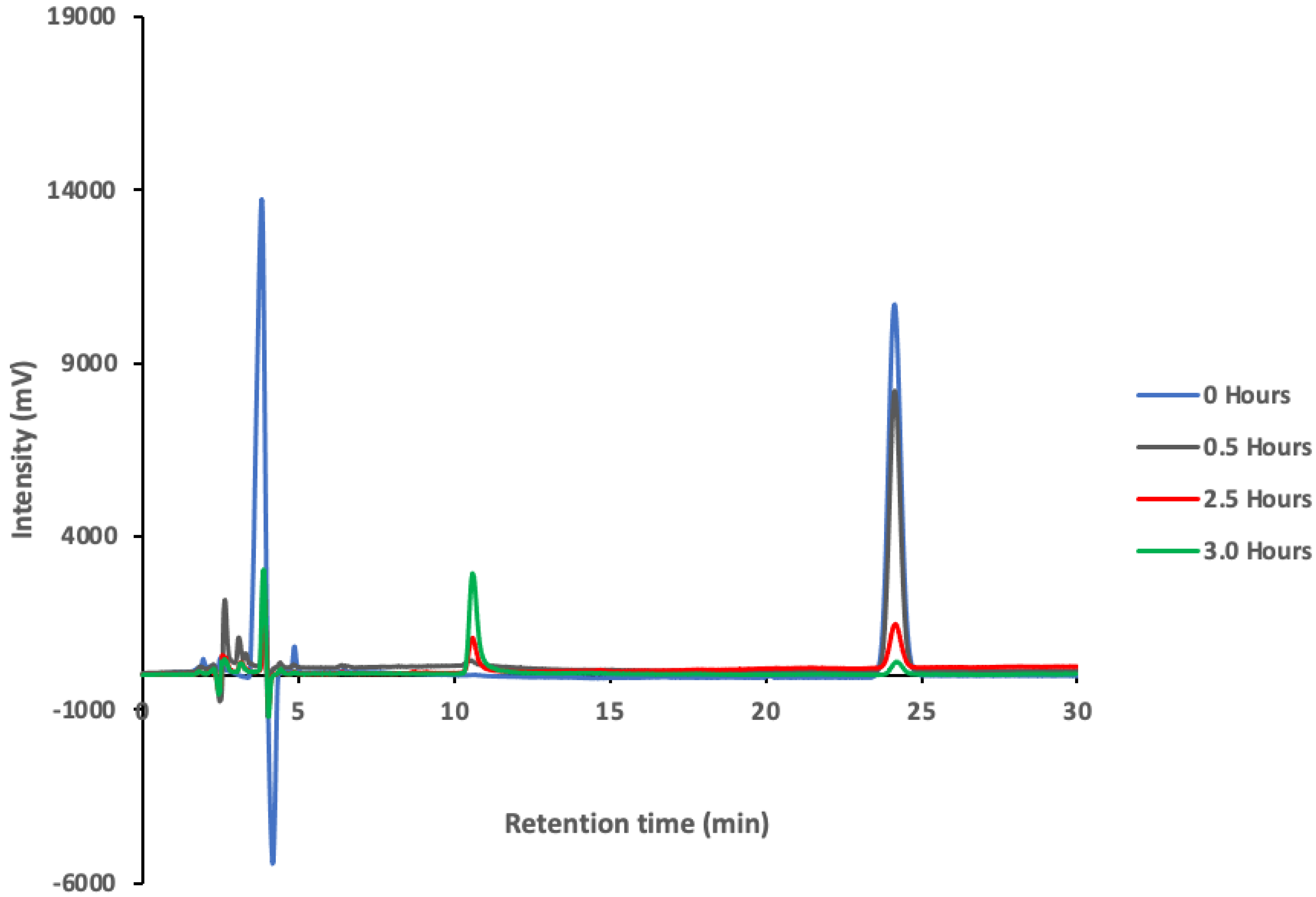
3.3. Degradation Products of Paraoxon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaushal, J.; Khatri, M.; Arya, K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Birolli, G.; Ferreira, M.; Porto, M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef] [PubMed]
- Hrvat, N.; Kovarik, Z. Counteracting poisoning with chemical warfare nerve agents. Arch. Ind. Hyg. Toxicol. 2020, 71, 266–284. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Li, J.; Ai, D.; Xi, G.; Zhao, M. Electrochemical Nonenzymatic Sensor Based on NiOFe2O3@carbon Nanotubes Nanocomposite for Determination of Paraoxon in Fruits and Water. Int. J. Electrochem. Sci. 2021, 16, 210711. [Google Scholar] [CrossRef]
- Kitagawa, S.; Cavalcante, F.; de Paula, L.; Rodrigues, B.; Bernardo, B.; da Silva, C.J.; da Silva, N.; dos Santos, V.; Granjeiro, M.; de Almeida, S. In Vitro Evaluation of Neutral Aryloximes as Reactivators for Electrophorus eel Acetylcholinesterase Inhibited by Paraoxon. Biomolecules 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafiza, A.A.; El Awadia, Y.M.; Badawia, M.A.; Mokhtar, M.S. Catalytic Destruction of Paraoxon by Metallomicelle Layers of Co(II) and Cr(III)., Paper no. S1447. J. Surfactants Deterg. 2005, 8, 203–206. [Google Scholar] [CrossRef]
- Mello, V.S.; Coutures, C.; Leblanc, M.R.; Cheng, T.; Rastogi, K.V.; DeFrank, J.J. Interaction between organophosphorus hydrolase and Paraoxon studied by surface chemistry in situ at air-water interface. Chemosphere 2001, 55, 881–887. [Google Scholar]
- Chi, W.; Shi, H.; Shi, W.; Guo, Y.; Guo, T. 4-Nitrophenol surface molecularly imprinted polymers based on multiwalled carbon nanotubes for the elimination of Paraoxon pollution. J. Hazard. Mater. 2012, 228, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Gao, J. The Reaction Mechanism of Paraoxon Hydrolysis by Phosphotriesterase from Combined QM/MM Simulations. Biochemistry 2007, 46, 13352–13369. [Google Scholar] [CrossRef]
- Wu, C.; Cha, J.H.; Valdes, J.J.; Bentley, E.W. GFP-Visualized immobilized Enzymes degradation of Paraoxon via organophosphorus Hydrolase in a packed column. Biotechnol. Bioeng. 2000, 77, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, K.J.; Calamini, B.; Wild, R.J.; Mesecar, D.A. Structural and mutational studies of organophosphorus hydrolase reveal a cryptic and functional allosteric-binding site. Arch. Biochem. Biophys. 2005, 442, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Kim, H.Y.J.; Cha, J.H. Enhanced detoxification of organophosphates using recombinant Escherichia coli with co-expression of organophosphorus hydrolase and bacterial hemoglobin. Biotechnol. Lett. 2002, 24, 879–883. [Google Scholar] [CrossRef]
- Singh, N.; Karpichev, Y.; Sharma, R.; Gupta, B.; Sahu, K.; Satnami, L.; Ghosh, K.K. From α-nucleophiles to functionalized aggregates: Exploring the reactivity of hydroxamate ion towards esterolytic reactions in micelles. Org. Biomol. Chem. 2015, 13, 2827–2848. [Google Scholar] [CrossRef]
- Rao, W.; Lv, G.; Wang, D.; Liao, L. Enhanced Degradation of Rh 6G by Zero Valent Iron Loaded on Two Typical Clay Minerals with Different Structures Under Microwave Irradiation. Front. Chem. 2018, 6, 463. [Google Scholar] [CrossRef]
- Prasad, K.G.; Ramacharyulu, K.R.P.V.; Praveen Kumar, J.; Srivastava, R.A.; Singh, B. Photocatalytic degradation of Paraoxon-ethyl in aqueous solution using titania nanoparticulate film. Thin Solid Film. 2012, 520, 5597–5601. [Google Scholar] [CrossRef]
- Xiudong, Y.; Mengfu, Z.; Hongbo, Z.; Ping, C.; Cheng, D. Study on antibacterial efficacy and catalytic capability of nanocrystalline magnesia. Adv. Mater. Res. 2011, 335, 515–518. [Google Scholar]
- Bromberg, L.; Chen, L.; Chang, P.E.; Wang, S.; Hatton, T. Reactive Silver and Cobalt Nanoparticles Modi fied with Fatty Acid Ligands Functionalized by Imidazole Derivatives. J. Chem. Mater. 2010, 22, 5383–5391. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Zhang, L.; Guo, Y.T. Core/shell molecular imprinting microparticles using RAFT technology for degradation of Paraoxon. Macromol. Res. 2011, 19, 1202–1209. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Wang, X. Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef]
- El-Shafei, M.M.; Hamdy, A.; Hefny, M.M. Zero-valent iron nanostructure: Synthesis, characterization and application. J. Environ. Biotechnol. 2018, 7, 1–10. [Google Scholar]
- Lin, Y.; Tseng, H.; Wey, M.; Lin, M. Characteristics of two types of stabilized nano zero-valent iron and transport in porous media. Sci. Total Environ. 2010, 408, 2260–2267. [Google Scholar] [CrossRef]
- Talebzadeh, S.; Forato, F.; Bujoli, B.; Trammell, A.; Grolleau, S.; Pal, H.; Queffélec, C.; Knight, D. Non-photochemical catalytic hydrolysis of methyl parathion using core–shell Ag@TiO2 nanoparticles. RSC Adv. 2018, 8, 42346–42352. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M. Influence of particle size of zero valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7. Chem. Eng. J. 2014, 237, 487–496. [Google Scholar] [CrossRef]
- Wu, C.; Linden, G. Degradation and Byproduct Formation of Parathion in Aqueous Solutions by UV and UV/H2O2 Treatment. Water Res. 2008, 42, 4780–4790. [Google Scholar] [CrossRef] [Green Version]
- Zhan, S.; Tseng, W.; Tseng, W. Impact of nanoceria shape on degradation of diethyl Paraoxon: Synthesis, catalytic mechanism, and water remediation application. Environ. Res. 2020, 188, 109653. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okello, V.A.; K’Owino, I.O.; Masika, K.; Shikuku, V.O. Reduction and Degradation of Paraoxon in Water Using Zero-Valent Iron Nanoparticles. Sustainability 2022, 14, 9451. https://doi.org/10.3390/su14159451
Okello VA, K’Owino IO, Masika K, Shikuku VO. Reduction and Degradation of Paraoxon in Water Using Zero-Valent Iron Nanoparticles. Sustainability. 2022; 14(15):9451. https://doi.org/10.3390/su14159451
Chicago/Turabian StyleOkello, Veronica A., Isaac O. K’Owino, Kevin Masika, and Victor O. Shikuku. 2022. "Reduction and Degradation of Paraoxon in Water Using Zero-Valent Iron Nanoparticles" Sustainability 14, no. 15: 9451. https://doi.org/10.3390/su14159451
APA StyleOkello, V. A., K’Owino, I. O., Masika, K., & Shikuku, V. O. (2022). Reduction and Degradation of Paraoxon in Water Using Zero-Valent Iron Nanoparticles. Sustainability, 14(15), 9451. https://doi.org/10.3390/su14159451






