Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia
Abstract
:1. Introduction
2. Materials and Methods
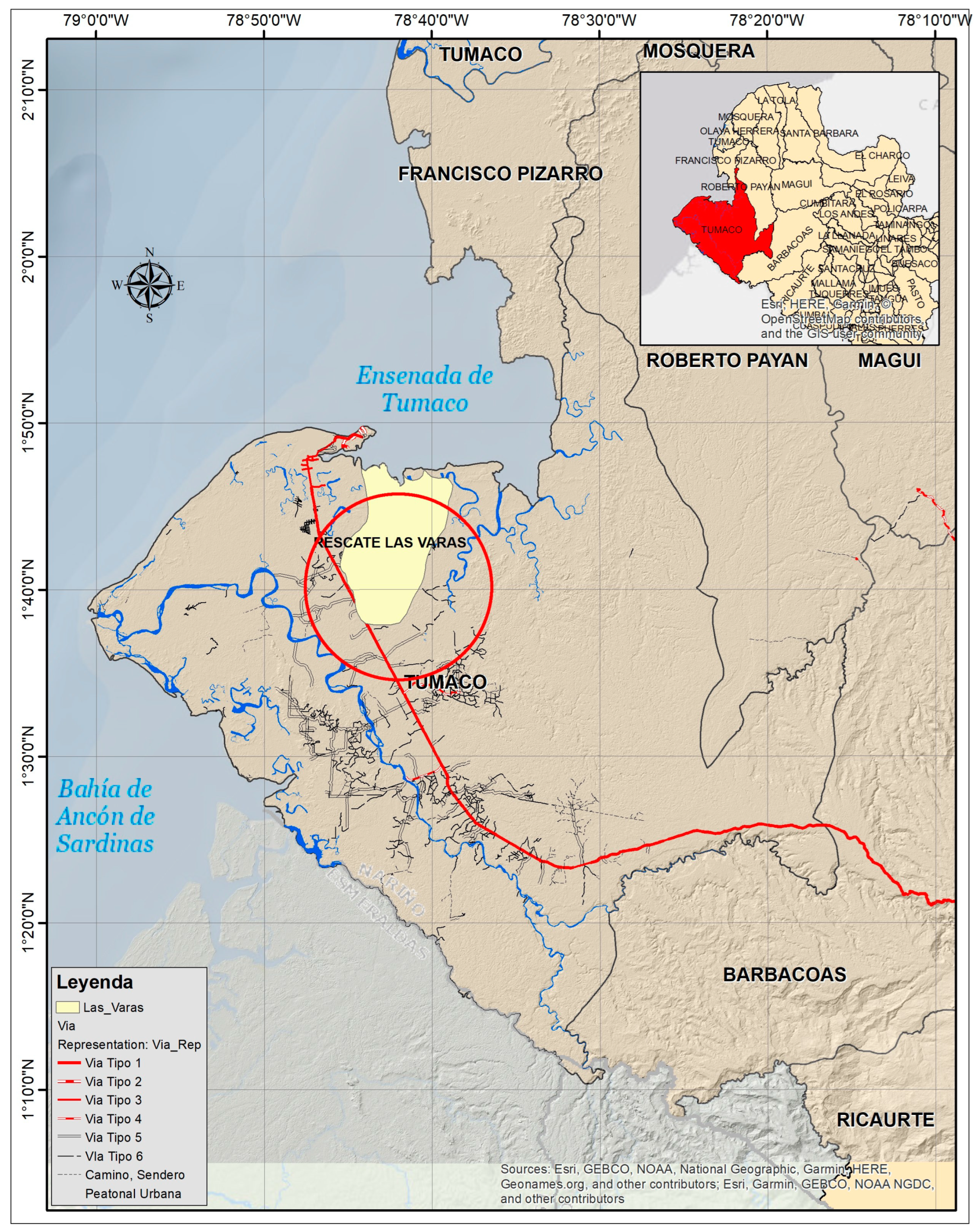
2.1. Study Area
2.2. Tree Sampling
2.3. Data Processing
2.4. Data Analysis
2.5. Economic Analysis
3. Results
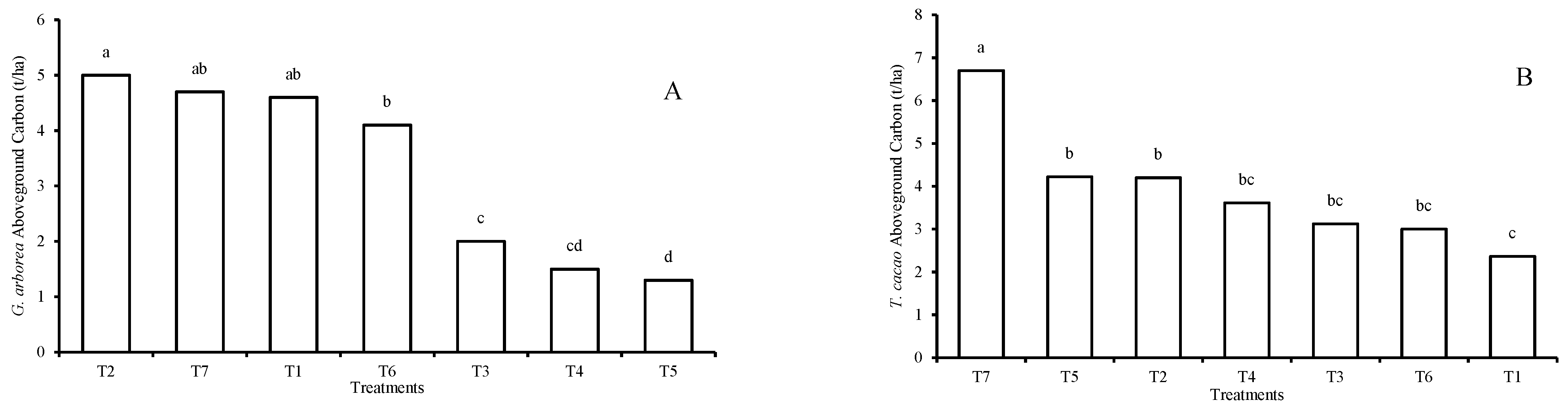
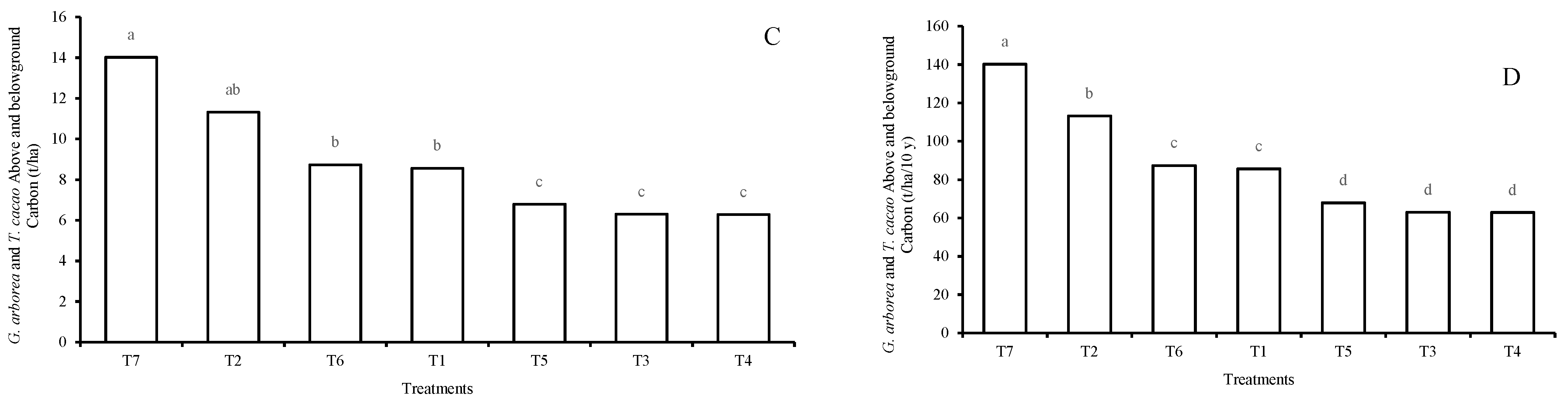
3.1. Carbon Storage Potential of Agroforestry Arrangements
3.2. Biomass Allometric Equations
3.3. Cocoa Yield
3.4. Economic Analysis
4. Discussions
4.1. Carbon Stored by the AFS
4.2. Allometric Models
4.3. Cocoa Yield in the Agroforestry Arrangements
4.4. Economic Assessment of the Agroforestry Arrangements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change: Synthesis Report. Contribution of Working Groups I.; II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate, Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- United Nations Organization—UNO. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 23 January 2021).
- Lipper, L.; Thornton, P.; Campbell, B.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- FAO. Climate-Smart Agriculture Case Studies. Successful Approaches from Different Regions; FAO: Rome, Italy, 2018; 44p. [Google Scholar]
- Anguiano, J.M.; Aguirre, J.; y Palma, J.M. Cunnigham y Pennisetum purpureum. Cuba CT—115. Av. Investig. Agropecu.-Aia 2013, 17, 149–160. [Google Scholar]
- Udawatta, R.P.; Jose, S. Carbon sequestration potential of agroforestry practices in temperate North America. In Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges, Advances in Agroforestry, 1st ed.; Kumar, B., Mohan Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 17, pp. 17–42. [Google Scholar]
- Pardos, J.A. Los Ecosistemas Forestales y el Secuestro de Carbono Ante el Cambio Climático; Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA): Madrid, Spain, 2010; 253p. [Google Scholar]
- Van Noordwijk, M.; Coe, R.; Sinclair, F.L. Central Hypotheses for the Third Agroforestry Paradigm within Common Definition; Working Paper, No 233; World Agroforestry Center (ICRAF) Southeast Asia Regional Program: Bogor, Indonesia, 2016; Available online: https://www.cabdirect.org/cabdirect/abstract/20163398164 (accessed on 8 February 2021).
- Torquebiau, E. A renewed perspective on agroforestry concepts and classification. Life Sci. 2000, 323, 1009–1017. [Google Scholar] [CrossRef]
- Nair, P.K.R. Carbon sequestration studies in agroforestry systems: A reality-check. Agrofor. Syst. 2012, 86, 243–253. [Google Scholar] [CrossRef]
- Alvarado, J.; Andrade, H.; Segura, M. Almacenamiento de carbono orgánico en suelos en sistemas de producción de café (Coffea arabica L.) en el municipio del Líbano, Tolima, Colombia. Colomb. For. 2013, 16, 21–31. [Google Scholar]
- Nair, P.K.R.; Nair, V.D.; Kumar, B.M.; Showalter, J. Carbon sequestration in agroforestry systems. Adv. Agron. 2010, 108, 237–307. [Google Scholar] [CrossRef]
- Brandle, J.R.; Hintz, D.L.; Sturrock, J.W. Windbreak Technology; Elsevier: Amsterdam, The Netherlands, 1988; 598p. [Google Scholar]
- Koohafkan, P.; Altieri, M.A. Globally Important Agricultural Heritage Systems: A Legacy for the Future; UN-FAO: Rome, Italy, 2010; 41p. [Google Scholar]
- Arévalo-Gardini, E.; Canto, M.; Alegre, J.; Loli, O.; Julca, A.; Baligar, V. Changes in soil physical and chemical properties in long term improved natural and traditional agroforestry management systems of cacao genotypes in Peruvian Amazon. PLoS ONE 2015, 10, e0132147. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia, A.G. Fungal diversity in coffee plantation systems and in a tropical montane cloud forest in Veracruz, Mexico. Agrofor. Syst. 2014, 88, 921–933. [Google Scholar] [CrossRef]
- Solis, R.; Vallejos-Torres, G.; Arévalo, L.; Marín-Díaz, J.; Ñique-Alvarez, M.; Engedal, T.; Bruun, T. Carbon stocks and the use of shade trees in different coffee growing systems in the Peruvian Amazon. J. Agric. Sci. 2020, 158, 450–460. [Google Scholar] [CrossRef]
- Mortimer, R.; Saj, S.; David, C. Supporting and regulating ecosystem services in cacao agroforestry systems. Agrofor. Syst. 2018, 92, 1639–1657. [Google Scholar] [CrossRef]
- Saj, S.; Durot, C.; Mvondo Sajouma, K.; Tayo Gamo, T.; Avana-Tientcheu, M.L. Contribution of associated trees to long-term species conservation, carbon storage and sustainability: A functional analysis of tree communities in cacao plantations of Central Cameroon. Int. J. Agr. Sustain. 2017, 15, 282–302. [Google Scholar] [CrossRef]
- Toledo-Hernández, M.; Wanger, T.C.; Tscharntke, T. Neglected pollinators: Can enhanced pollination services improve cocoa yields? A review. Agr. Ecosyst. Environ. 2017, 247, 137–148. [Google Scholar] [CrossRef]
- Murray, N.R.M.; Bojórquez, S.J.; Hernández, J.A.; Orozco, M.G.; García., J.D.; Gómez, A.R.; Ontiveros, G.H.; Aguirre, O.J. Efecto de la materia orgánica sobre las propiedades físicas del suelo en un sistema agroforestal de la llanura costera norte de Nayarit, México. Rev. Bio Cienc. 2011, 1, 27–35. [Google Scholar]
- Schroth, G.; Jeusset, A.; da Silva Gomez, A.; Florence, C.T.; Pinto Coelho, A.; Faria, D.; Laderach, P. Climate friendliness of cocoa agroforests is compatible with productivity increase. Mitig. Adapt. Glob. Chang. 2016, 21, 67–80. [Google Scholar] [CrossRef]
- Andersson, L. Achieving the Global Goals through Agroforestry. Stocolm. Available online: http://agroforestrynetwork.org.hemsida.eu/wp-content/uploads/2018/09/Achieving-the-Global-Goals-through-agroforestry.pdf (accessed on 18 July 2022).
- Harvey, C.A.; Komar, O.; Chazdon, R.; Ferguson, B.G.; Finegan, B.; Griffith, D.M.; Martinez-Ramos, M.; Morales, H.; Nigh, R.; Soto-Pinto, L.; et al. Integrating agricultural landscapes with biodiversity conservation in the Mesoamerican hotspot. Conserv. Biol. 2008, 22, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.K.R. Agroforestry systems and environmental quality: Introduction. J. Environ. Qual. 2011, 40, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Preciado, O.; Ocampo, C.; Possú, W.B. Caracterización del sistema de producción tradicional de cacao de cacao (Theobroma cacao L.) en seis núcleos productivos del municipio de Tumaco, Nariño. Rev. Cienc. Agrícolas 2011, 20, 58–69. [Google Scholar]
- Espinosa-Alzate, J.A.; Ríos-Osorio, L.A. Caracterización de sistemas agroecológicos para el establecimiento de cacao (Theobroma cacao L.); en comunidades afrodescendientes del Pacífico Colombiano (Tumaco-Nariño, Colombia). Acta Agronómica 2016, 65, 211–217. [Google Scholar] [CrossRef]
- Cardenas-Pardo, N.J.; Darghan, A.; Sosa-Rico, M.D.; Rodriguez, A. Spatial analysis of diseases incidence in different cocoa genotypes (Theobroma cacao L.) in Yopal (Casanare).; Colombia. Acta Biol. Colomb. 2017, 22, 209–220. [Google Scholar] [CrossRef]
- Agronet. Agricultural Statistics, Cocoa, Production, Yield Share in the Department. Available online: https://www.agronet.gov.co/Documents/6-CACAO_2017.pdf (accessed on 8 February 2021).
- Tennhardt, L.; Lazzarini, G.; Weisshaidinger, R.; Schader, C. Do environmentally-friendly cocoa farms yield social and economic co-benefits? Ecol. Econ. 2022, 197, 107428. [Google Scholar] [CrossRef]
- Fountain, A.; Huetz-Adams, F. Cocoa Barometer. 2018. Available online: https://voicenetwork.cc/wp-content/uploads/2019/07/2018-Cocoa-Barometer.pdf (accessed on 18 July 2022).
- Mithöfer, D.; Roshetko, J.M.; Donovan, J.A.; Nathalie, E.; Robiglio, V.; Wau, D.; Blare, T. Unpacking ‘sustainable’ cocoa: Do sustainability standards, development projects and policies address producer concerns in Indonesia, Cameroon and Peru? Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 444–469. [Google Scholar] [CrossRef] [Green Version]
- Meier, C.; Sampson, G.; Larrea, C.; Schlatter, B.; Voora, V.; Dang, D.; Bermudez, S.; Wozniak, J.; Willer, H. The State of Sustainable Markets 2020: Statistics and Emerging Trends; ITC: Geneva, Switzerland, 2020. [Google Scholar]
- Grabs, J.; Carodenuto, S.L. Traders as sustainability governance actors in global food supply chains: A research agenda. Strateg. Environ. 2021, 30, 1314–1332. [Google Scholar] [CrossRef]
- Busquet, M.; Bosma, N.; Hummels, H. A multidimensional perspective on child labor in the value chain: The case of the cocoa value chain in West Africa. World Dev. 2021, 146, 105601. [Google Scholar] [CrossRef]
- Ebagnerin Tondoh, J.; N’guessan Kouamé, F.; Martinez Guéi, A.; Sey, B.; Wowo Koné, A.; Gnessougou., N. Ecological changes induced by full-sun cocoa farming in Côte d’Ivoire. Glob. Ecol. Conserv. 2015, 3, 575–595. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Verma, K.S.; Kaushal, R. Biomass and carbon sequestration in different agroforestry systems of a Western Himalayan watershed. Biol. Agric. Hortic. 2013, 30, 88–96. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P.; Gordon, A.M. Carbon sequestration in tropical and temperate agroforestry systems: A review with examples from Costa Rica and southern Canada Agriculture. Ecosyst. Environ. 2004, 104, 359–377. [Google Scholar] [CrossRef]
- Dhyani, S.K.; Ram, A.; Dev, I. Potential of agroforestry systems in carbon sequestration in India. Indian J. Agric. Sci. 2016, 86, 1103–1112. [Google Scholar]
- Ballesteros Possu, W.; Brandle, J.R.; Domke, G.M.; Schoeneberger, M.; Blankenship, E. Estimating carbon storage in windbreak trees on U.S. agricultural lands. Agrofor. Syst. 2016, 90, 889–904. [Google Scholar] [CrossRef] [Green Version]
- Chirwa, P.W.; Mala, W. Trees in the landscape: Towards the promotion and development of traditional and farm forest management in tropical and subtropical regions. Agrofor. Syst. 2016, 90, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Holdridge, L. Ecología Basada en Zonas de Vida; IICA: San José, Costa Rica, 1982; p. 216. [Google Scholar]
- Instituto de Hidrología, Meteorología y Estudios Ambientales-IDEAM. Cartas Climatológicas del Aeropuerto la Florida, Tumaco. Nariño. Available online: www.ideam.gov.co (accessed on 10 March 2020).
- MacDicken, K.G. A guide to monitoring carbon storage in forestry and agroforestry projects. Report of the forest carbon monitoring program; Winrock Internationl Institute for Agricultural Development: Arlington, VA, USA, 1997; p. 92. [Google Scholar]
- Picard, N.; Saint-André, L.; Henry, M. Manual for Building Tree Volumen and Biomass Allometric Equations: From Field Measurement to Prediction; Food and Agricultural Organization of the United Nations: Rome, Italy; Centre de Coopération Internationale en Recherche Agronomique pour le Développement: Montpellier, France, 2012; p. 215. [Google Scholar]
- Skog, K.E.; Nicholson, G.A. Carbon cycling through wood products: The role of wood and paper products in carbon sequestration. For. Prod. J. 1998, 48, 75. [Google Scholar]
- Jenkins, J.C.; Birdsey, R.A.; Pan, Y. Biomass and NPP estimation for the mid-Atlantic region (USA) using plot-level forest inventory data. Ecol. Appl. 2001, 11, 1174–1193. [Google Scholar] [CrossRef]
- Crow, T.R. Biomass and production in three contiguous forests in northern Wisconsin. Ecology 1978, 59, 265–273. [Google Scholar] [CrossRef]
- Loetsch, F.; Zohrer, F.; Haller, K.E. Forest Inventory; BLV Verlagsgesellschaft: Munchen, Germany, 1973; p. 469. [Google Scholar]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Furnival, G.M. An index for comparing equations used in constructing volume tables. For. Sci. 1961, 7, 337–341. [Google Scholar]
- Nakamura, T.; Judd, K.; Mess, M.I.; Small, M. A comparative Study of Information Criteria for Model Selection. Available online: http://staffhome.ecm.uwa.edu.au/~00027830/pdf/IJBC16-2.pdf (accessed on 9 February 2021).
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J. Applied Linear Regression Models, 4th ed.; Irwin Inc.: New York, NY, USA, 2004; p. 701. [Google Scholar]
- Whitesell, C.D.; Miyasaka, S.C.; Strand, R.F.; Schubert, T.H.; McDuSe, K.E. Equations for Predicting Biomass in 2- to 6-year-old Eucalyptus Saligna in Hawaii; USAD Forest Service Research Notes, PSW-402; Pacific Southwest Forest and Range Experiment Station: Albany, CA, USA, 1988; p. 5. [Google Scholar]
- Busing, R.T.; Clebsch, E.E.C.; White, P.S. Biomass production of southern Appalachian cove forests reexamined. Can. J. For. Res. 1993, 23, 60–65. [Google Scholar] [CrossRef]
- Andrade, H.; Segura, M.; Somarriba, E.; Villalobos., M. Valoración biofísica y financiera de la fijación de carbono por uso del suelo en fincas cacaoteras indígenas de Talamanca, Costa Rica. Agroforestería En Las Américas 2008, 46, 45–50. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 9 February 2020).
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Telles Antonio, R.; Alanís Rodríguez, E.; Jiménez Pérez, J.; Aguirre Calderón, O.A.; Treviño Garza, E.J. Accumulated carbon estimation in Gmelina arborea Roxb. from Tlatlaya.; Estado de México with allometric equations. Rev. Mex. Cienc. For. 2019, 10, 135–153. [Google Scholar] [CrossRef] [Green Version]
- Monteith, J.; Chin, L.; Ong, K.; Corlett, J.E. Microclimatic interactions in agroforestry system. For. Ecol. Manag. 1991, 45, 31–44. [Google Scholar] [CrossRef]
- Ayegboyin Kayode, O.; Famaye Amos, O.; Adeosun Seun, A.; Idrisu, M.; Ugioro, O.; Asowata, F.E.; Okunade Favour, A. Effect of high density planting on the vigour and yield of Theobroma cacao L. in the Southwest of Nigeria. World J. Adv. Res. Rev. 2020, 8, 217–223. [Google Scholar] [CrossRef]
- Spaggiari Souza, C.A.; dos Santos Dias, L.A.; Galeas Aguila, M.A.; Songheti, S.; Oliveira, J.; Andrade Costa, J.L. Cacao yield in different planting densities. Braz. Arch. Biol. Technol. 2009, 52, 1313–1320. [Google Scholar] [CrossRef]
- Abou Rajab, Y.; Leuschner, C.; Barus, H.; Tjoa, A.; Hertel, D. Cacao cultivation under diverse shade tree cover allows high carbon storage and sequestration without yield losses. PLoS ONE 2016, 11, e0149949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, Y. Análisis mensual de acumulación de biomasa y fijación de carbono en una plantación de Gmelina arborea Roxb. Los Chiles, Alajuela, Costa Rica. Ph.D. Thesis, Ingeniero forestal-Instituto tecnológico de Costa Rica, Escuela de Ingeniería Forestal Costa Rica, Alajuela, Costa Rica, 2016; p. 39. [Google Scholar]
- Bohre, P.; Chaubey, O.P.; Singhal, P.K. Biomass Accumulation and Carbon Sequestration in Tectona grandis Linn. f. and Gmelina arborea Roxb. Int. J. Bio-Sci. Bio-Technol. 2013, 5, 153–174. [Google Scholar]
- Cámara, C.D.C.; Arias, M.; Martínez, S.J.L.; Castillo, A.O. Carbono almacenado en selva mediana de Quercus oleoides y plantaciones de Eucalyptus urophylla y Gmelina arborea en Huamanguillo, Tabasco. In Estado Actual del Conocimiento del Ciclo del Carbono y Sus Interacciones en México: Síntesis a 2013; Pellat, F.P., Julio, W.G., Maira y, B., Saynes, V., Eds.; Universidad Autónoma de Chapingo: Texcoco, Mexico, 2013; p. 702. [Google Scholar]
- Melo Cruz, O.A. Modelación del Crecimiento.; Acumulación de Biomasa y Captura de Carbono en Árboles de Gmelina arborea Roxb., Asociados a Sistemas Agroforestales y Plantaciones Homogéneas en Colombia. Ph.D. Thesis, Facultad de Ciencias Agrarias, Universidad Nacional de Colombia, Sede Medellín, Medellín, Colombia, 2015; 166p. [Google Scholar]
- Patiño Forero, S.; Suárez Santos, L.N.; Andrade Castañeda, H.J.; Segura Madrigal, M.A. Captura de carbono en biomasa en plantaciones forestales y sistemas agroforestales en Armero-Guayabal, Tolima, Colombia. Rev. De Investig. Agrar. Y Ambient. 2018, 9, 121–134. [Google Scholar] [CrossRef]
- Onyekwelu, C.J. Above-ground biomass production and biomass equations for even-aged Gmelina arborea (ROXB) Plantations in South-Western Nigeria. Biomass Bioenergy 2004, 26, 39–46. [Google Scholar] [CrossRef]
- Lasco, R.; Come, R.; Estrella, R.; Saplaco, S.R.; Castillo, A.S.A.; Cruz, R.; Pulhin, F. Carbon stock assessment of two agroforestry systems in a tropical forest reserve in the Philippines. Philipp. Agric. Sci. 2001, 84, 401–407. [Google Scholar]
- Owusu, S.; Anglaaere, L.C.N.; Abugre, S. Aboveground biomass and carbon content of a cocoa—Gliricida sepium agroforestry system in Ghana. Ghana Jnl Agric. Sci. 2018, 53, 45–60. [Google Scholar] [CrossRef]
- Aristizábal, J.; Guerra, A. Estimación de la Tasa de Fijación de Carbono en el Sistema Agroforestal Nogal Cafetero Cordia alliodora, cacao Theobroma cacao y Plátano Musa paradisíaca. Ph.D. Thesis, Ingeniero Forestal, Universidad Distrital, Bogotá, Colombia, 2002; 108p. Available online: http://www.sidalc.net/repdoc/a4836e/a4836e.pdf (accessed on 8 February 2021).
- Vespa, A. Relaciones Hídricas e Intercambio de Gases en Theobroma cacao var. Guasare Bajo Períodos de Déficit Hídrico. Rev. Fac. Agron. Univ. Zulia 2008, 2, 112–120. [Google Scholar]
- Chijioke, E.O. Impart on Soils of Fast Growing Species in Lowland Humid Tropics; FAO Forestry Paper, No.21; Food and Agricultural Organization: Rome, Italy, 1980; 111p. [Google Scholar]
- Schoeneberger, M.M. Agroforestry: Working trees for sequestering carbon on agricultural lands. Agrofor. Syst. 2009, 75, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Blandon, B.A.; Hernández-Álvarez, E.; Rodríguez-Macias, R.; Salcedo-Pérez, E. Mensuration assessment and biomass production in Gmelina arborea Roxb. ex Sm. established in pure and mixed stands. Rev. Mex. Cienc. For. 2020, 11, 94–117. [Google Scholar] [CrossRef]
- Claesson, S.; SahlTen, K.; Lundmark, T. Functions for biomass estimation of young Pinus sylvestris, Picea abies and Betula spp from stands in Northern Sweden with high sand densities. Scand. J. For. Res. 2001, 16, 138–146. [Google Scholar] [CrossRef]
- Nam, V.T.; van Kuijk, M.; Anten, N.P.R. Allometric equations for aboveground and belowground biomass estimations in an evergreen forest in Vietnam. PLoS ONE 2016, 11, e0156827. [Google Scholar] [CrossRef]
- Somarriba, E.; Cerda, R.; Orozco, L.; Deheuvels, O.; Cifuentes, M.; Dávila, H.; Espin, T.; Mavisoy, H.; Ávila, G.; Alvarado, E.; et al. Carbon stocks and cocoa yields in agroforestry systems of Central America. Agr. Ecosyst. Environ. 2013, 173, 46–57. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Montoya-Restrepo, I.A.; Montoya-Restrepo, L.A.; Lowy Cerón, P.D. Oportunidades para la actividad cacaotera en el municipio de Tumaco, Nariño, Colombia. Entramado 2015, 11, 48–59. [Google Scholar] [CrossRef]
- Armengot, L.; Barbieri, P.; Andres, C.; Milz, J.; Schneider, M. Cacao agroforestry systems have higher return on labor compared to full-sun monocultures. Agron. Sustain. Dev. 2016, 36, 70. [Google Scholar] [CrossRef] [Green Version]
- Beer, J.; Muschler, R.; Somarriba, E.; Kass, D. Shade management in coffee and cocoa plantations. Agrofor. Syst. 1997, 38, 139–164. [Google Scholar] [CrossRef]
- Koko, L.K.; Snoeck, D.; Lekadou, T.T.; Assiri, A.A. Cacao-fruit tree intercropping effects on cocoa yield, plant vigour and light interception in Côte d’Ivoire. Agrofor. Syst. 2013, 87, 1043–1052. [Google Scholar] [CrossRef]
- Blaser, W.J.; Oppong, J.; Hart, S.P.; Landolt, J.; Yeboah, E.; Six, J. Climate-smart sustainable agriculture in low-to-intermediate shade agroforests. Nat. Sustain. 2018, 1, 234. [Google Scholar] [CrossRef]
- Andres, C.; Blaser, W.J.; Dzahini-Obiatey, H.K.; Ameyaw, G.A.; Domfeh, O.K.; Awiagah, M.A.; Gattinger, A.; Schneider, M.; Offei, S.K.; Six, J. Agroforestry systems can mitigate the severity of cocoa swollen shoot virus disease. Agric. Ecosyst. Environ. 2018, 252, 83–92. [Google Scholar] [CrossRef]
- Asare, R.; Markussen, B.; Asare, R.A.; Anim-Kwapong, G.; Ræbild, A. On-farm cocoa yields increase with canopy cover of shade trees in two agro-ecological zones in Ghana. Clim. Dev. 2018, 11, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Vernon, A.J.; Sundaram, S. Current cocoa research. In Proceedings of the 4th International Cocoa Research Conference, St. Augustine, Trinidad and Tobago, 8–18 January 1972; pp. 689–693. [Google Scholar]
- Lahive, F.; Hadley, P.; Daymond, A.J. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron. Sustain. Dev. 2019, 39, 5. [Google Scholar] [CrossRef] [Green Version]
- Yalta, H. Identificación y Rentabilidad de Sistemas Agroforestales Asociados al Cultivo de Cacao (Theobroma cacao L.) en Tingo Maria. Ph.D. Thesis, Universidad Nacional Agraria de la Selva, Tingo Maria, Peru, 2003; 101p. [Google Scholar]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Cerda, R.; Deheuvels, O.; Calvache., D.; Niehaus, L.; Saenz, Y.; Kent, J.; Vilchez, S.; Villota, A.; Martinez, C.; Somarriba, E. Contribution of cocoa agroforestry systems to family income and domestic consumption: Looking toward intensification. Agrofor. Syst. 2014, 88, 957–981. [Google Scholar] [CrossRef]
- Notaro, M.; Gary, C.; Deheuvels, O. Plant diversity and density in cocoa-based agroforestry systems: How farmers’ income is affected in the Dominican Republic. Agrofor. Syst. 2020, 94, 1071–1084. [Google Scholar] [CrossRef]
- Bisseleua, D.H.B.; Missoup, A.D.; Vidal, S. Biodiversity conservation, ecosystem functioning, and economic incentives under cocoa agroforestry intensification. Conserv. Biol. 2009, 23, 1176–1184. [Google Scholar] [CrossRef]
- Egbewole, Z.T.; Falade, L.O.; Rotowa, O.J.; Kuje, E.D.; Mairafi, H.H. Evaluation of the Effect of Agricultural Crop on the Growth Performance of Gmelina arborea under Agroforestry System. Available online: https://www.researchgate.net/publication/343502140_Evaluation_of_the_Effect_of_Agricultural_Crop_on_the_Growth_Performance_of_Gmelina_arborea_under_Agroforestry_System (accessed on 18 July 2022).
- Jose, S.; Bardhan, S. Agroforestry for biomass production and carbon sequestration: An overview. Agrofor. Syst. 2012, 86, 105–111. [Google Scholar] [CrossRef]
- Biswas, B.; Chakraborty, D.; Timsina, J.; Rudra Bhowmick, U.; Pratap Kumar, D.; Dipak Kumar, G.; Sarkar, A.; Mondal, M.; Adhikary, S.; Kanthal, S.; et al. Agroforestry offers multiple ecosystem services in degraded lateritic soils. J. Clean. Prod. 2022, 365, 132768. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018; 630p. [Google Scholar]
- United Nations Organization. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 18 July 2022).
- Kuyah, S.; Whitney, C.W.; Jonsson, M.; Sileshi, G.W.; Öborn, I.; Muthuri, C.W.; Luedeling, E. Agroforestry delivers a win-win solution for ecosystem services in sub-Saharan Africa. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 47. [Google Scholar] [CrossRef] [Green Version]
- Carsan, S.; Stroebel, A.; Dawson, I. Can agroforestry option values improve the functioning of drivers of agricultural intensification in Africa? Curr. Opin. Environ. Sustain. 2014, 6, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Syampungani, S.C. The potential of using agroforestry as a win-win solution to climate change mitigation and adaptation and meeting food security challenges in Southern Africa. Agric. J. 2010, 5, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Nunoo, I.; Fromm, I.; Frimpong, B.N. Factors Influencing the Adoption of Cocoa Agroforestry Systems in Mitigating Climate Change in Ghana: The Case of Sefwi Wiawso in Western Region. Environ. Sustain. Clim. Chang. 2020, 2, 1–4. Available online: https://researchopenworld.com/wp-content/uploads/2020/07/ESCC-2-1-202.pdf (accessed on 18 July 2022).

| Treatments | Cacao | Melina | ||
|---|---|---|---|---|
| Spacing (m2) | Trees/ha | Spacing (m2) | Trees/ha | |
| 1 | 3 × 3 | 1111 | 3 × 3 | 1111 |
| 2 | 4 × 4 | 625 | 4 × 4 | 625 |
| 3 | 3 × 4 | 833 | 8 × 6 | 208 |
| 4 | 3 × 4 | 833 | 12 × 6 | 139 |
| 5 | 3 × 4 | 833 | 16 × 6 | 104 |
| 6 | 3 × 4 | 833 | 3 × 3 × 7.5 | 440 |
| 7 * | 7 × 7 | 200 | 12 × 12 | 69 |
| Number | Author | Equation |
|---|---|---|
| 1 | Berkhout | bm = a + b × dbh |
| 2 | Kopezky | bm = a + b × dbh2 |
| 3 | Hohenadl-Krenn | bm = a + b × dbh + c × dbh2 |
| 4 | Husch | Ln bm = a + b × ln dbh |
| 5 | Spurr | bm = a + b × dbh2 × ht |
| 6 | Stoate | bm = a + b × dbh2 + c × dbh2 × ht + d × ht |
| 7 | Meyer | bm = a + b × dbh2 + c × dbh2 × ht + d × dbh2 × h |
| 8 | Schumacher-Hall | Log bm = a + b × ln dbh + c × ln × ht |
| 9 | Brenack | Log bm = a + b × dbh + c × 1/dbh |
| 10 | This study | Sqrt (bm) = a + b × dbh |
| 11 | This study | bm = a + b ln(dbh) + ln(ht) |
| 12 | This study | Sqrt (bm) = a + b × dbh + c × ht |
| 13 | This study | bm = a × exp(b × dbh) |
| 14 | This study | bm = a × dbh^b |
| 15 | This study | bm = a + b ln(dbh)) |
| Model | R2 | AIC | BIC | PRESS | Cp | VIF | FI |
|---|---|---|---|---|---|---|---|
| 1 | 0.93 | 399.7 | 404 | 560.3 | 14.4 | 1.0 | 3.9 |
| 2 | 0.93 | 401.6 | 406.4 | 55.8 | 16.5 | 1.0 | 59.1 |
| 3 | 0.93 | 400 | 405 | 48.2 | 5.8 | 14.5 | 33.8 |
| 4 | 0.91 | 399.8 | 404.5 | 153.6 | 2.00 | 1.0 | 65.8 |
| 5 | 0.93 | 70.8 | 66.00 | 134. 8 | 16.4 | 139.6 | 59.8 |
| 6 | 0.94 | 405.5 | 410.2 | 0.30 | 2.80 | 144.8 | 19.4 |
| 7 | 0.94 | 401.6 | 407.9 | 0.30 | 15.2 | 66.1 | 3.8 |
| 8 | 0.91 | 70.8 | 66.00 | 155.5 | 18.7 | 13.7 | 67.5 |
| 9 | 0.91 | 68.8 | 62.5 | 150.1 | 1.80 | 144.8 | 65.5 |
| 10 | 0.92 | 118.0 | 125.9 | 143.1 | 2.80 | 1.0 | 62.6 |
| 11 | 0.92 | 403.5 | 411.4 | 134.8 | 17.4 | 124.5 | 60.6 |
| 12 | 0.92 | 114.6 | 119.3 | 147.7 | 4.80 | 13.7 | 63.3 |
| 13 | 0.93 | 402.9 | 412.4 | 155.5 | 2.60 | 1.0 | 7.4 |
| 14 | 0.93 | 402.8 | 413.9 | 134.9 | 5.60 | 1.0 | 33.24 |
| 15 | 0.93 | 399.8 | 404.5 | 160 | 16.4 | 1.0 | 68.7 |
| Model Number | Model 1 | R2 | α | β | MSPE | Rank |
|---|---|---|---|---|---|---|
| 1 | Berkouth | 0.93 | −534.339 | 33.282 | 5.74 | 1 |
| 14 | This study 3 | 0.93 | 1.8048 | 1.6469 | 5.78 | 2 |
| 4 | Husch | 0.91 | 0.3982 | 1.6982 | 5.82 | 3 |
| 10 | This study 1 | 0.92 | 4.64771 | 0.58219 | 5.84 | 4 |
| 2 | Kopezky | 0.93 | 141.54096 | 0.39794 | 5.89 | 5 |
| 15 | This study 4 | 0.93 | −4153.61 | 1346.99 | 6.11 | 6 |
| 13 | This study 2 | 0.93 | 164 | 0.0386 | 6.21 | 7 |
| Treatments | NPV1 | IRR (%) | BCR |
|---|---|---|---|
| 1 | 1,737,024 | 26 | 1.14 |
| 2 | 1,262,004 | 25 | 1.16 |
| 3 | 1,308,798 | 30 | 1.22 |
| 4 | 1,302,763 | 29 | 1.20 |
| 5 | 1,287,711 | 29 | 1.18 |
| 6 | 1,446,457 | 42 | 1.67 |
| 7 | 651,650 | 13 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros-Possú, W.; Valencia, J.C.; Navia-Estrada, J.F. Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia. Sustainability 2022, 14, 9447. https://doi.org/10.3390/su14159447
Ballesteros-Possú W, Valencia JC, Navia-Estrada JF. Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia. Sustainability. 2022; 14(15):9447. https://doi.org/10.3390/su14159447
Chicago/Turabian StyleBallesteros-Possú, William, Juan Carlos Valencia, and Jorge Fernando Navia-Estrada. 2022. "Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia" Sustainability 14, no. 15: 9447. https://doi.org/10.3390/su14159447
APA StyleBallesteros-Possú, W., Valencia, J. C., & Navia-Estrada, J. F. (2022). Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia. Sustainability, 14(15), 9447. https://doi.org/10.3390/su14159447







