Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments
Abstract
:1. Introduction
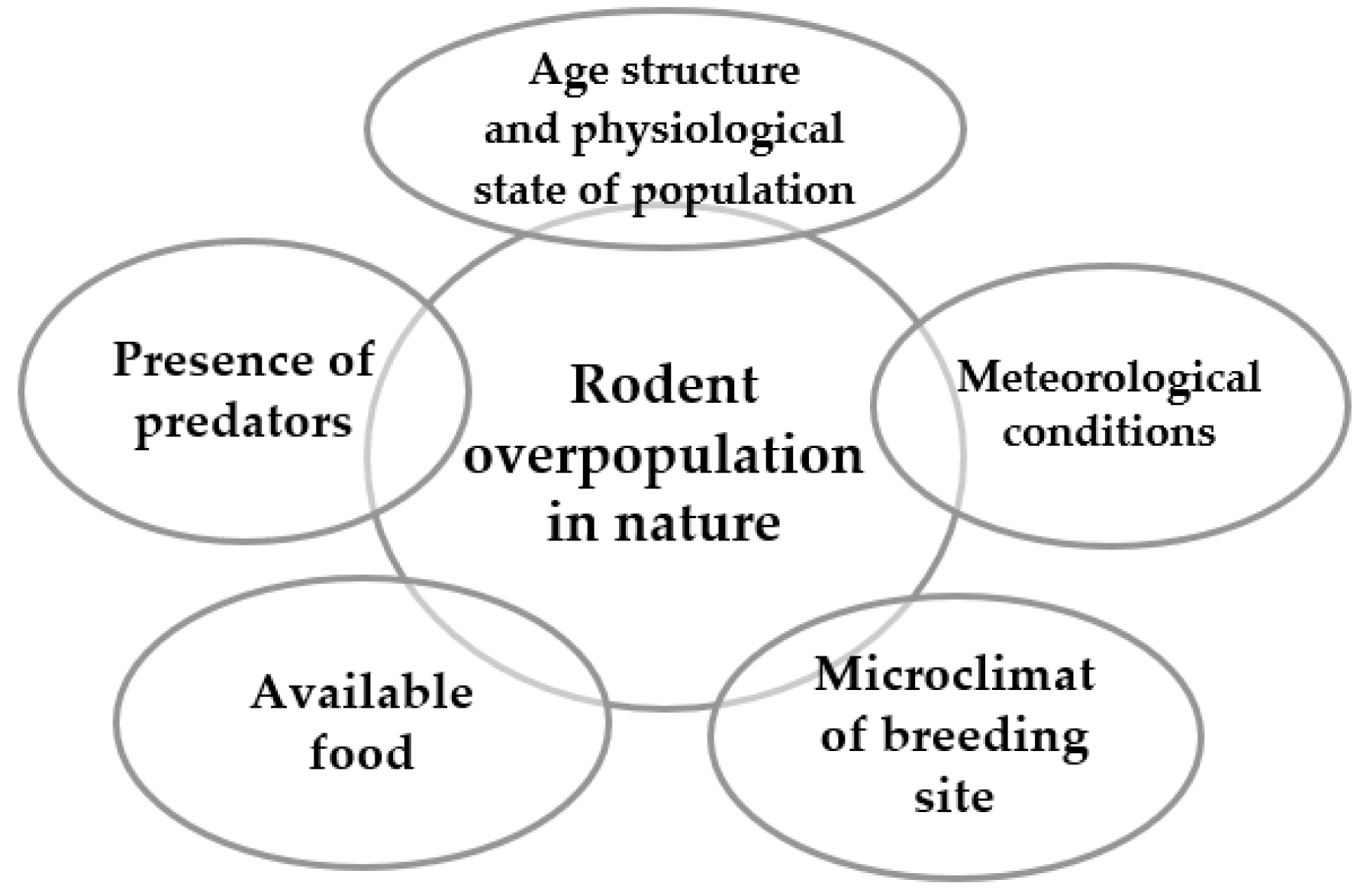
Mouselike Rodents’ Overpopulation in Nature
2. Family Cricetidae
2.1. Myodes glareolus (Schreber 1780)–Bank Vole
2.2. Microtus arvalis (Pallas, 1778)–Common Vole
2.3. Microtus subterraneus (Selys-Longchamps, 1836)–Common Pine Vole
2.4. Microtus agrestis (Linnaeus, 1761)–Field Vole or Short Tailed Vole
2.5. Arvicola terrestris (Linnaeus, 1758)–Eurasian Water Vole
2.6. Ondatra zibethicus (Linnaeus, 1766)–Common Muskrat
3. Family Muridae
3.1. Apodemus agrarius (Pallas, 1771)–Striped Field Mouse
3.2. Apodemus flavicollis (Melchior, 1834)–Yellow Necked Field Mouse
3.3. Apodemus sylvaticus (Linnaeus, 1758)–Wood Mouse, Long Tailed Field Mouse
3.4. Mus musculus Linnaeus 1758–House mouse
3.5. Rattus norvegicus (Berkenhout 1769)–Brown Rat
3.6. Rattus rattus (Linnaeus 1758)–Black Rat
4. Family Myoxidae
4.1. Glis glis (Linnaeus, 1766)–Fat Dormouse
4.2. Muscardinus avellanarius (Linnaeus 1758)–Dormouse or Common Dormouse
4.3. Dryomys nitedula (Pallas, 1778)–Forest Dormouse
5. Family Sciuridae
Sciurus vulgaris Linnaeus, 1758–Eurasian Red Squirrel
6. Family Castoridae
6.1. Castor fiber Linnaeus, 1758–European Beaver
6.2. Important Note
7. Damage, Consequences and Benefits of Rodents
7.1. Direct Damages Caused by Rodents in Agricultural Production and Forestry
7.1.1. Damages to Crops and Vegetables
7.1.2. Damage and Contamination in Food Intended for Human and Animal Consumption
7.1.3. Damage of Buildings, Riverbanks, and Railway Embankments Accompanied by Accidents and Fires
7.1.4. Damage to Textile, Leather, and Other Items
7.1.5. Damage to Other Animal Species
7.1.6. Damage to Seeds, Fruits and Seedlings in Nurseries, Orchards and Forestry Production
7.2. Rodent Danger to Human and Animal Health
7.3. Fear and Anxiety of Rodents
8. Identification of the Rodents and Other Mammals Presence Based on Their Tracks
9. Methods for Estimating Rodent Population Density
9.1. Methods for Estimating Rodent Numbers in Agricultural Production
9.2. Methods for Estimating Rodent Numbers in Communal Hygiene Practice
9.2.1. Method of Marking the Animals (Lincoln Index)
9.2.2. Placebo Bait Method
9.3. Methods for Estimating Rodent Numbers in Forestry Practice
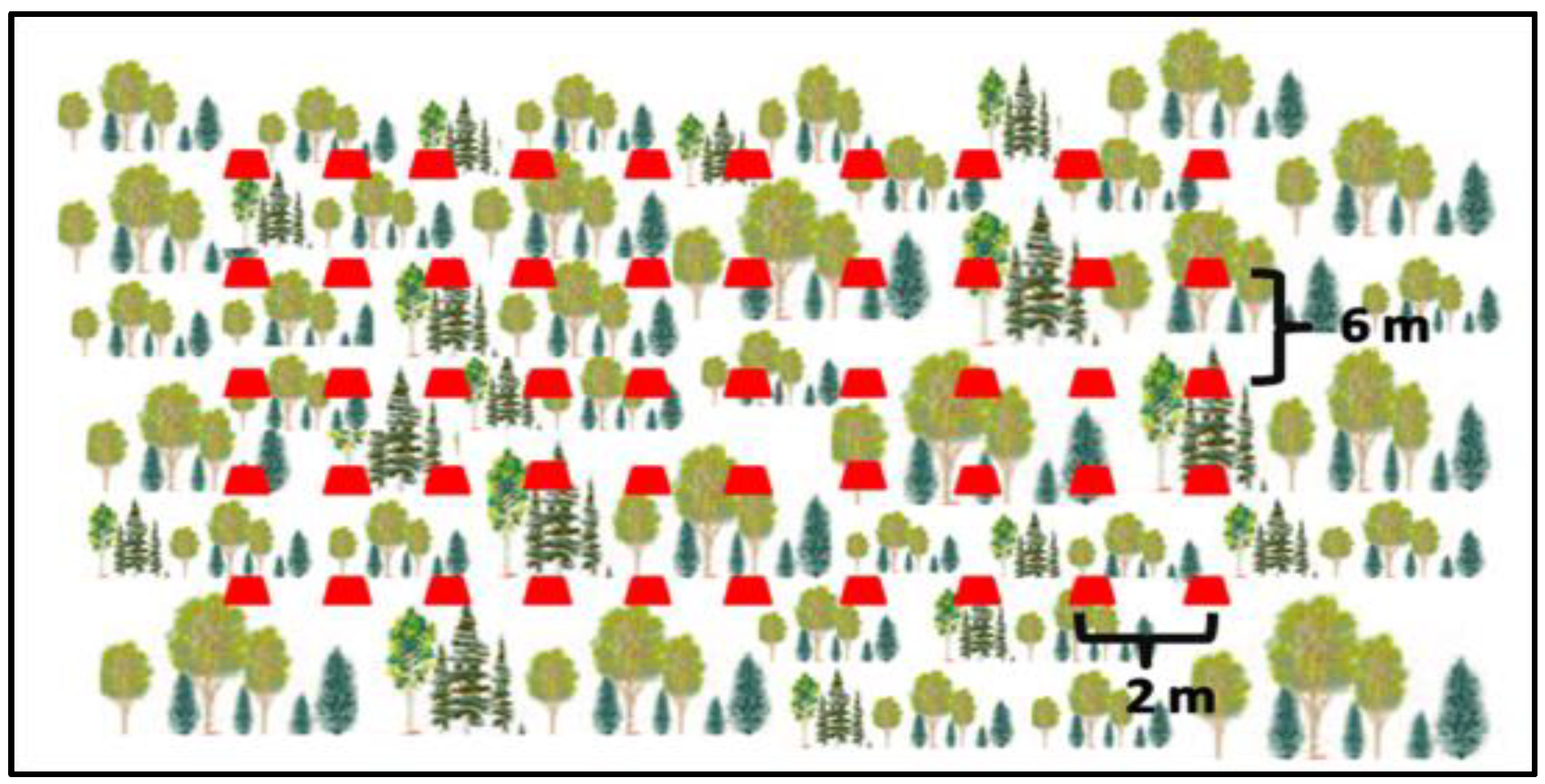
9.3.1. Method of Sampling Area
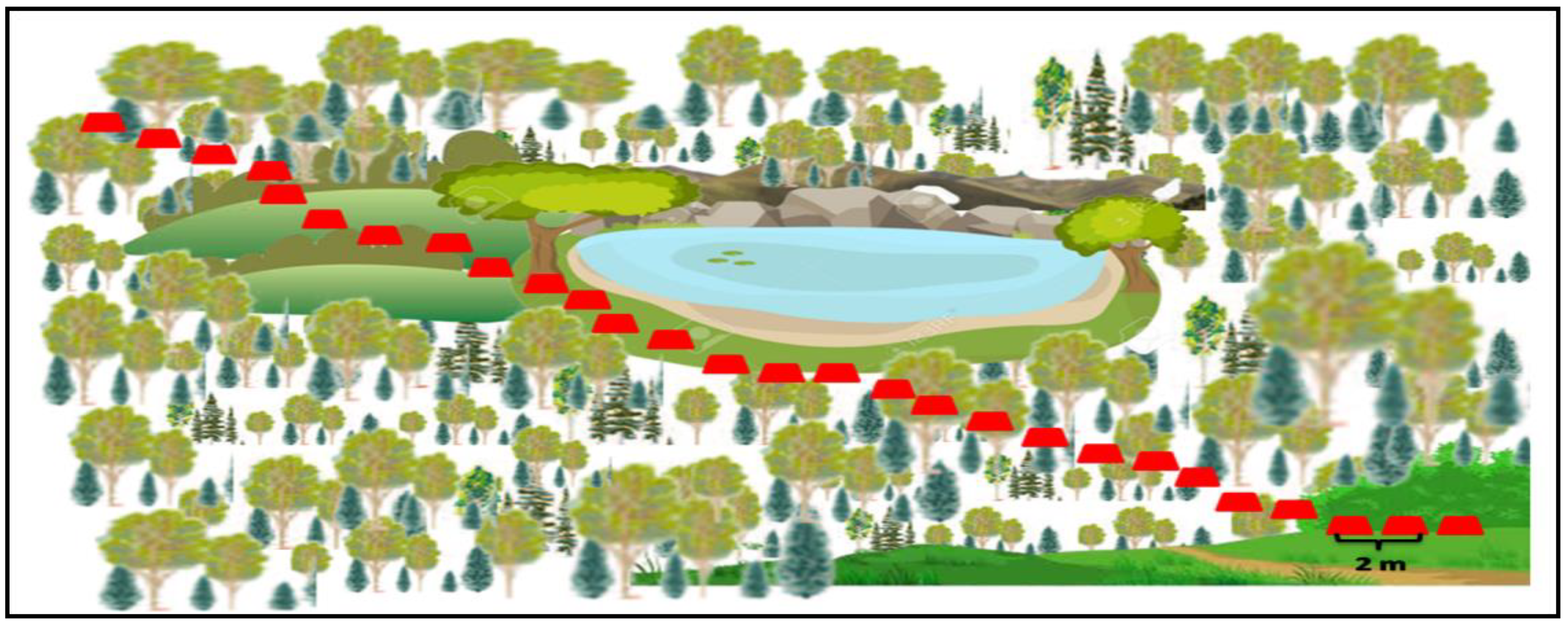
9.3.2. Transect Method (Sampling Line)
- -
- Relative number < 20%: no need to apply control measures
- -
- Relative number 20–30%: control measures can be applied
- -
- Relative number > 30%: protection measures should be applied
9.3.3. Method of Counting Active Holes
- -
- Threshold of active holes in forest is 2–3 active (open) holes/250 m2 (≥8/1.000 m2);
- -
- Threshold in orchatds is 8 active holes/250 m2 (≥32/1.000 m2).
9.3.4. Method of Setting Plant Shoots as Baits
- -
- If there is damage on >20% of shoots by the second day, control measures should be carried out.
9.3.5. Method of Counting Water Vole Holes
9.3.6. Least-Squares Method
9.3.7. Y Method
10. Identification Keys for the Most Damaging Species of Rodents
- (a)
- Body length (measured on ventral side from the end of the muzzle to the anal opening)
- (b)
- Tail length (measured from base to end without distal hairs)
- (c)
- Paw length (measured from the end of the heel to the end of the longest toe without claw)
- (d)
- Number of toes (front and back paws)
- (e)
- Claw shape (blunt, sharp, retractable, non-retractable)
- (f)
- The cuticle between the fingers (exists, does not exist)
- (g)
- Pads on the feet
- (h)
- The shape of the tail and its coverage with different hair shapes
- (i)
- Ratio of body and tail length
- (j)
- The shape of the lateral and predial protrusions on the skull
- (k)
- Formula and structure of teeth (incisors, canines, premolars, and molars)
- (l)
- Diastema-toothless (distance without teeth between incisors and molars)
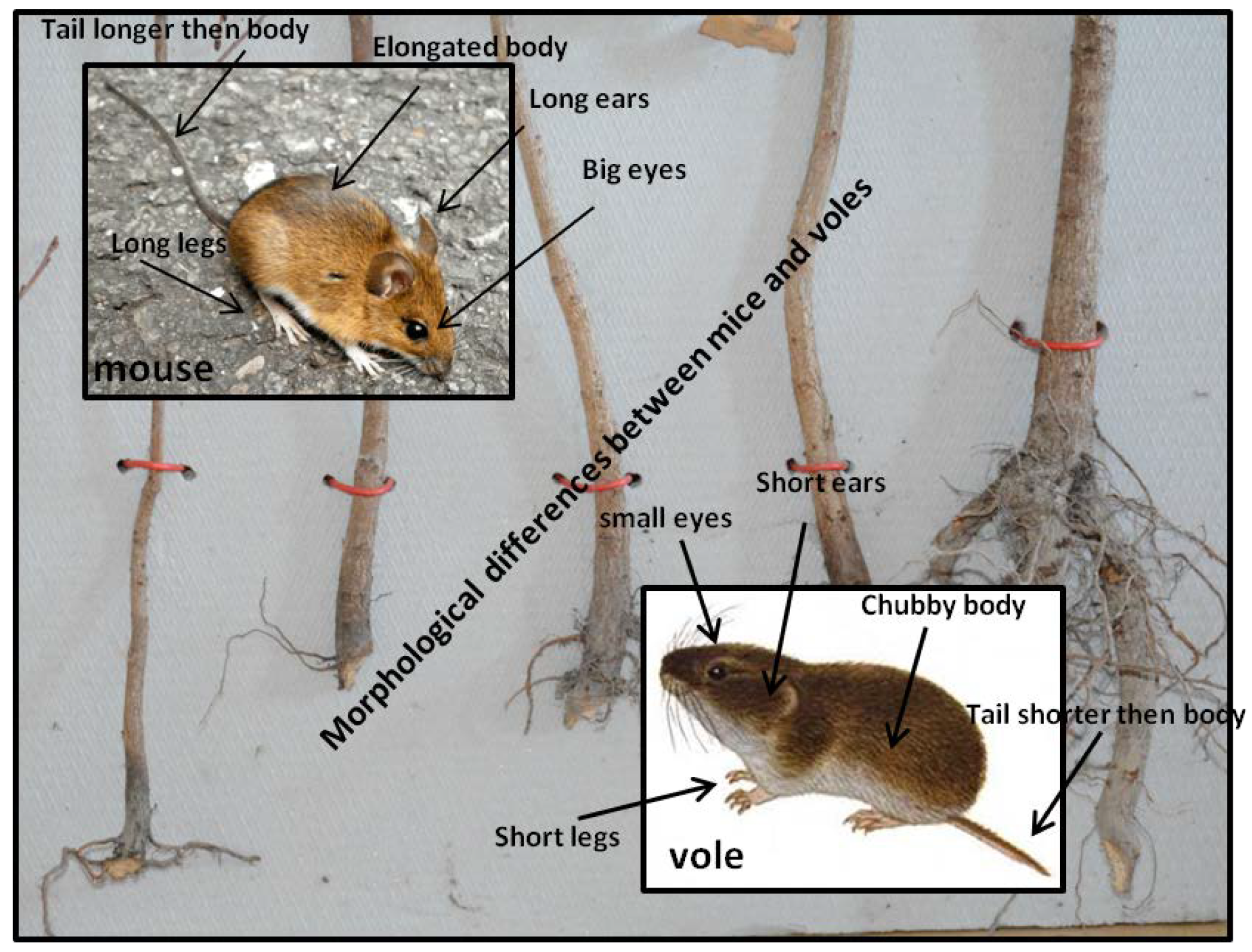
10.1. Identification Key for Family Muridae, Subfamily Murinae: Mouse-Like Rodents
- (a)
- Tail shorter than body, ear straightened, toes connected by cuticles, dorsal region gray, abdominal region whitish (R. norvegicus)
- (b)
- The tail is longer than both body and head, the ear reaches the eyes, the fingers are free, the dorsal region is black, the abdomen is dark gray (R. rattus)
- (a)
- Body and head longer than 70 mm, ears longer than 1/3 of the head, dark longitudinal stripe present on the back, females have four pairs of breasts (A. agrarius)
- (b)
- The dorsal region is brown or reddish. It always has a small yellowish to brown chest pattern. The foot is shorter than 23 mm, the length of the upper molars is <3.9 mm (A. sylvaticus)
- (c)
- There is a large colored pattern on the chest, feet longer than 23 mm (A. flavicollis).
10.2. Identification Key for Family Muridae, Subfamily Arvicolinae (Microtinae): Voles
- (a)
- Body length 120–180 mm. The length of the tail exceeds half of the body length. The ventral region is dark, the number of pads on the feet is five, the number of breasts is eight (A. terestris);
- (b)
- Body length is less than 120 mm. The length of the tail does not exceed ½ of the body length. The eyes are extremely small. The number of pads on the feet is five. Behind the fourth interdigital pad there is only one tarsal. Females have four breasts (Pitymys subterraneus);
- (c)
- The eyes are big, the number of pads on the feet is six, females have eight breasts. The dorsal region is rusty-colored (Myodes glareolus);
- (d)
- The dorsal region is gray or gray-brown, the length of the tail corresponds to 1/3 of the body length, the foot is 14 to 17.3 mm long (M. arvalis);
- (e)
- The dorsal region is gray or gray-brown, the tail length is 1/3 of the body length, the foot length is 18.8–20 mm (M. agrestis);
- (f)
- The fur is brown, the ventral abdominal region is lighter than the dorsal, (yellowish brown), the body length without tail is 35–40 cm, the tail is long, flattened, and covered with scales, eyes small and round, ears are small and hidden in dense fur, hind legs with strong claws (O. zibethicus).
10.3. Identification Key for Fam. Gliridae: Dormice
- (a)
- There is no black stripe between muzzle and ear, fur is gray-brown, tail along the entire length overgrown with dense hairs longer than 1.5 cm, upper teeth longer than 5.5 mm, on the mandible-lower jaw, there is no opening in the processus angularis (G. glis);
- (b)
- Orange-brown fur, tail overgrown with sparse hair shorter than 1 cm, upper dentition shorter than 5.5 mm, on the mandible there is an opening in the processus angularis (M. avellanarius);
- (c)
- The black stripe is extended behind the ear, in front of the tip of the tail there is a black colored area, and on the top of the tail is a white tuft; the upper dentition is longer than 4.6 mm (E. quercinus);
- (d)
- The black stripe ends before the ear, the tail on the dorsal side is equally colored, the upper dentition is shorter than 4.6 mm (D. nitedula).
10.4. Identification Key for Fam. Sciuridae: Squirrel
- (a)
- Eyes and ears normally developed, tail partly covered with hairs, in the lower jaw on each side are four hind teeth (one premolar and three molars), hind leg length is >3 cm, in the upper jaw on each side are five hind teeth (two premolars and three molars). The most common species in our country is the red squirrel (S. vulgaris).
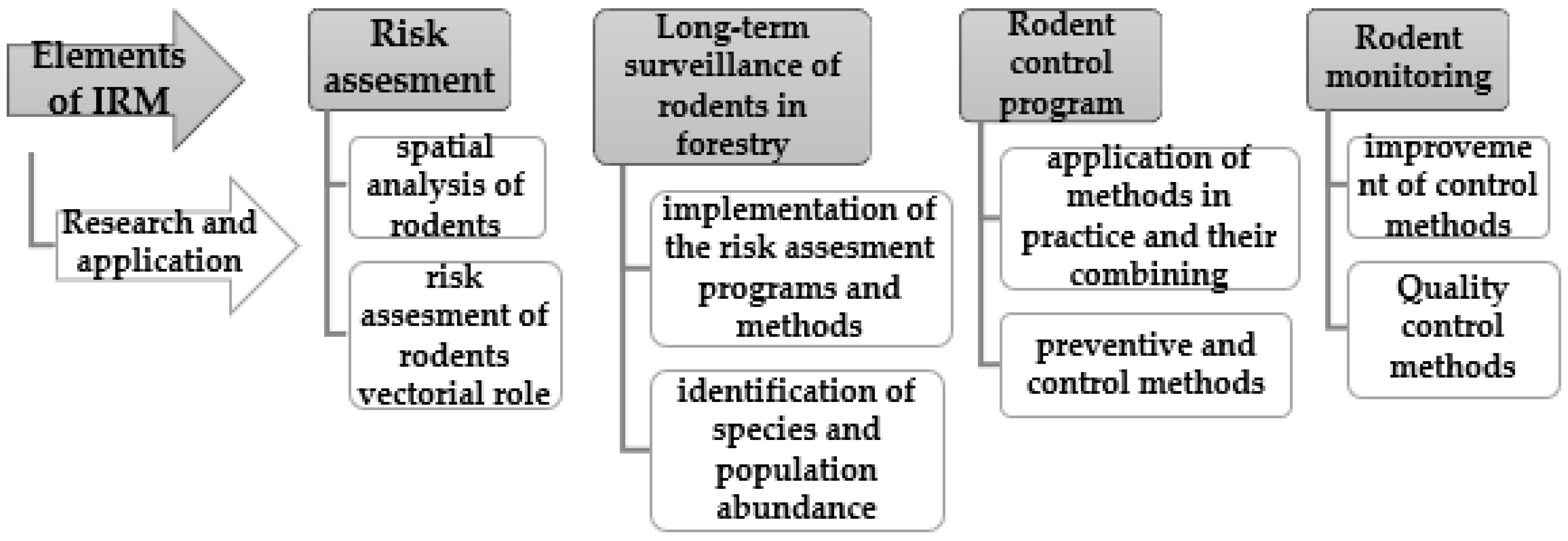
11. Integrated Rodent Management (IRM)
12. Forest Protection and FSC Standards
13. Evaluation of Rodent Control Measures (Treatment Quality Control)
Henderson–Tilton Formula
14. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krunić, M.; Vidović, V.; Pujin, V.; Petrović, Z.; Šapkarev, J.; Stevanović, D.; Horvatović, A.; Brajković, M. Sistematika Invertebrate sa Praktikumom; I deo. Univ. u Beogradu; Institute for Textbooks and Teaching Aids: Belgrade, Serbia, 1983. [Google Scholar]
- Hickman, C.; Roberts, L.; Keen, S.; Larson, A.; Anson, H.; Eisenhour, D. Integrated Principles of Zoology, 14th ed.; McGraw-Hill: Boston, MA, USA, 2006; p. 910. [Google Scholar]
- Sullivan, T.P.; Granatstein, D.M. Influence of living mulches on vole populations and feeding damage to apple trees. Crop Prot. 2018, 108, 78–86. [Google Scholar] [CrossRef]
- Bertolino, S.; Asteggiano, L.; Saladini, M.A.; Giordani, L.; Vittone, G.; Alma, A. Environmental factors and agronomic practices associated with Savi’s pine vole abundance in Italian apple orchards. J. Pest Sci. 2015, 88, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, J.; Šipoš, J.; Košulič, O. Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest. Forests 2020, 11, 1320. [Google Scholar] [CrossRef]
- Boone, S.; Mortelliti, A. Small mammal tree seed selection in mixed forests of the Eastern United States. For. Ecol. Manag. 2019, 449, 117487. [Google Scholar] [CrossRef]
- Stejskal, V.; Hubert, J.; Aulicky, R.; Kucerova, Z. Overview of present and past and pest-associated risks in stored food and feed products: European perspective. J. Stored Prod. Res. 2014, 64, 122–132. [Google Scholar] [CrossRef]
- Hornok, S.; Földvári, G.; Rigó, K.; Meli, M.L.; Gönczi, E.; Répási, A.; Farkas, R.; Papp, I.; Kontschán, J.; Hofmann-Lehmann, R. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasites Vectors 2015, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Rabiee, M.H.; Mahmoudi, A.; Siahsarvie, R.; Kryštufek, B.; Mostafavi, E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl. Trop. Dis. 2018, 12, e0006256. [Google Scholar] [CrossRef] [Green Version]
- Buesching, C.D.; Newman, C.; Twell, R.; Macdonald, D.W. Reasons for arboreality in wood mice Apodemus sylvaticus and Bank voles Myodes glareolus. Mamm. Biol. 2008, 73, 318–324. [Google Scholar] [CrossRef]
- Đukić, N.; Horvatović, A.; Kataranovski, D.; Maletin, S.; Matavulj, M.; Pujin, V.; Sekulić, R.; Jurišić, A. Poljoprivredna Zoologija; University of Novi Sad, Faculty of Agriculture: Novi Sad, Serbia, 2018; ISBN 978-86-7520-418-3. [Google Scholar]
- Jacob, J.; Tkadlec, E. Rodent Outbreaks in Europe: Dynamics and Damage. In Rodent Outbreaks—Ecology and Impacts; Singleton, G.R., Belmain, S., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–223. [Google Scholar]
- Faber, M.S.; Ulrich, R.G.; Frank, C.; Brockmann, S.O.; Pfaff, G.M.; Jacob, J.; Krüger, D.H.; Stark, K. Steep rise in notified hantavirus infections in Germany. Eurosurveillance 2010, 15, 19574. [Google Scholar] [CrossRef]
- Mushtaq, M.; Khanam, S. Grain losses caused by the commensal rodents in rural Pothwar, Pakistan. Asian J. Agric. Biol. 2017, 5, 263–269. [Google Scholar]
- Bijelić-Čabrilo, O.; Čabrilo, B.; Popović, E. Helminth fauna of rodents (Mammalia, Rodentia) from Zasavica (Serbia). Biol. Serbica 2013, 35, 43–47. [Google Scholar]
- Kataranovski, M.; Mirkov, I.; Belij, S.; Popov, A.; Petrović, Z.; Gačić, Z.; Kataranovski, D. Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): The effect of sex, age and habitat. Parasite 2011, 18, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.; Imholt, C.; Caminero-Saldaña, C.; Couval, G.; Giraudoux, P.; Herrero-Cófreces, S.; Horváth, G.; Luque-Larena, J.; Tkadlec, E.; Wymenga, E. Europe-wide outbreaks of common voles in 2019. J. Pest. Sci. 2020, 93, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, P.; Koskela, E.; Mappes, T. Does risk of predation by mammalian predators affect the spacing behaviour of rodents? Two large-scale experiments. Oecologia 2000, 122, 487–492. [Google Scholar] [CrossRef]
- Hanski, I.; Henttonen, H.; Korpimäki, E.; Oksanen, L.; Turchin, P. Small-rodent dynamics and predation. Ecology 2001, 82, 1505–1520. [Google Scholar] [CrossRef]
- Kollmann, J.; Buschor, M. Edges effects on seed predation by rodents in deciduous forests of northern Switzerland. Plant Ecol. 2003, 164, 249–261. [Google Scholar] [CrossRef]
- Klemola, T.; Korpimäki, E.; Norrdahl, K. Does avian predation risk depress reproduction of voles? Oecologia 1998, 115, 149–153. [Google Scholar] [CrossRef]
- Klaa, K.; Mill, P.J.; Incoll, L.D. Distribution of small mammals in a silvoarable agroforestry system in Northern England. Agrofor. Syst. 2005, 63, 101–110. [Google Scholar] [CrossRef]
- Bjedov, L.; Vucelja, M.; Margaletić, J. Priručnik o Glodavcima Šuma Hrvatske; Hrvatski Šumarski Institute: Jastrebarsko, Croatia, 2016; ISBN 978-953-7909-04-8. [Google Scholar]
- Christiansen, E. Fluctuations in some small rodent populations in Norway 1971–1979. Holarct. Ecol. 1983, 6, 24–31. [Google Scholar] [CrossRef]
- Vukša, M.; Đedović, S. Rodent Control in Agricultural Fields and Introduction of New Ecologically-Friendly Products. In Proceedings of the 2nd International Conference on Rural Health and 1st International Conference on Occuptional and Environmental Health in Mediterranean, South East and Central European Countries, Belgrade, Serbia, 26–29 May 2004; pp. 62–63. [Google Scholar]
- Heroldová, M.; Šipoš, J.; Suchomel, J.; Zejda, J. Influence of crop type on common vole abundance in Central European agroecosystems. Agric. Ecosyst. Environ. 2021, 315, 107443. [Google Scholar] [CrossRef]
- Jurišić, A.; Petrović, A.; Ivanović, I. Glodari u ratarskim usevima. Biljni Lek. 2017, 45, 494–498. [Google Scholar]
- Đedović, S.; Vukša, M.; Jokić, G.; Šćepović, T. Environmentally safe performance of products for management of commensal rodents in Serbia. J. Process. Energy Agric. 2013, 17, 51–53. [Google Scholar]
- Vukša, M. Glodari u Skladištima i Njihovo Suzbijanje. In Zaštita Uskladištenih Biljnih Proizvoda od Štetnih Organizama; Kljajić, P., Ed.; Institute for Pesticides and Environmental Protection: Belgrade, Serbia, 2008; pp. 215–227. [Google Scholar]
- Margaletić, J.; Glavaš, M.; Bäumler, W. The development of mice and voles in an oak forest withsurplus of acorns. Anz. Schädlingskunde/J. Pest Sci. 2002, 75, 95–98. [Google Scholar] [CrossRef]
- Viitala, J.; Hoffmeyer, I. Social organization in Clethrionomys compared with Microtus and Apodemus: Social odours, chemistry and biological effects. Ann. Zool. Fenn. 1985, 22, 359–371. [Google Scholar]
- Miklós, P.; Žiak, D. Microhabitat selection by three small mammal species in oak-elm forest. Folia Zool. 2002, 51, 275–288. [Google Scholar]
- Torre, I.; Arrizabalaga, A. Habitat preferences of the bank vole Myodes glareolus in a Mediterranean mountain range. Acta Theriol. 2008, 53, 241–250. [Google Scholar] [CrossRef]
- Giraudoux, P.; Villette, P.; Quéré, J.P.; Damange, J.P.; Delattre, P. Weather influences M. arvalis reproduction but not population dynamics in a 17-year time series. Sci. Rep. 2019, 9, 13942. [Google Scholar] [CrossRef] [Green Version]
- Bryja, J.; Nesvadbová, J.; Heroldová, M.; Jánová, E.; Losík, J.; Trebatická, L.; Tkadlec, E. Common vole (Microtus arvalis) population sex ratio: Biases and process variation. Can. J. Zool. 2005, 83, 1391–1399. [Google Scholar] [CrossRef]
- Jokić, G.; Blažić, T. Control of Common Vole (Microtus arvalis) in Alfalfa Crops Using Reduced Content of Anticoagulants. Agronomy 2022, 12, 53. [Google Scholar] [CrossRef]
- Turchin, P.; Batzli, G.O. Availability of food and the population dynamics of arvicoline rodents. Ecology 2001, 82, 1521–1534. [Google Scholar] [CrossRef]
- Mathias, M.D.; Hart, E.B.; Ramalhinho, M.D.; Jaarola, M. Microtus agrestis (Rodentia: Cricetidae). Mamm. Species 2017, 49, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.P.; Bright, P.W. Reedbeds as refuges for water voles (Arvicola terrestris) from predation by introduced mink (Mustela vison). Biol. Conserv. 2003, 111, 371–376. [Google Scholar] [CrossRef]
- Abt, K.; Bock, W. Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol. 1998, 43, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Marsh, A.C.W.; Harris, S. Partitioning of woodland habitat resources by two sympatric species of Apodemus: Lessons for the conservation of the yellow-necked mouse (A. flavicollis) in Britain. Biol. Conserv. 2000, 92, 275–283. [Google Scholar] [CrossRef]
- Juškaitis, R. Spatial distribution of the yellow-necked mouse (Apodemus flavicollis) in large forest areas and its realtion with seed crop of forest trees. Mamm. Biol. 2002, 67, 206–211. [Google Scholar] [CrossRef]
- Muňoz, A.; Bonal, R. Are you strong enough to carry that seed? Seed size/body size ratios influence seed choices by rodents. Anim. Behav. 2008, 76, 709–715. [Google Scholar] [CrossRef]
- Díaz, M.; Santos, T.; Tellería, J.L. Effects of forest fragmentation on the winter body condition and population parameters of an habitat generalist, the wood mouse Apodemus sylvaticus: A test of hypotheses. Acta Oecol. 1999, 20, 39–49. [Google Scholar] [CrossRef]
- Margaletić, J.; Božić, M.; Grubešić, M.; Glavaš, M.; Baumler, W. Distribution and abundance of small rodents in Croatian forests. J. Pest Sci. 2005, 78, 99–103. [Google Scholar] [CrossRef]
- Daniels, M.J.; Hutchings, M.R. The response of cattle and sheep to feed contaminated with rodent faeces. Vet. J. 2001, 162, 211–218. [Google Scholar] [CrossRef]
- Jurišić, A.; Ivanović, I.; Poljaković-Pajnik, L.; Vasić, V.; Velojić, M.; Drekić, M.; Petrović, A.; Meseldžija, M.; Dabić, S.; Potkonjak, A. Priručnik za Kontrolu i Suzbijanje Glodara u Šumarstvu-Praktikum; JP Vojvodinašume: Petrovaradin, Serbia, 2021; ISBN 978-86-906665-8-4. [Google Scholar]
- Aria, A.K.; Morovati, M.; Naseri, M. Efficacy of the rodenticide bromethalin in the control of Microtus arvalis and Nesokia indica in alfalfa fields. Turk. J. Zool. 2008, 31, 261–264. [Google Scholar]
- Suchomel, J.; Šipoš, J.; C’epelka, L.; Heroldová, M. The impact of Microtus arvalis and Lepus europaeus on apple trees by trunk bark gnawing. Plant. Prot. Sci. 2019, 55, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Suchomel, J.; Šipoš, J.; Ourednícková, J.; Skalský, M.; Heroldová, M. Bark Gnawing by Rodents in Orchards during the Growing Season—Can We Detect Relation with Forest Damages? Agronomy 2022, 12, 251. [Google Scholar] [CrossRef]
- Desoky, A.E.; Abd-Alla, A.E.; Mahmoud, E.A. Mahmoud. Control of Rodents in food Processing Unit “As Model”. Int. J. Res. Agric. For. 2020, 7, 23–25. [Google Scholar]
- Kataranovski, D. Distribution of the Norway Rat Rattus norvegicus BERK., 1769 (Rodentia) on the territory of former and present Yugoslavia. In Proceedings of the International Symposium: Contribution to the Zoogeography and Ecology of the Eastern Mediterranean Region, Kavala, Greece, 17–21 May 1999; Hellenic Zoological Society: Athens, Greece, 1999; Volume 1, pp. 99–104. [Google Scholar]
- Mulungu, L. Control of rodent pests in maize cultivation: The case of Africa. In Achieving Sustainable Maize Cultivation; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2017; Volume 2, pp. 317–338. [Google Scholar] [CrossRef]
- Stojnić, B. Poljoprivredna Zoologija; University of Belgrade, Faculty of Agriculture: Zemun, Serbia, 2019; ISBN 978-86-7834-334-6. [Google Scholar]
- Ratajac, R. Zoologija za Student Poljoprivrednog Fakulteta; PMF i MP Stylos: Novi Sad, Serbia, 1995. [Google Scholar]
- Battersby, J.E. Public health policy—can there be an economic imperative? An examination of one such issue. J. Environ. Health Res. 2004, 3, 19–28. [Google Scholar]
- Bajomi, D. Chronology of a rat control programme with lasting success. Public Health 1993, 10, 14–19. [Google Scholar]
- Fernandez, M.S.; Cavia, R.; Cueto, G.R.; Suarez, O.V. Implementation and evaluation of an integrated program for rodent control in a shanty town of Buenos Aires city, Argentina. EcoHealth 2007, 4, 271–277. [Google Scholar] [CrossRef]
- Brooks, J.E.; Rowe, F.P.; World Health Organization. Rodents: Training and Information Guide; Division of Vector Biology and Control, World Health Organization: Geneva, Switzerland, 1987. Available online: https://apps.who.int/iris/handle/10665/61081 (accessed on 25 May 2022).
- Childs, J.E.; McLafferty, S.L.; Sadek, R.; Miller, G.L.; Khan, A.S.; DuPree, E.R.; Advani, R.; Mills, J.N.; Glass, A.G. Epidemiology of rodent bites and prediction of rat infestation in New York city. Am. J. Epidemiol. 1998, 148, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Kataranovski, D. Analiza Osnovnih Parametara Populacija Vrsterattus Norvegicus Berkenhout, 1769, u Uslovima Urbanih i Suburbanih Staniãta. Ph.D. Thesis, Prirodno-matematički fakultet, Univerzitet u Beogradu, Beograd, Srbija, 1988. [Google Scholar]
- Olson, R.A.; Brewer, M.J. Small mammal populations occurring in a diversified winter wheat cropping system. Agric. Ecosyst. Environ. 2003, 95, 311–319. [Google Scholar] [CrossRef]
- Suchomel, J.; Krojerová-Prokešová, J.; Heroldová, M.; Purchart, L.; Barančeková, M.; Homolka, M. Habitat preferences of small terrestrial mammals in the mountain forest clearings. Mendelova Zemědělská A Lesn. Univerzita V Brně 2009, 2, 195–200. [Google Scholar]
- Suchomel, J.; Heraldova, M.; Šipoš, J.; Čepelka, L.; Dukulilova, M.; Purchart, L. Bark Gnawing of forest trees by voles during the Growing Season. Eur. J. For. Res. 2021, 140, 1431–1440. [Google Scholar] [CrossRef]
- Somoano, A.; Ventura, J.; Miñarro, M. Continuous breeding of fossorial water voles in northwestern Spain: Potential impact on apple orchards. Folia Zool. 2017, 66, 37–49. [Google Scholar] [CrossRef]
- Miñarro, M.; Montiel, C.; Dapena, E. Vole pests in apple orchards: Use of presence signs to estimate the abundance of Arvicola terrestris cantabriae and Microtus lusitanicus. J. Pest. Sci. 2012, 85, 477–488. [Google Scholar] [CrossRef]
- Obigala, A.; Jeske, K.; Augustin, M.; Król, N.; Fischer, S.; Mertens-Scholz, K.; Imholt, C.; Suchomel, J.; Heroldova, M.; Tomaso, H.; et al. Highly prevalent bartonellae and other vector-borne pathogens in small mammal species from the Czech Republic and Germany. Parasites Vectors 2019, 12, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkadlec, E.; Václavík, T.; Široký, P. Rodent host abundance and climate variability as predictors of tickborne disease risk 1 year in advance. Emerg. Infect. Dis. 2019, 25, 1738–1741. [Google Scholar] [CrossRef]
- Jurišić, A. Iksodidni Krpelji (Acari: Ixodidae)—Vektori i Rezervoari Uzročnika Oboljenja Kod Ljudi i Životinja. Ph.D. Thesis, University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia, 2012. [Google Scholar]
- Gryczyńska, A.; Sokół, M.; Gortat, T.; Kowalec, M. Borrelia miyamotoi infection in Apodemus spp. mice populating an urban habitat (Warsaw, Poland). Int. J. Parasitol. Parasites Wildl. 2021, 14, 138–140. [Google Scholar] [CrossRef]
- Kurtenbach, K.; Kampen, H.; Dizij, A.; Arndt, S.; Seitz, H.; Schaible, U.; Simon, M. Infestation of Rodents with Larval Ixodes ricinus (Acari; Ixodidae) Is an Important Factor in the Transmission Cycle of Borrelia burgdorferi s.l. in German Woodlands. J. Med. Entomol. 1995, 32, 807–817. [Google Scholar] [CrossRef]
- Matuschka, F.; Endepols, S.; Richter, D.; Ohlenbusch, A.; Eiffert, H.; Spielman, A. Risk of Urban Lyme Disease Enhanced by the Presence of Rats. J. Infect. Dis. 1996, 174, 1108–1111. [Google Scholar] [CrossRef]
- Craine, N.G.; Nuttall, P.A.; Marriott, A.C.; Randolph, S.E. Role of grey squirrels and pheasants in the transmission of Borrelia burgdorferi sensu lato, the Lyme disease spirochaete, in the U.K. Folia Parasitol. 1997, 44, 155–160. [Google Scholar]
- Humair, P.; Gern, L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 1998, 69, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Institute of Public Health of Serbia “Dr Milan Jovanovic Batut”. Available online: https://www.batut.org.rs (accessed on 25 May 2022).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report for 2016, Echinoccocosis; ECDC: Stockholm, Sweeden, 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/echinococcosis-annual-epidemiological-report-2016 (accessed on 10 June 2022).
- Elliott, S. Rat Bite Fever and Streptobacillus moniliformis. Clin. Microbiol. Rev. 2007, 20, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, M.H.; Janda, J.M. Rat-bite fever (Streptobacillus moniliformis): A potential emerging disease. Int. J Infect Dis. 2001, 5, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Ojukwu, I.C.; Christy, C. Rat-bite fever in children: Case report and review. Scand. J. Infect. Dis. 2002, 34, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Witmer, G.W.; Hakim, A.A.; Moser, B.W. Investigations of Methods to Reduce Damage by Voles. 2000. Available online: https://digitalcommons.unl.edu/icwdm_wdmconfproc/49 (accessed on 20 May 2022).
- Zejda, J.; Nesvadbová, J. Abundance and reproduction of the common vole, Microtus arvalis in crop rows and associated agricultural habitats. Folia Zool. 2000, 49, 261–268. [Google Scholar]
- Frankova, M.; Kaftanova, B.; Aulicky, R.; Rodl, P.; Frynta, D.; Stejskal, V. Temporal production of colored faeces in wild roof rats (Rattus rattus) following consumption of fluorescent non-toxic bait and a comparison with wild R. norvegicus and Mus musculus. J. Stored Prod. Res. 2019, 81, 7–10. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Integrated Pest Management: Conducting Urban Rodent Surveys; US Department of Health and Human Services: Atlanta, GA, USA, 2006. Available online: http://www.cdc.gov/nceh/ehs/docs/ipm_manual.pdf (accessed on 12 June 2022).
- Krebs, C.J. Ecological Methodology, 2nd ed.; Longman: Menlo Park, CA, USA, 1999. [Google Scholar]
- Anderson, D.R.; Burnham, K.P.; White, G.C.; Otis, D.L. Density estimation of small-mammal populations using a trapping web and distance sampling methods. Ecology 1983, 64, 674–680. [Google Scholar] [CrossRef]
- Efford, M. Density estimation in live-trapping studies. Oikos 2004, 106, 598–610. [Google Scholar] [CrossRef]
- Motro, Y.; Ghendler, Y.; Muller, Y.; Goldin, Y.; Chagina, O.; Rimon, A.; Harel, Y.; Motro, U. A comparison of trapping efficacy of 11 rodent traps in agriculture. Mamm. Res. 2019, 64, 435–443. [Google Scholar] [CrossRef]
- Lambin, X.; MacKinnon, J. The relative efficiency of two commercial live-traps for small mammals. J. Zool. 1997, 242, 400–404. [Google Scholar] [CrossRef]
- Jacob, J.; Ylönen, H.; Hodkinson, C.G. Trapping efficiency of Ugglan traps and Longworth traps for house mice in south-eastern Australia. Wildl. Res. 2002, 29, 101–103. [Google Scholar] [CrossRef]
- Ricankova, V.; Sumbera, R.; Sedlacek, F. Familiarity and partner preferences in female common voles, Microtus arvalis. J. Ethol. 2006, 25, 95–98. [Google Scholar] [CrossRef]
- Anthony, N.M.; Ribic, C.A.; Bautz, R.; Garland, T., Jr. Comparative effectiveness of Longworth and Sherman live traps. Wildl. Soc. B 2005, 33, 1018–1026. [Google Scholar] [CrossRef]
- Jokić, G.; Vukša, M.; Đedović, S. Efficacy of rodent control in alfalfa and wheat crops using chemical and natural rodenticides. Pestic. Phytomed. 2007, 22, 241–246. [Google Scholar]
- Kataranovski, D.; Kataranovski, M. Second generations rodenticides: Comparative Study. Arch. Toxicol. Kinet. Xenobiot. Metab. 1997, 5, 91–99. [Google Scholar]
- Simeonovska-Nikolova, D.M. Interspecific social interactions and behavioral responses of Apodemus agrarius and Apodemus flavicollis to conspecific and heterospecific odors. J. Ethol. 2007, 25, 41–48. [Google Scholar] [CrossRef]
- Petrović, A. Sezonske Fluktuacije Voluharica i Miševa (RODENTIA: Muridae) i Njihova Uloga kao Vektora Iksodidnih Krpelja (Acari: Ixodidae). Ph.D. Thesis, University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia, 2015. [Google Scholar]
- Wenk, M. Mäuse, Waldschutz-Merkblatt 53. Hrsg. Ministerium für Ländliche Entwicklung, Umwelt und Verbraucherschutz des Landes Brandenburg und Landesforstanstalt Eberswalde, 34 S. 2007. Available online: http://forst.brandenburg.de/sixcms/media.php/9/ws_maeuse2016.pdf (accessed on 10 June 2022).
- Tobin, M.E.; Richmond, M.E. Vole Management in Fruit Orchards; Biological Report 5; U.S. Fish and Wildlife Service: Bailey’s Crossroads, VA, USA, 1993; pp. 1–24. [Google Scholar]
- Tobin, M.; Fall, M. Pest Control: Rodents. Wildlife Damage Management; Internet Center for USDA National Wildlife Research Center, University of Nebraska: Lincoln, NE, USA, 2004; Available online: http://digitalcommons.unl.edu/icwdmusdanwrc/67 (accessed on 12 June 2022).
- Desoky, A.E.A.S.S. Integrated Pest Management for Rodent in Buildings. Curr. Investig. Agri. Curr. Res. 2018, 4, 563–565. [Google Scholar] [CrossRef]
- World Health Organization: Instructions for Determining the Susceptibility or Resistence of Rodents to Anticoagulant Rodenticides. WHO Vector Control, Ser. 75, I. 1975. Available online: http://apps.who.int/iris/handle/10665/263521 (accessed on 12 June 2022).
- Forest Stewardship Council. Available online: https://www.fsc.org/en/document-center/documents/cc238e58-1db6-4e69-bf99-4771b47458da (accessed on 27 May 2022).
- Uzin, I. Suzbijanje Glodavaca u Voćnjacima; Specijalistički Rad; Josip Juraj Strossmayer University of Osijek, Faculty of Agriculture: Osjek, Croatia, 2008. Available online: http://zpio.unios.hr/wp-content/uploads/radovi/spec.rad/ivica.uzin.pdf (accessed on 10 June 2022).
- Janjić, V. Phytopharmacy; Plant Protection Society of Serbia: Belgrade, Serbia, 2005. [Google Scholar]
- Forest Stewardship Council. Available online: https://fsc.org/en/document-centre/documents/resource/315 (accessed on 27 May 2022).
- Forest Stewardship Council. Available online: https://fsc.org/en/details-page/pesticides-policy (accessed on 27 May 2022).
- Sahni, K.; Prabha, C. Comparative Efficacy of Zinc Phosphide and Bromadiolone against Rats in Houses. J. Life Sci. 2012, 4, 67–68. [Google Scholar] [CrossRef]
- Suárez, O.V.; Cueto, G.R. Comparison of efficacy of second-generation anticoagulant rodenticides: Effect of active ingredients, type of formulation and commercial suppliers. Cogent Food Agric. 2018, 4, 1525147. [Google Scholar] [CrossRef]
- Available online: https://eur-.europa.eu/legalcontent/en/TXT/?uri=CELEX%3A32018R12 (accessed on 12 June 2022).
- Frankova, M.; Stejskal, V.; Aulicky, R. Efficacy of rodenticide baits with decreased concentrations of brodifacoum: Validation of the impact of the new EU anticoagulant regulation. Sci. Rep. 2019, 9, 16779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Office of Consumer Protection and Food Safety (BVL). Available online: https://apps2.bvl.bund.de/psm/jsp/ListeMain (accessed on 1 June 2022).
- Hamel, D. Posljedice zabrane upotrebe rodenticida na poljoprivrednim površinama. Glas. Biljn. Zaštite 2017, 17, 404–412. [Google Scholar]
- Ministry of Agriculture. List of Registered Plant Protection Products. Available online: https://fis.mps.hr/trazilicaszb/ (accessed on 11 May 2022).
- Henderson, C.F.; Tilton, E.W. Tests with Acaricides against the brow Wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]



| Bacterial Diseases | Virus- and Rickettsia-Caused Diseases | Parasitic Diseases |
|---|---|---|
| Tularemia | Foot-and-mouth disease | Trichinosis |
| Brucellosis | Rabies | Leishmaniasis |
| Leptospirosis | Lymphocytic choriomeningitis | Coccidiosis |
| Listeria | Hemorrhagic fever | Protozoan-induced toxoplasmosis |
| Tuberculosis | Rickettsia | Fungal dermatomycoses |
| Plague | ||
| Salmonellosis | ||
| Borreliosis |
| Category | Population Density | Criteria |
|---|---|---|
| I | very low number | <10 holes/ha |
| II | low number | 10–500 holes/ha |
| III | medium number | 500–5000 holes/ha |
| IV | high number | 5000–20,000 holes/ha |
| V | very high number | 20,000–50,000 holes/ha |
| VI | calamity | >50,000 holes/ha |
| Category | Population Density | Criteria |
|---|---|---|
| I | very low number | <10 holes/ha |
| II | low number | 10–50 holes/ha |
| III | medium number | 50–500 holes/ha |
| IV | high number | 500–2000 holes/ha |
| V | very high number | 2000–10,000 holes/ha |
| VI | calamity | >10,000 holes/ha |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurišić, A.; Ćupina, A.I.; Kavran, M.; Potkonjak, A.; Ivanović, I.; Bjelić-Čabrilo, O.; Meseldžija, M.; Dudić, M.; Poljaković-Pajnik, L.; Vasić, V. Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments. Sustainability 2022, 14, 9233. https://doi.org/10.3390/su14159233
Jurišić A, Ćupina AI, Kavran M, Potkonjak A, Ivanović I, Bjelić-Čabrilo O, Meseldžija M, Dudić M, Poljaković-Pajnik L, Vasić V. Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments. Sustainability. 2022; 14(15):9233. https://doi.org/10.3390/su14159233
Chicago/Turabian StyleJurišić, Aleksandar, Aleksandra Ignjatović Ćupina, Mihaela Kavran, Aleksandar Potkonjak, Ivana Ivanović, Olivera Bjelić-Čabrilo, Maja Meseldžija, Milica Dudić, Leopold Poljaković-Pajnik, and Verica Vasić. 2022. "Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments" Sustainability 14, no. 15: 9233. https://doi.org/10.3390/su14159233
APA StyleJurišić, A., Ćupina, A. I., Kavran, M., Potkonjak, A., Ivanović, I., Bjelić-Čabrilo, O., Meseldžija, M., Dudić, M., Poljaković-Pajnik, L., & Vasić, V. (2022). Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments. Sustainability, 14(15), 9233. https://doi.org/10.3390/su14159233










