Sustainable Production of Biodiesel Using UV Mutagenesis as a Strategy to Enhance the Lipid Productivity in R. mucilaginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Microbes
2.2. Physical Mutation of Microbes Using UV Radiation Method
2.3. Molecular Sequencing
2.4. Culture and Growth Conditions of Selected Strains
2.5. Analysis of Cell Dry Weight
2.6. Extraction of Lipids
2.7. Quantification of Lipids
2.8. Gas Chromatography and Mass Spectroscopy
2.9. Fourier Transformed Infrared Spectroscopy
2.10. Transesterification
2.11. Biodiesel Properties
3. Results and Discussion
3.1. Screening of High Lipid-Yielding Microbe
3.2. Screening and Selection of UV-Mutated Strains
3.3. Molecular Identification of Wild and Mutated Strains
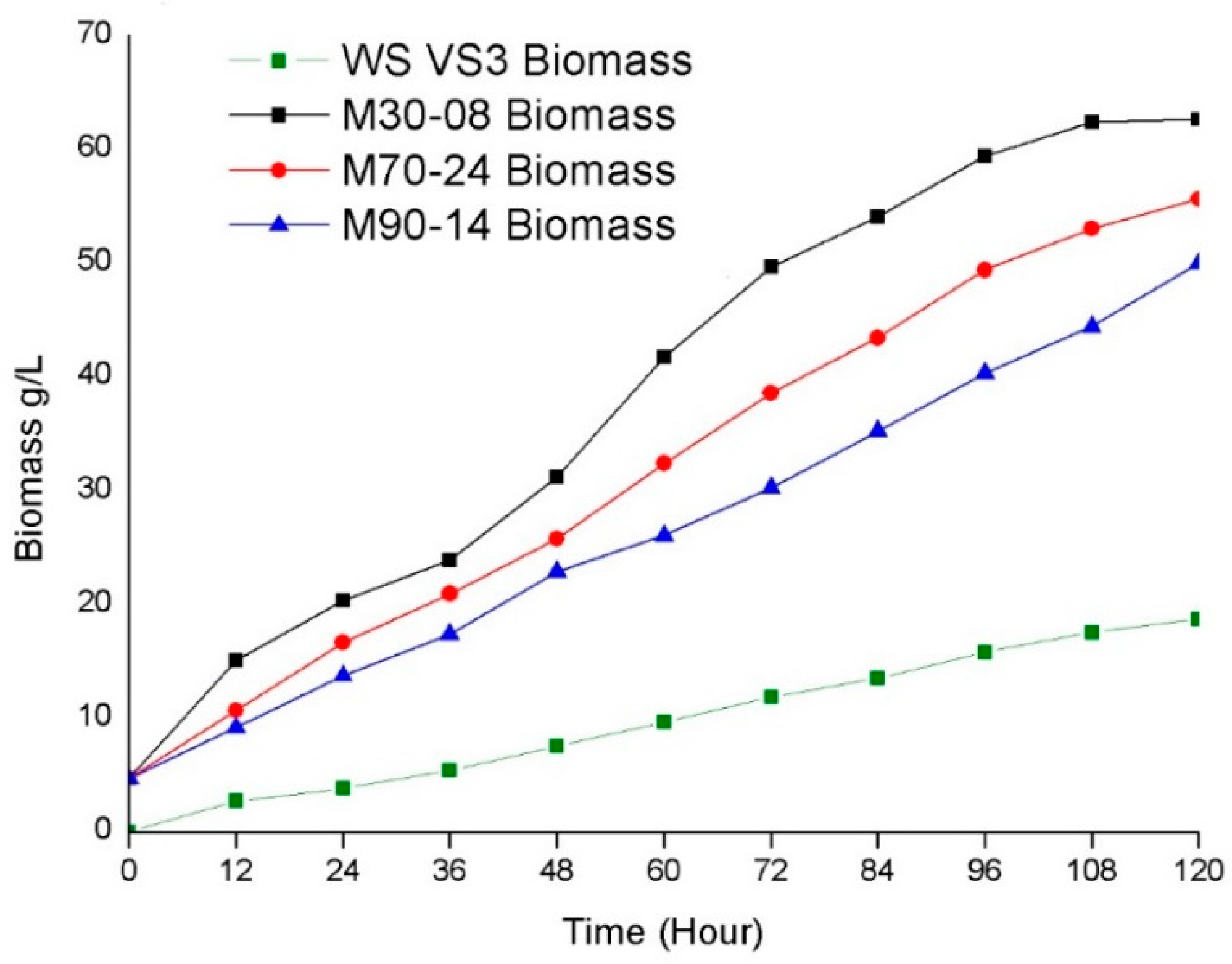
3.4. Production of Biomass and Lipids from Wild and UV-Mutated Strain
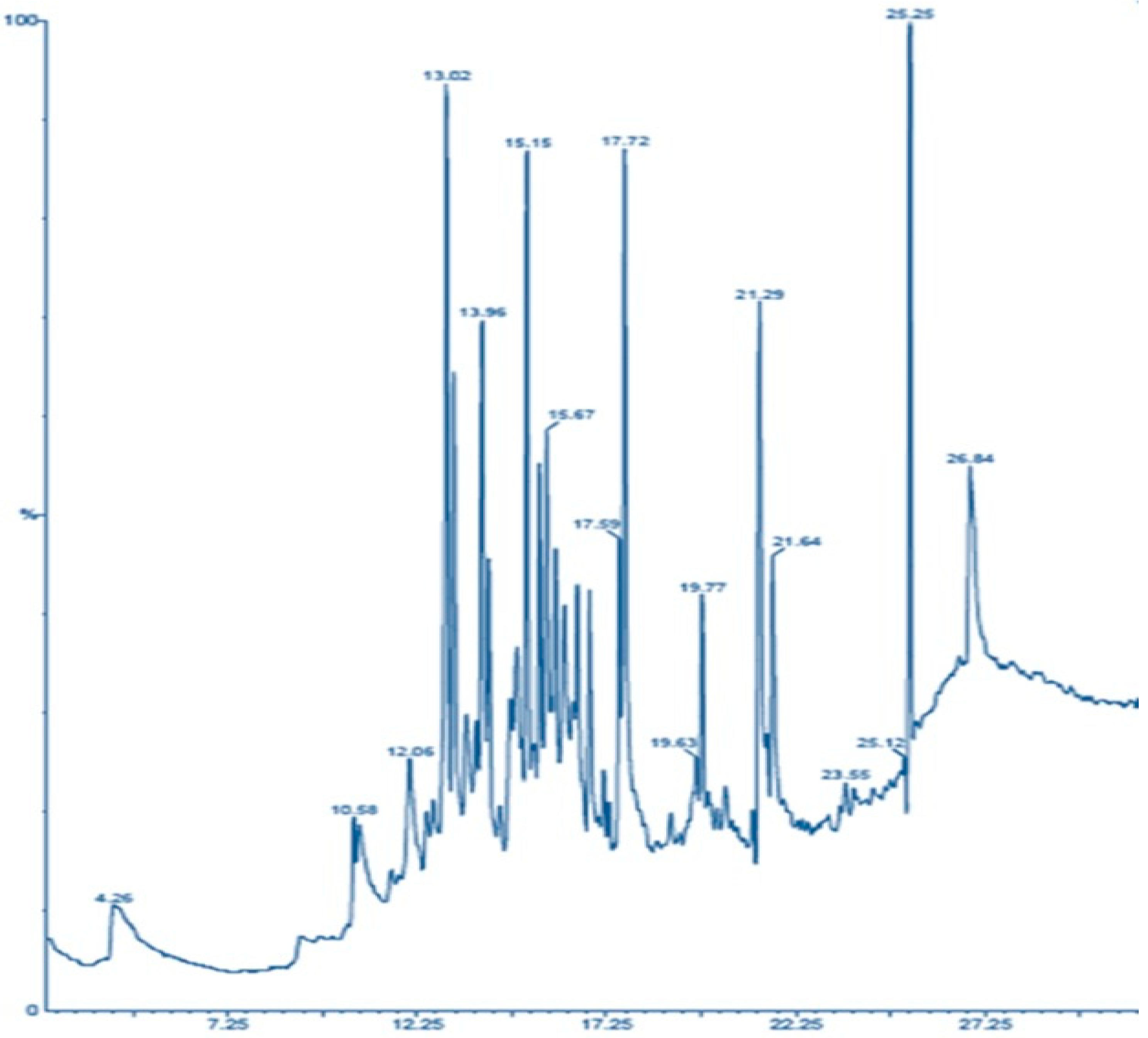
3.5. GCMS Analysis of Lipids Extracted from VS3 and M30-8 Strain
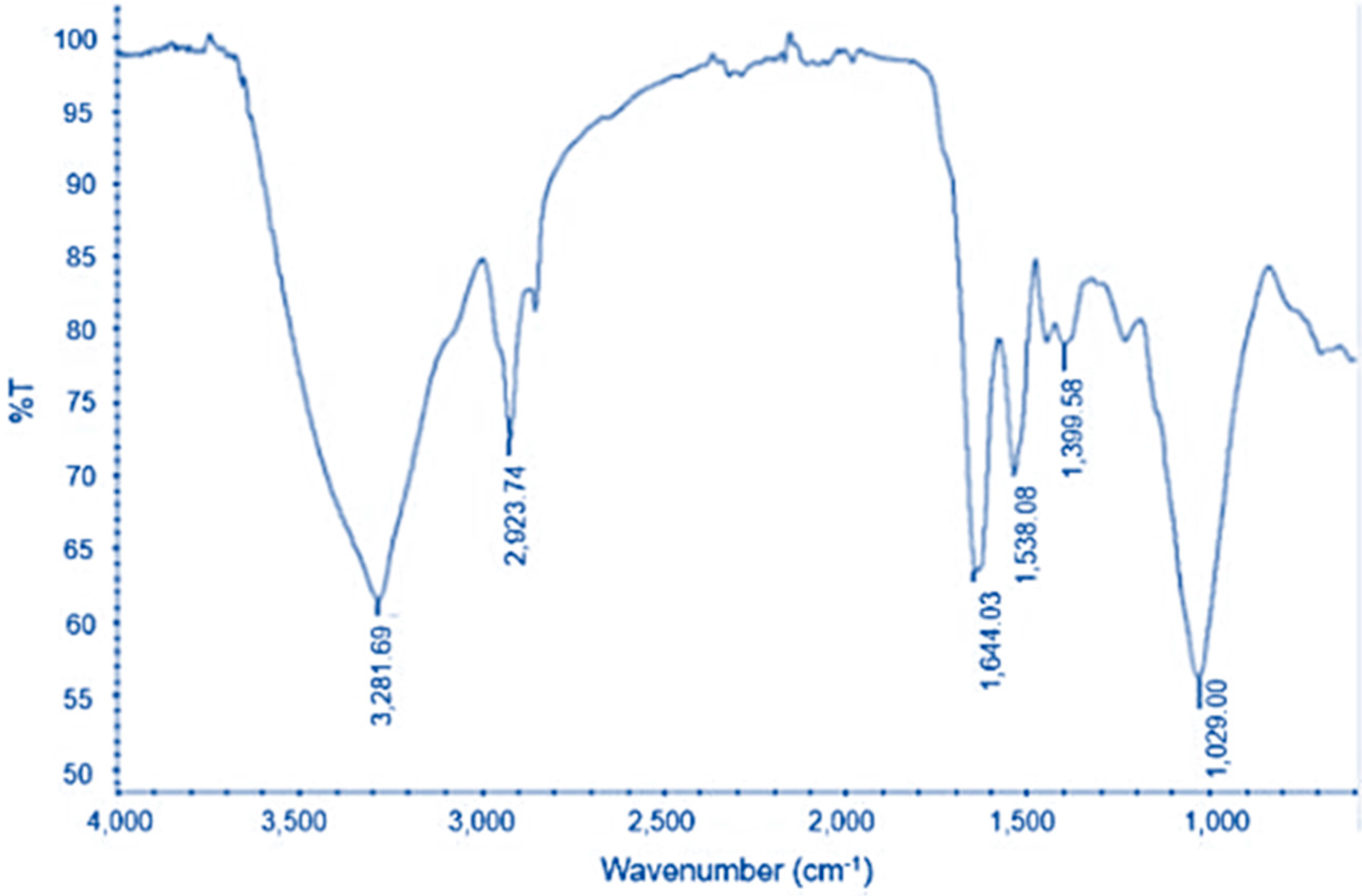
3.6. FTIR Analysis of Lipids Extracted from VS3 and M30-8 Strain
3.7. Biodiesel Properties of VS3 and M30-8 Strain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogul, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel, and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Aloklah, B.; Alhajali, A.; Yaziji, S. Identification of some yeasts by fatty acid profiles. Pol. J. Microbiol. 2014, 63, 467–472. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel Production from High FFA Rubber Seed Oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Demirbas, A. Progress, and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Kodym, A.; Afza, R. Physical and Chemical Mutagenesis. Methods Mol. Biol. 2003, 236, 189–203. [Google Scholar]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energy J. 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment. Bioresour. Technol. 2017, 235, 366–370. [Google Scholar] [CrossRef]
- Rachmayati, R.; Agustriana, E.; Rahman, D.Y. UV Mutagenesis as a Strategy to Enhance Growth and Lipid Productivity of Chlorella sp. 042. J. Trop. Biodivers. Biotechnol. 2020, 5, 218–227. [Google Scholar] [CrossRef]
- Kumar, A.K. UV mutagenesis treatment for improved production of endoglucanase and β-glucosidase from newly isolated thermotolerant actinomycetes, Streptomyces griseoaurantiacus. Bioresour. Bioprocess. 2015, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Paneerselvam, P.; Venkadesan, G.; Panithasan, M.S.; Alaganathan, G.; Wierzbicki, S.; Mikulski, M. Evaluating the Influence of Cetane Improver Additives on the Outcomes of a Diesel Engine Characteristics Fueled with Peppermint Oil Diesel Blend. Energies 2021, 14, 2786. [Google Scholar] [CrossRef]
- Panithasan, M.S.; Gopalakichenin, D.; Venkadesan, G.; Malairajan, M. Evaluating the working characters of a diesel engine fueled with biodiesel blends added with rice husk Nano particles. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Panithasan, M.S.; Gopalakichenin, D.; Venkadesan, G. Assessing the combined outcome of rice husk nano additive and water injection method on the performance, emission and combustion characters of the low viscous pine oil in a diesel engine. SAE Stud./Young Prof. Tech. Pap. Compet. 2019. [Google Scholar] [CrossRef]
- Yao, R.; Li, M.; Deng, S.; Hu, H.; Wang, H.; Li, F. Mutagenesis of Trichoderma viride by ultraviolet and plasma. Plasma Sci. Technol. 2012, 14, 353–356. [Google Scholar] [CrossRef]
- Choi, T.-O.; Kim, K.-H.; Kim, G.-D.; Choi, T.-J.; Jeon, Y.J. The Evaluation of UV-induced Mutation of the Microalgae, Chlorella vulgaris in Mass Production Systems. J. Life Sci. 2017, 27, 1137–1144. [Google Scholar]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic engineering of microalgal based biofuel production: Prospects and challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- Kanakdande, A.P.; Khobragade, C.N.; Mane, R.S. Ultraviolet-induced random mutagenesis in Bacillus amyloliquefaciens (MF 510169) for improving biodiesel production. Fuel 2021, 304, 121380. [Google Scholar] [CrossRef]
- Huang, G.-H.; Chen, G.; Chen, F. Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenergy 2009, 33, 1386–1392. [Google Scholar] [CrossRef]
- Pick, U.; Rachutin-Zalogin, T. Kinetic anomalies in the interactions of Nile red with microalgae. J. Microbiol. Methods 2012, 88, 189–196. [Google Scholar] [CrossRef]
- Uprety, B.K.; Dalli, S.S.; Rakshit, S.K. Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidiumtoruloides ATCC 10788 capable of growing in the presence of impurities. Energy Convers. Manag. 2017, 1, 117–128. [Google Scholar] [CrossRef]
- Halim, R.; Webley, P.A. Nile Red Staining for Oil Determination in Microalgal Cells: A New Insight through Statistical Modelling. Int. J. Chem. Eng. 2015, 2015, 695061. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods. 2009, 77, 41–47. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Liu, L.; Ao, X.; Ma, L.; Wu, M. Improving cell growth and lipid accumulation in green microalgae Chlorella sp. via UV irradiation. Appl. Biochem. Biotechnol. 2015, 175, 3507–3518. [Google Scholar] [CrossRef]
- Ramadhani, A.P.; Prashantyo, M.H.; Soedarmodjo, T.P.; Widjaja, A. The Effect of UV-B Mutation on Biodiesel from Microalgae Botryococcusbraunii using Esterification, Transesterification, and Combination of Esterification Transesterification. AIP Conf. Proc. 2020, 2217, 030021. [Google Scholar]
- Cheirsilp, B.; Kitcha, S.; Torpee, S. Co-culture of an oleaginous yeast Rhodotorulaglutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann. Microbiol. 2012, 62, 987–993. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of methods to extract and quantify lipids from Synechocystis PCC 6803. Bioresour. Technol. 2011, 102, 1697–1703. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fernandez, C.M.; Ramos, M.J.; Perez, A.; Rodriguez, J.F. Production of biodiesel from winery waste: Extraction refining and transesterification of grape seed oil. Bioresour. Technol. 2010, 101, 7019–7024. [Google Scholar] [CrossRef]
- Bradley, D.; Michael, R.W.; Alex, T.M.; Robert, M.W.; Michael, D.M.; Daniel, J.D.; Dye, D.J.; Bugbee, B.; Byard, D. Wood, and Lance, C. Seefeldt. Biodiesel from Microalgae, Yeast, and Bacteria: Engine Performance and Exhaust Emissions. Energy Fuels 2013, 27, 220–228. [Google Scholar]
- Chen, W.; Sommerfeld, M.; Hu, Q. Microwave-assisted Nile red method for in-vivo quantification of neutral lipids in microalgae. Bioresour. Technol. 2011, 102, 135–141. [Google Scholar] [CrossRef]
- Kuan, I.-C.; Kao, W.-C.; Chen, C.-L.; Yu, C.-Y. Microbial Biodiesel Production by Direct Transesterification of Rhodotorulaglutinis Biomass. Energy J. 2018, 11, 1036. [Google Scholar]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafntseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, A.; Khan, M.R.; Islam, M.A.; Wahid, Z.A.; Pirozzi, D. Technical difficulties, and solutions of direct transesterification process of microbial oil for biodiesel synthesis. Biotechnol. Lett. 2017, 39, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Hoekmana, S.K.; Brocha, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Gohain, M.; Bardhan, P.; Laskar, K.; Sarmah, S.; Mandal, M.; Bora, U.; Kalita, M.C.; Goud, V.V.; Deka, D. Rhodotorula mucilaginosa: A source of heterogeneous catalyst for biodiesel production from yeast single cell oil and waste cooking oil. Renew. Energy 2020, 160, 220–230. [Google Scholar] [CrossRef]
- Arora, N.; Yen, H.-W.; Philippidis, G.P. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 2020, 12, 5125. [Google Scholar] [CrossRef]
- Mohammad, B.; Najafi, H.; Pezeshki, P. Bacterial mutation; Types, mechanisms and mutant detection methods: A review. Eur. Sci. J. 2013, 4, 1857–7881. [Google Scholar]
- Lemmer, K.C.; Zhang, W.; Langer, S.J.; Dohnalkova, A.C.; Hu, D.; Lemke, R.A.; Piotrowski, J.S.; Orr, G.; Noguera, D.R.; Donohue, T.J. Mutations That Alter the Bacterial Cell Envelope Increase Lipid Production. mBio 2017, 8, e00513-17. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.K.; Rai, A.; Kundu, K.; Karmakar, R.; Roy, P.; Chowdhury, A.R. Effect of process parameters for standardization of esterification of cottonseed oil for the production of biodiesel. Int. J. Curr. Sci. 2013, 8, 74–78. [Google Scholar]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, A.A.; Gadela, R.; Bharali, B.; Deshavath, N.N.; Dasu, V.V. Development of high biomass and lipid yielding medium for newly isolated Rhodotorula mucilaginosa. Fuel 2019, 239, 874–885. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Kumar, V.; Nanda, M.; Kumar, S.; Chauhan, P.K. The effects of ultraviolet radiation on growth, biomass, lipid accumulation, and biodiesel properties of microalgae. Energy Sources 2018, 40, 787–793. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Katlekha, P.; Mongkolwatee, W.; Boonrawd, S. Biodiesel production from crude palm oil with a high content of free fatty acids and fuel properties. Chiang Mai Univ. J. Nat. Sci. 2009, 8, 115–124. [Google Scholar]
- Elangovan, T.; Anbarasu, G. Analysis of biodiesel properties from various oil resources and develop relationships among the properties. Asian J. Inf. Technol. 2016, 7, 2658–2664. [Google Scholar]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]







| Bacterial Colonies | Concentration of Lipid (mg/mL) | Fungal Colonies | Concentration of Lipid (mg/mL) |
|---|---|---|---|
| BS1 | 0.28 | FS1 | 0.27 |
| BS2 | 0.58 | FS2 | 0.34 |
| BS3 | 0.04 | FS3 | 0.13 |
| BS4 | 0.39 | FS4 | 0.14 |
| BS5 | 0.27 | FS5 | 0.12 |
| BS6 | 0.14 | FS6 | 0.22 |
| BS7 | 0.09 | FS7 | 0.13 |
| BS8 | 0.21 | FS8 | 0.13 |
| BS9 | 0.14 | FS9 | 0.4 |
| BS10 | 0.13 | FS10 | 0.12 |
| S.No | Relative Fluorescence Unit of UV-Mutated R. mucilaginosa of Different Time Intervals at 540 nm | ||
|---|---|---|---|
| M-30 Strain | M-75 Strain | M-90 Strain | |
| 1 | 0.6 | 1.6 | 0.9 |
| 2 | 0.7 | 1.1 | 1.1 |
| 3 | 1.3 | 0.8 | 0.7 |
| 4 | 1.6 | 0.5 | 1.2 |
| 5 | 0.9 | 1.2 | 0.4 |
| 6 | 1.8 | 0.9 | 0.6 |
| 7 | 0.7 | 0.2 | 0.3 |
| 8 | 3.7 | 1.2 | 0.8 |
| 9 | 0.6 | 1.7 | 0.1 |
| 10 | 0.4 | 1 | 1.1 |
| 11 | 1.1 | 0.9 | 1.4 |
| 12 | 0.3 | 0.6 | 0.6 |
| 13 | 0.9 | 1.4 | 1.6 |
| 14 | 0.4 | 0.1 | 0.9 |
| 15 | 0.8 | 1.3 | 0.5 |
| 16 | 1.4 | 0.8 | 0.8 |
| 17 | 0.31 | 0.22 | 0.2 |
| 18 | 0.4 | 0.7 | 0.6 |
| 19 | 0.47 | 0.6 | 0.1 |
| 20 | 0.81 | 1.1 | 0.7 |
| 21 | 0.5 | 0.3 | 0.2 |
| 22 | 0.7 | 0.4 | 0.5 |
| 23 | 1.3 | 0.9 | 1.1 |
| 24 | 2.1 | 1.8 | 0.4 |
| 25 | 2 | 1.3 | 0.3 |
| 26 | 0.9 | 0.4 | 1 |
| 27 | 0.7 | 0.7 | 0.5 |
| 28 | 0.9 | 1.2 | 0.6 |
| 29 | 0.4 | 0.1 | 0.8 |
| 30 | 1.1 | 0.7 | 0.3 |
| 31 | 0.6 | 0.5 | 0.1 |
| 32 | 1.2 | 0.3 | 0.9 |
| 33 | 0.9 | 0.6 | 0.7 |
| 34 | 1.6 | 1.1 | 0.3 |
| 35 | 1.4 | 0.9 | 1.1 |
| 36 | 1.3 | 0.5 | 0.8 |
| 37 | 0.6 | 0.2 | 0.5 |
| 38 | 0.7 | 0.4 | 0.2 |
| 39 | 0.5 | 1.2 | 0.7 |
| 40 | 0.3 | 0.7 | 0.1 |
| 41 | 0.2 | 0.1 | 0.6 |
| 42 | 0.4 | 0.5 | 0.3 |
| 43 | 0.8 | 0.3 | 0.5 |
| 44 | 1.2 | 1.1 | 1 |
| 45 | 1.4 | 0.9 | 0.8 |
| 46 | 1.6 | 0.6 | 0.9 |
| 47 | 1.3 | 1.1 | 0.2 |
| 48 | 1.1 | 0.5 | 0.7 |
| 49 | 0.4 | 0.3 | 0.1 |
| 50 | 1.2 | 1.1 | 0.6 |
| Micro-Organism | Accession Number | Sequence Length | Percentage Identity |
|---|---|---|---|
| Rhodotorula mucilaginosa VS3 | OL635991 | 536 | 100% |
| Rhodotorula mucilaginosa M30-08 | OL658823 | 538 | 98.52% |
| Rhodotorula mucilaginosa M70-24 | OL658824 | 537 | 98.88% |
| Rhodotorula mucilaginosa M90-14 | OL658825 | 536 | 98.70% |
| Micro-Organism | Production of Biomass (mg/L) | Percentage of Lipids (%) |
|---|---|---|
| Rhodotorula mucilaginosa VS3 | 18.7 | 41.3 |
| Rhodotorula mucilaginosa M30-08 | 62.1 | 68.5 |
| Rhodotorula mucilaginosa M70-24 | 53.2 | 56.3 |
| Rhodotorula mucilaginosa M90-14 | 48.5 | 48.6 |
| Peak No | RT (Min.) | Compound Name | Fatty Acid (%) | ||
|---|---|---|---|---|---|
| VS3 | M30-8 | VS3 | M30-8 | ||
| 1 | 17.58 | 17.72 | Tetra decanoic acid | 5.07 | 11.26 |
| 2 | 19.29 | 19.63 | Pentadecanoic acid | 9.24 | 13.16 |
| 3 | 19.49 | 19.77 | n-hexadecenoic acid | 11.24 | 15.24 |
| 4 | 22.66 | 21.64 | hexadecenoic acid | 6.85 | 9.29 |
| 5 | - | 25.25 | 9-octadecanoic acid | - | 13.58 |
| 6 | - | 26.84 | Oleic acid | - | 6.77 |
| S.No | Properties | Units | Indian Standard | American Standard | Biodiesel | Comparison of Oils | Test Procedure | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VS3 | M30-8 | Jatropha | Peppermint | Pine | ||||||
| 1 | Density | kg/m3 | 860–900 | 860 | 865 | 897 | 890 | 886 | ASTM D4052 | |
| 2 | Specific gravity | 0.86 | 0.88 | |||||||
| 3 | Kinematic viscosity | mm2/s | 2.5–6.0 | 1.9–6.0 | 5.2 | 5.5 | 4.74 | 3.3 | 1.3 | ASTM D445 |
| 4 | Flashpoint | °C | 120 | 150 | 160 | 155 | 135 | 82 | 53 | ASTM D93 |
| 5 | Fire point | °C | 130 | 160 | 165 | 167 | 160 | 166 | 64 | ASTM D93 |
| 6 | Pour point | °C | −2 to 8 | −2 to 6 | −7 | ASTM D2500 | ||||
| 7 | Calorific value | kJ/kg | 38,500 | - | 37,500 | 37,650 | 39,600 | 32,000 | 41,900 | Demirbas, 2008 |
| 8 | Acid value | mg/KOH | 0.50 max | 0.80 max | 0.1 | 0.3 | FFA Titration | |||
| 9 | Cetane number | min | 51 | 47 | 56 | 56 | 54 | 18 | 32 | Krisnangkura, 1986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tensingh, J.A.S.; Shankar, V. Sustainable Production of Biodiesel Using UV Mutagenesis as a Strategy to Enhance the Lipid Productivity in R. mucilaginosa. Sustainability 2022, 14, 9079. https://doi.org/10.3390/su14159079
Tensingh JAS, Shankar V. Sustainable Production of Biodiesel Using UV Mutagenesis as a Strategy to Enhance the Lipid Productivity in R. mucilaginosa. Sustainability. 2022; 14(15):9079. https://doi.org/10.3390/su14159079
Chicago/Turabian StyleTensingh, Joseph Antony Sundarsingh, and Vijayalakshmi Shankar. 2022. "Sustainable Production of Biodiesel Using UV Mutagenesis as a Strategy to Enhance the Lipid Productivity in R. mucilaginosa" Sustainability 14, no. 15: 9079. https://doi.org/10.3390/su14159079
APA StyleTensingh, J. A. S., & Shankar, V. (2022). Sustainable Production of Biodiesel Using UV Mutagenesis as a Strategy to Enhance the Lipid Productivity in R. mucilaginosa. Sustainability, 14(15), 9079. https://doi.org/10.3390/su14159079






