Abstract
Historically, microorganisms have proven to be efficient alternatives for the removal of PCBs, since these contaminants continue to be a major problem for human health and the environment. In this work, the removal of decachlorobiphenyl (PCB-209) was evaluated using native bacterial strains individually and in consortia through biostimulation and bioaugmentation processes. Bacillus sp. DCB13, Staphylococcus sp. DCB28, and Acinetobacter sp. DCB104 were biostimulated in a minimal medium that initially contained biphenyl and later PCB-209 for adaptation as a carbon source. The removal potential of PCB-209 by bacterial strains was evaluated in a bioaugmentation process under aerobic conditions. Using a completely randomized design, ten different treatments were evaluated. Finally, the bacterial growth (CFU/g of soil) and the chemical characteristics of the bioaugmented soil were determined, as was the content of PCB-209 removed by gas chromatography–mass spectrometry. Strains DCB13, DCB28, and DCB104 showed cell growth (>3.4 × 105 CFU/mL) during 120 h of biostimulation, with a marked difference between treatments with biphenyl compared with those where PCB-209 was added. Strains DCB13 and DCB104 (3.4 × 105 CFU/mL and 2.0 × 106 CFU/mL, respectively) grew better with PCB-209, while DCB28 grew better with biphenyl (4.5 × 106 CFU/mL). In bioaugmented soils contaminated with PCB-209, the strains showed maximum growth when inoculated in a consortium (>2.0 × 104 CFU/g). The results showe that the range of the bacterial elimination of PCB-209 in the treatments was from 9.58 to 17.33 mg/kg. The highest elimination potential of PCB-209 was obtained when the bacterial strains were inoculated in a consortium. These findings open a wide perspective for the use of native bacteria for the cleaning and restoration of soils contaminated by toxic chemicals.
1. Introduction
Pollution by xenobiotic compounds due to anthropogenic activities has caused serious damage to the natural environment and toxic effects on human health. Currently, biostimulation and bioaugmentation processes for the bioremediation of contaminants have proven to be the most efficient and used technologies for the elimination of polychlorinated biphenyls (PCBs). During biostimulation, the application of pretreatments with chemical compounds (e.g., biphenyl) and the modification of the physical conditions in the contaminated sites increase the potential of microorganisms to eliminate PCBs. During bioaugmentation, the application of bacteria with the potential to degrade PCBs in contaminated sites promotes the mineralization of toxic compounds, such that they are assimilated by the native microorganisms of the affected site [1]. PCBs are part of the compounds that pollute the environment the most; within their organic structure, there are theoretically 209 congeners, and each congener can vary according to the number and positions of chlorine atoms, thus increasing its lipophilicity and toxicity [2,3]. Decachlorobiphenyl (PCB-209) is the congener with the highest number of chlorines as well as the highest lipophilicity, toxicity, and recalcitrance [4].
In Mexico, the presence and accumulation of PCBs in different aquatic sediments and soils has been reported as an effect of industrial activities or due to the spillage of fluids from electrical equipment that contain this contaminant [5,6]. These sediments are now becoming biologically important areas, as substantial sources of micro-organisms with a biodegradable potential for PCBs. Despite their high toxicity, a great diversity of bacteria has been found living in soils contaminated with PCBs. Some of these bacteria have shown the ability to biodegrade these compounds [7]. In the biodegradation processes of PCBs, the solubilization of these compounds is very important for their elimination, where the presence of biosurfactants produced by bacteria plays a fundamental role [8]. Furthermore, the presence of bacterial enzymes such as dioxygenase, hydrolases, and dehydrogenases is important in defining the potential use of bacteria in the aerobic bioremediation of PCBs [9]. Individual bacterial strains, consortia, and variations in consortium designs are currently being studied and are associated with PCB biodegradation. Therefore, the complexity and variability of the results obtained historically are due to the multiplicity of PCB congeners in contaminated sites, to the variations in nutritional and physical–chemical parameters, to the genetic properties of the strains, and possibly to the production of toxic metabolites during the bioremediation process [10]. For this reason, biostimulation and bioaugmentation processes have gained importance in recent times due to their high efficiency in the elimination of PCBs. The stimulation of a micro-organism to degrade chemical contaminants during its cell growth is known as biostimulation, while bioaugmentation is defined as the process of increasing the amount of micro-organisms that can remove contaminants from soil or water [11]. Therefore, biostimulation and bioaugmentation using indigenous microflora can be good strategies to improve the degradation of PCBs [12].
Consequently, several authors have carried out different investigations associated with the use of bacterial consortia in bioremediation processes, such as bacterial bioaugmentation. Egorova et al. [11] evaluated the biodegradation of different PCB congeners in natural industrial soils using two bacterial strains (Rhodococcus ruber P25 and Microbacterium sp. B51). Likewise, Horváthová et al. [13] evaluated the biodegradation of different PCB congeners using 21 consortia of 4 bacterial strains (Achromobacter xylosoxidans, Stenotrophomonas maltophilia, Ochrobactrum anthropi, and Rhodococcus ruber). Steliga et al. [14] evaluated the individual biodegradation of PCBs from three strains (Mycolicibacterium frederiksbergense IN53, Rhodococcus erythropolis IN129, and Rhodococcus sp. IN306) as well as the biodegradation with mixed cultures in bacterial bioaugmentation processes. The results of the aforementioned study show high percentages of biodegradation for specific PCB congeners in bacterial bioaugmentation processes using contaminated natural soils. In the same way, in previous works, we have contributed to the study of highly efficient alternatives for the elimination and degradation of PCBs in contaminated soils. We examined vermicomposting systems and symbiotic bacteria from the earthworm Eisenia fetida and found that large amounts of PCB-209 could be removed [15]. Likewise, rhizospheric and endophytic bacteria associated with the Ocimun basilicum plant had the ability to remove up to 500 mg/L of PCB-209 [16]. From another study, in agricultural and forest soils contaminated with PCBs, we isolated bacteria classified within the genera Burkholderia, Bacillus, Acinetobacter, Comamonas, and Cupriavidus, which showed high efficiency in removing and degrading PCB-209 as well as Aroclor 1260 (mixture of PCBs) [6]. We recently demonstrated that the native strain Pseudomonas extremaustralis ADA-5 has the biochemical ability to bioaccumulate and biodegrade PCB-209 at concentrations of 250 to 1000 mg/L [17]. We hypothesize that the native bacterial strains Bacillus sp. DCB13, Staphylococcus sp. DCB28, and Acinetobacter sp. DCB104 have the biochemical and genetic capacity to degrade DCB-209.
Therefore, the objectives of this work were (i) to analyze the bioremediation potential of PCB-209 through biostimulation and bioaugmentation processes using native bacterial strains, both individually and in a consortium; (ii) to evaluate bacterial viability through the growth of cells in systems contaminated with PCB-209; and (iii) to correlate the variations in the physical and chemical characteristics as well as the removal of PCB-209 during the process of bacterial bioaugmentation.
2. Materials and Methods
2.1. Chemical Reagents
The chemicals used were analytical grade (>98%). Pentane, organic reagents, biphenyl, and decachlorobiphenyl (PCB-209) were purchased from Sigma-Aldrich® (St. Louis, MO, USA). The medium components were obtained from J.T. Baker Media (Phillipsburg, NJ, USA).
2.2. Soil Samples
The soils used in this study were collected in Chiapas, Mexico. Soil (A), with clay characteristics, was obtained in “La Herradura”, Raudales Malpaso (17.11° N, 93.36° W; 136 masl), from a site near a hydroelectric dam. Forest soil (B) was collected at the ecological reserve “El Ocote”, ubicated in the Ocozocoautla municipality (16.81° N, 93.37° W; 985 masl). Soil (C) was collected in an agricultural plot located in “La Gloria”, in the Suchiapa municipality (16.11° N, 93.06° W; 620 masl). The soils were dried at 80 °C for 24 h, ground, and sieved to 150 mm.
2.3. Bacterial Strains
Bacterial strains with PCB degradation ability, Bacillus sp. DCB13 (MZ544391), Staphylococcus sp. DCB28 (MZ544389), and Acinetobacter sp. DCB104 (MZ544388) (Table 1), were used in this study. The strains were isolated from a historically PCB-contaminated soil (the content of PCBs in terms of Aroclor 1260 mixtures had a final concentration of 100 mg/kg) and identified via 16S rRNA gene sequencing [6]. Complete-genome sequencing of the three bacterial strains was performed in order to detect Bph genes that encode biphenyldioxygenase, which are located in chromosomal and plasmid DNA (data not shown).

Table 1.
Phylogenetic affiliation of bacterial strains isolated from PCB-contaminated soils.
2.4. Master Cell Bank
A master cell bank (MCB) was carried out in order to maintain the viability and quality of the bacterial strains. Pure cultures of the individual strains were cultivated in a minimal medium (2.0 g/L (NH4)2SO4, 1.0 g/L K2HPO4, 0.3 g/L NaCl, 0.3 g/L MgSO4 7H2O, and 0.03 g/L FeSO4 7H2O) to which 200 mg/L of biphenyl was added (as a sole carbon) at 120 rpm using an orbital shaker (Shaker®, SD-300d, Suzhou, China) at 30 °C, according to the methodology recommended by Del Puerto et al. [18]. The bacterial cultures were cryopreserved at −20 °C in microtubes with a binary mixture of glycerol and a minimal medium with biphenyl in a 7:3 (v/v) ratio. The cellular biomass was obtained when the bacterial strains reached the mid-exponential phase (OD600 = 0.8) of their growth.
2.5. Biostimulation of Bacterial Strains in a Liquid Medium Contaminated with PCB
Strains DCB13, DCB28, and DCB104 were biologically stimulated in a liquid medium for their cellular adaptation and to increase the PCB-209 assimilation efficiency. Strains were inoculated individually in a 250 mL Erlenmeyer flask with a cotton wool stopper with 100 mL of a minimal medium composed of 2.0 g/L (NH4)2SO4, 1.0 g/L K2HPO4, 0.3 g/L NaCl, 0.3 g/L MgSO4 7H2O, and 0.03 g/L FeSO4 7H2O, to which 200 mg/L of biphenyl (as a sole carbon) was added in a proportion of 10% (v/v) in constant agitation at 120 rpm using an orbital shaker (Shaker®, SD-300d (Suzhou, China) for 120 h [17]. The biomass produced was used as a preinoculum in flasks with a minimal medium to which 200 mg/L of PCB-209 was added in a proportion of 10% (v/v), keeping it under constant stirring at 120 rpm for 120 h at 30 °C. Bacterial growth was measured via serial dilutions (1 × 10−1 to 1 × 10−9) from 1.0 mL of the medium. Sampling was performed every 6 h, and 0.1 mL of each dilution was inoculated in a solid culture medium and incubated at 30 °C for 36 h in a 50 L Luzeren® (Luzerne, PA, USA) digital incubator. The response variables were cell growth measured in CFU/mL of the medium. All experiments were performed in triplicate.
2.6. Bioaugmentation Assay
The potential for PCB removal by native bacterial strains individually and in consortia was determined through a bioaugmentation assay. For this purpose, a total of 10 treatments with triplicates distributed according to a completely randomized design were evaluated (Table 2). Bioaugmentation was carried out in a 100 mL Erlenmeyer flask that was closed with a cotton stopper. Each flask contained 30 g of a soil mixture (40% soil (A), 30% soil (B), and 30% soil (C)). All soil flasks were sterilized at 121 °C for 30 min every 24 h for three consecutive days (Autoclave vertical Evar®, Tlajomulco de Zuñiga, Mexico). In each flask, the relative humidity was adjusted to 70% with sterilized distilled water. Only flasks with the treatments T1, T2, T3, T4, and CQ were contaminated with 200 mg/kg of PCB-209 using pentane as a solvent (Table 2). Strains DCB13, DCB28, and DCB104 were cultivated aerobically in 100 mL of a minimal culture medium with PCB-209 at 30 °C and 120 rpm (Shaker®, SD-300d, Suzhou, China) until they reached the mid-exponential phase (OD600 = 0.8). For the application of the treatments, two schemes were carried out, as described by Horváthová et al. [13]. In the first scheme, bacterial strains were inoculated individually (treatments T1, T2, and T3). Upon cultivation, bacteria were centrifuged at 3200 rpm for 20 min (Centrifuge Eppendorf® 5811F, Dortmund, Hamburg, Germany), and the biomass suspension was added into the flasks as an inoculum in a concentration of 1.0 g/L. In the second scheme, three bacterial degraders were inoculated in consortia (treatments T4 and C4). The concentration of the added suspension of each strain was 0.33 g/L. The flasks were incubated at 30 °C (50 L Luzeren® digital incubator, Luzerne, PA, USA) in a stationary position for 21 days in the dark.

Table 2.
Treatments evaluated in the bioaugmentation process of PCBs.
2.7. Measurement of Bacterial Growth in the Bioaugmentation Process
Of the soil collected aseptically from each experimental unit, 1.0 g was mixed with 100 mL of distilled water in an Erlenmeyer flask and kept stirring at 120 rpm (Shaker®, SD-300d, Guangzhou, China) for 40 min. Next, 1.0 mL of the supernatant was subjected to serial dilutions (1 × 10−1 to 1 × 10−9). Subsequently, 0.1 mL of each dilution was inoculated into a solid culture medium and incubated at 30 °C (50 L Luzeren® digital incubator, Luzerne, PA, USA) for 36 h. This evaluation was performed on days 0, 3, 6, 9, 12, 15, 18, and 21 of the bioaugmentation process for all of the treatments. Bacterial growth was measured by plate count (CFU/g of soil) [17].
2.8. Physicochemical Characterization of Bioaugmented Soil
The soil characteristics were determined in all of the evaluated treatments at the end of the bioaugmentation process (day 21) to analyze the changes produced between the treatments. The pH and electric conductivity (EC) were measured by using a digital pH meter, a Mettler Toledo® (Columbus, OH, USA) Model S220 (New York, NY, USA), in a 1:10 (weight/volume) aqueous solution [15]. The soil organic matter (SOM) content, total carbon, and C:N ratio were analyzed according to AOAC methods [19]. The total nitrogen was measured by the Kjeldhal method [20]. The total phosphorus was determined with the solubilization method of HNO3/HClO4.
2.9. PCB-209 Quantification in Bioaugmented Soil
To measure the removal potential of PCB-209 in the bioaugmentation processes evaluated, the concentration of PCB-209 in the experiment was measured at an initial time (day 0) and at a final time (day 21) in the treatments that contained the contaminant (T1, T2, T3, T4, and CQ). For this, the soil samples of the evaluated treatments were dried at 80 °C (drying oven, Novatech® HS45-ED, Tlaquepaque, México) for 24 h, and the extraction of the PCB-209 content was carried out as described by Zenteno-Rojas et al. [6]. Briefly, 5.0 g of soil was taken from each treatment and mixed with 25 mL of pentane, mixing in vortex for 5 min and 40 min in ultrasound (Ultrasonic Power, Skymen®, Guangzhou, China). The supernatant was separated by centrifugation at 4000 rpm for 5 min and placed in a 50 mL falcon tube; the procedure was repeated twice. The accumulated pentane was then concentrated using a rotary evaporator and resuspended (Rotovapor digital® RE100-S, Boston, MA, USA) in 1.0 mL of pentane HPLC. The concentration of PCB-209 was determined in an Agilent Technologies 7890 chromatograph coupled with MSD VL 5975 C mass spectrometry (Wilmington, USA) by using the 8270D method [21] described by Villalobos et al. [22].
2.10. Statistic Analysis
The variables in the chemical characterization and concentration of PCB-209 were analyzed by ANOVA at a significance level of alpha = 0.05 by using the statistical software Statgraphics XV.2. The comparison of means was carried out by the Tukey test (p < 0.05). Positive and negative variations between the physicochemical parameters, PCB-209 content, and bacterial growth between the treatments were correlated by principal component analysis (PCA) by using Minitab v. 18.1.
3. Results
3.1. Biostimulation of Bacterial Strains
The strains Acinetobacter sp. DCB104, Staphylococcus sp. DCB28, and Bacillus sp. DCB13 showed cell growth during the 120 h of biostimulation with a marked difference between the biphenyl treatments with respect to those added with PCB-209 as a carbon source. With respect to the DCB104 and DCB13 strains, they showed better growth with PCB-209 (3.4 × 105 ± 0.000051 CFU/mL and 2.0 × 106 ± 0.000053 CFU/mL, respectively), unlike the DCB28 strain, which showed higher growth with biphenyl as a carbon source (4.5 × 106 ± 0.000042) CFU/mL (Figure 1).
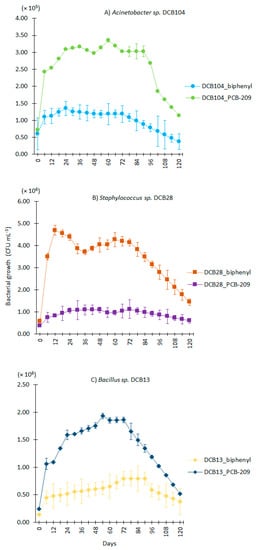
Figure 1.
Bacterial growth of native strains evaluated in biostimulation processes in a liquid medium to which biphenyl and decachlorobiphenyl (PCB-209) were added. (A) Acinetobacter sp. DCB104, (B) Staphylococcus sp. DCB28, and (C) Bacillus sp. DCB13.
3.2. Bioaugmentation of PCB-Contaminated Soil by Individual Bacterial Strains or in Consortia
The growth of the strains DCB13, DCB28, and DCB104 during the bioaugmentation process showed some positive effects due to the presence of PCB-209, specifically with the DCB104 strain, where a remarkable difference was observed between treatment T1, with PCB-209 (>1.5 × 104 ± 0.0011 CFU/g of soil), and treatment C1 (biological control), with 0.8 × 104 ± 0.0001 CFU/g of soil, while the maximum growth between all of the treatments, >2.0 × 104 CFU/g of soil, was observed in treatment T2 with the DCB28 strain and treatment T4 with bacterial consortia (DCB13, DCB128, and DCB104) (Figure 2).
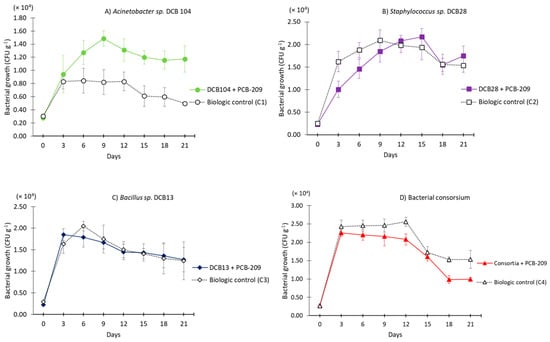
Figure 2.
Bacterial growth with different treatments evaluated in the bioaugmentation process contaminated with PCB-209 and biological controls (without PCB-209) using individual native strains and in consortia. (A) Acinetobacter sp. DCB104, (B) Staphylococcus sp. DCB28, (C) Bacillus sp. DCB13, and (D) bacterial consortium.
3.3. Physicochemical Characteristics of Bioaugmented Soil
The physicochemical characteristics of soil at day 21 of the bioaugmentation process are shown in Table 3. The physicochemical parameters of the different treatments showed significant differences between strain types, mainly related to the presence of the contaminant (p < 0.05). The registered pH values were slightly acidic (5.9–6.8), while the electrolytic conductivity (<1 dS m−1) did not show significant differences in any of the treated soils. With respect to the soil organic matter (SOM), significant differences (p < 0.05) were shown between the treatments evaluated. The soil organic matter (SOM) content was higher in the soils of bacterial consortia (DCB104, DCB28, and DCB13) contaminated with decachlorobiphenyl, PCB-209, compared with the rest of the treatments. The uninoculated and without-PCB-209 soil had a higher phosphorus content value (5.6 mg/kg) compared with the other treatments. The total carbon content ranged from 7.1 to 9.1 mg/kg, while the total N ranged from 0.57 to 0.9 mg/kg. The C:N ratio is considered an important parameter related to soil fertility. In our study, the soil inoculated with the strain Bacillus sp. DCB13 had a higher C:N ratio (16.4) compared with the soils inoculated with other strains and contaminated with PCBs.

Table 3.
Physicochemical characteristics at the final time of the bioaugmentation process in soil contaminated with decachlorobiphenyl, PCB-209.
3.4. PCB-209 Removal Potential by Bacterial Strains in the Bioaugmentation Process
The removal potential of PCB-209 was measured in treatments T1 to T4, calculating it with respect to CQ (Table 4). In this study, a material balance was carried out by identifying and quantifying the remaining amounts of PCB-209 in the evaluated treatments. In Figure 3, the GC-MS chromatograms are shown with the identification of PCB-209 with a retention time of 14.23 min (Figure 3). The identification was carried out at the final time (day 21) of the bioaugmentation process, and PCB-209 was quantified by using a standard reagent with the concentrations determined on standard curves. The results show that the elimination range of PCB-209 was from 9.58 to 17.33 mg/kg in the evaluated treatments. The most efficient treatment was T4 (10.51%), formulated with a bacterial consortium (DCB13, DCB128, and DCB104 strains) (Table 2). On the other hand, no significant differences were observed between the treatments formulated with individual strains (T1 to T3) (Table 4). Additionally, the presence of cometabolites, such as 1,3 bis-(1,1 dimethyl ethyl) benzene and benzoates, which can be products of the biodegradation of PCB-209, was detected by gas chromatography.

Table 4.
Removed potential of PCB-209 by individual native strains and consortia in bioaugmentation processes.
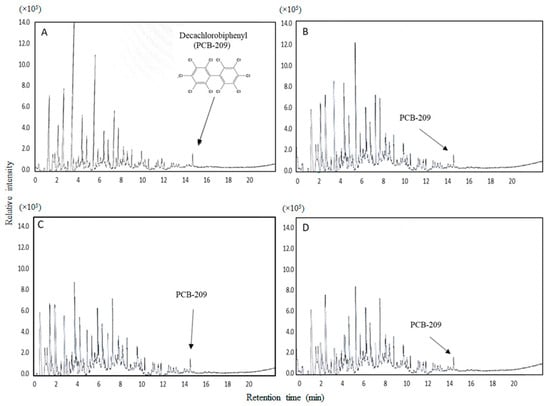
Figure 3.
Chromatograms of decachlorobiphenyl (PCB-209) detected during the bacterial bioaugmentation process in the treatments evaluated. (A) Acinetobacter sp. DCB104, (B) Staphylococcus sp. DCB28, (C) Bacillus sp. DCB13, and (D) bacterial consortium (DCB108 + DCB28 + DCB13).
3.5. Principal Component Analysis (PCA)
The principal component analysis (PCA), considering the variations in the physicochemical characteristics and the removal of PCB-209 in the treatments T1 to T4 and C1 to C4, clearly separated the treatments (Figure 4). The CN, T1, T2, T3, and T4 treatments were characterized by a positive PC1 in a proportion that explained 39.9% of the variation, while the other treatments (C1, C2, C3, C4, and CQ) were of a negative PC1 (Figure 4). The PCA also separated some of the chemical characteristics (C/N ratio, total P, and SOM) that positively influenced the CN, T2, and T4 treatments in a proportion that explained PC2 with 35.2%, while the total C and total N contents showed a negative effect on the other treatments (C1, C2, C3, C4, T1, and T3).
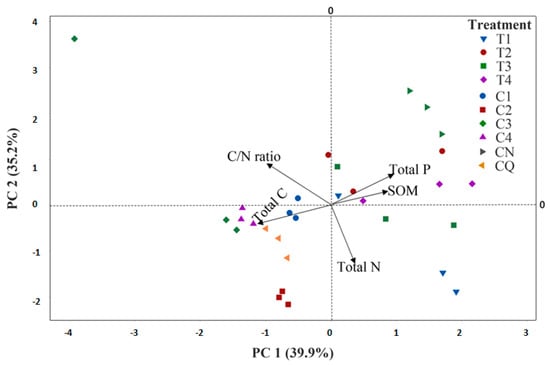
Figure 4.
Principal component analysis considering the changes in the characteristics of the soils and the removal variations in PCB-209 in the bioaugmented treatments.
4. Discussion
The bacterial strains within the genera Acinetobacter, Staphylococcus, and Bacillus that were analyzed in this study have been previously identified in PCB biodegradation processes due to their ability to adapt and tolerate PCBs, their genetic characteristics, and their potential to promote biodegradation processes [6,23]. They were selected for their potential to grow and assimilate biphenyl as a carbon source (Table 1). In this study, biphenyl was used as a biostimulant inducer for the selection of strains and to improve the biodegradability potential of PCB-209 (Figure 1), taking into account the components of the medium reported by Gómez et al. [17]. The results show notable increases in bacterial growth for the PCB-209 treatments with Acinetobacter sp. DCB104 and Bacillus sp. DCB13 compared with Staphylococcus sp. DCB28, which maintained higher growth with biphenyl as a carbon source (Figure 1). However, the bacterial growth in all of the treatments was not higher than that reported by Gómez et al. [17], with the Pseudomonas extremaustralis ADA-5 strain with 2.0 × 108 CFU/mL of a medium. The above can be described as a process of prior bacterial adaptability to the bioremediation process [1]. The biphenyl molecule acts as a stimulant in the assimilation of PCB-209 for the degradation of the PCB carbonic structure, since it is of vital importance in aerobic biostimulation processes for bioavailability, which, in turn, accelerates the biodegradation of these compounds [24,25]. According to this, one of the previous procedures for PCB biodegradation has been adaptability strategies and the biostimulation of native bacteria to improve the biodegradability potential. Egorova et al. [26] used biphenyl molecules as a carbon source in enrichment cultures, obtaining increases in the biodegradation potential of some PCB congeners. Several microcosm studies of soils contaminated with a known PCB mix have revealed that this component can be biodegraded significantly, particularly when modified with biphenyl and oxygen and inoculated with PCB-degrading bacteria [27,28,29]. Bacteria induced for PCB biodegradation procedures regularly exceed the biomass of indigenous organisms in contaminated sites due to their potential for assimilation to the contaminant [12] and the appropriate content of available microbial and mineral nutrients, such that they should be analyzed during the biostimulation process [30]. Similarly, Chun et al. [31] evaluated the biostimulation of autochthonous micro-organisms through electrical potential to provide electron donors and acceptors in the degradation of PCBs, finding substantial increases between 40 and 60% degradation. Bhattacharya et al. [32] induced cells of the PCB-degrading strain Burkholderia xenovorans LB400 with 4-chlorobiphenyl, reaching up to 98% degradation, and Sánchez-Pérez et al. [16] demonstrated the importance of adapting bacterial strains to culture media enriched with phenol and PCBs to increase the assimilation capacity of PCB-209. In bioaugmentation processes, the biodegradation rate is improved, increasing the biomass of the degrading bacteria [27,33]. However, it is important to consider the viability of the bacterial strains during the process. In Figure 2, we show the growth dynamics of the three strains used in the different treatments during the bioaugmentation process (Table 2), where the presence of PCB-209 did not affect the exponential growth of the bioaugmented strains. In the study carried out by Saavedra et al. [34], increases of up to two orders of magnitude during the first 15 days of bioaugmentation in model systems for PCB mineralization using the strains Cupriavidus necator JMS34 and Burkholderia xenovorans LB400 were observed. In another investigation, using Rhodococcus ruber P25 and Microbacterium sp. B51, increases of up to three orders were obtained by calculating the 1.7–1.8 × 108 CFU/g of soil [11]. Likewise, Su et al. [35] studied Castellaniella sp. SPC4, and it showed high efficiency in the degradation of PCB-77 (3,3′, 4,4′-tetrachlorobiphenyl), with a maximum growth rate in PCB-77 of 2.6 × 107 CFU/mL of a medium, indicating an excellent potential for PCB bioremediation. Regarding the variations in the physicochemical characteristics evaluated in the bioaugmentation processes, the pH varied significantly between the experimental units. In the units treated with bacteria plus the contaminant, PCB-209, the pH was slightly acidic (6.3), and in those treated with the bacterial consortium plus PCB, the pH decreased to 5.9. This tendency towards strong acidity can be attributed to mineralization processes by bacteria using carbon from soil organic matter or using PCB as an alternative carbon source [31]. It is known that when the carbon content is optimal, there are good conditions for capturing other necessary nutrients, such as phosphorus and nitrogen, and through its chemical form it can promote the development of the bacteria involved [12]. On the other hand, organic matter is often a substantial source of energy and fertility in soils [36], and the elements involved in bacterial development are the C/N/P ratio [37]. According to the results obtained, the maximum variations were in the C content, N content, and P content (Table 3). The maximum C content was in treatment C4 (DCB104, DCB28, and DCB13), with the bacterial consortium, while the maximum N content was obtained with treatment C2 (strain DCB28). Delgado-Baquerizo et al. [37] mentioned that a soil with a high P content can support high bacterial diversity because C and N are relatively easy to obtain from the atmosphere for some microbial communities, but for carbon assimilation to occur in a microbial biomass, N must also be assimilated in amounts determined by the microbial biomass C/N ratio [38]. The results obtained in the C/N ratio are highly variable in the treatments evaluated, without a specific pattern, where the nutrient ratio in both of the soils was totally unbalanced, although the C/N ratio was higher in the bioaugmentation treatments with strain DCB13. In addition, the results obtained from the chromatographic analyses (Figure 3) show that the removal degree of PCB-209 increases (10.51%) with treatment T4 through the application of the bacterial consortium, compared with the treatments with individual strains, where the highest removal potential of PCB-209 in treatment T3 (Bacillus sp. DCB13) was 6.66% (Table 4). The bacterial genera, Acinetobacter, Staphylococcus, and Bacillus, analyzed in this study have been studied in contaminant bioremediation processes. The genus Acinetobacter has been identified as a representative and dominant genus in the biodegradation processes of soils contaminated with PCBs and other toxic compounds [39,40]. Meanwhile, the genus Staphylococcus has been little studied for its potential in PCB biodegradation processes, but there are reports that demonstrate its degradation capability and its application in the bioremediation of hydrocarbons and other contaminants [41,42,43,44]. The genus Bacillus has been one of the most representative, and it has been identified in sites contaminated with PCBs and hydrocarbons [45,46]. Bacillus has been reported by several authors to be a genus with significant capacities for the tolerance and assimilation of these compounds [47,48], while Bacillus subtilis has been extensively studied based on the biological mechanisms involved in the metabolism of PCBs and the genes involved [49].
Principal component analysis (PCA) allowed the establishment of a relationship between the evaluated treatments and the variations in the soil chemical characteristics during the PCB-209 removal process (Figure 4). Treatments T1, T2, and T3, formulated with bacterial strains (Acinetobacter sp. DCB104, Staphylococcus sp. DCB28, and Bacillus sp. DCB13, respectively), as well as the bacterial consortium (treatment T4), showed a positive effect with the removal of PCB-209 in PC1. Acinetobacter has been studied as a strain with the ability to degrade available PCBs in contaminated soils and for its genetic capabilities [39]. Similarly, Leaes et al. [50] reported the potential of Staphylococcus xylosus to degrade 0.01 ppm of several polychlorinated biphenyl congeners (PCB 10, 28, 52, 138, 153, and 180) during 168 h of incubation in a liquid medium, while Bacillus sp. has demonstrated capabilities for the biodegradation of PCBs—up to 80% of the xenobiotic in 96 h [46]. Treatment T4 (consortium) was positively related, according to PC1, with the C/N ratio, total P, and SOM. These soil parameters were the most important during the bioaugmentation due to carbon and nitrogen availability in the production of bacterial biomass. The results demonstrate the potential of native bacterial strains to tolerate and remove decachlorobiphenyl (PCB-209), as well as the potential biodegradation of the contaminant in the bioaugmentation process. It has been evaluated that, during the aerobic degradation of PCBs, the splitting of the biphenyl ring occurs, activating several genes that are mainly groups of bph (BphA, BphB, and BphC) [3]. Bph genes are involved in PCB transformation through dihydrodiol, biphenyl-2,3-dihydrodiol, 2,3-dihydroxybiphenyl, and phenylcatechol. The gene complementation by the bacteria involved in the consortium allows the sequenced biodegradation of PCB [51]; these genes were identified in this study. Therefore, treatment T4, through the bacterial consortium, obtained the greatest effect in the removal of PCB-209. The most relevant physicochemical factors in the PCB-209 bioaugmentation processes are the phosphorus content, the C content, and the N content [37]. It is known that carbon content is very important for the identification of pollutants in soils, such as hydrocarbons, but also as a source of energy for bacterial consortia in bioaugmentation processes. Nitrogen content, as a micronutrient, is an essential element in proteins, amino acids, enzymes, and the cell wall. As well as nitrogen, phosphorus is an essential nutrient for bacteria, and can become limiting, since it is a component of nucleic acids and phospholipids that influence the assimilation and biodegradation of PCBs [12]. Therefore, due to the variations in the factors studied in the bioaugmentation process, the identification of the bacterial genes involved in the process through bacterial consortia becomes an important factor to understand the processes of PCB-209 removal and biodegradation, as well as their relationship with physicochemical changes in the biostimulation and bacterial bioaugmentation processes.
5. Conclusions
In this study, the potential for the bioremediation of soils contaminated with decachlorobiphenyl (PCB-209) by native bacterial strains through biostimulation and bioaugmentation processes was evaluated under in vitro conditions. During biostimulation, the strains showed significant differences when they grew in the presence of highly chlorinated compounds. The medium contaminated with decachlorobiphenyl (PCB-209) better supported the growth of the strains Bacillus sp. DCB13 and Staphylococcus sp. DCB28, whereas with biphenyl, Acinetobacter sp. DCB104 showed higher growth. Based on the factors mentioned above, we infer that these bacteria have the biochemical and genetic machinery with which to use PCB-209 or biphenyl as a carbon source. In bioaugmentation, the strains showed differences when grown in the presence of PCB-209, individually or in consortia. An increased cell growth (>1.4 × 104 CFU/kg of soil) was recorded by the DCB104 strain when it grows alone, and when growing in a consortium together with the DCB13 and DCB28 strains, they reach a growth of >2.0 × 104 CFU/kg of soil. The highest removal potential of PCB-209 was given when the bacterial strains were inoculated in a consortium. These findings open a wide perspective for using native bacteria to clean and restore soils contaminated by recalcitrant toxic chemicals.
Author Contributions
Designed the study, R.R.-R.; performed laboratory experiments, A.Z.-R., R.I.C.R., J.J.V.M. and C.I.R.-M.; contributed new reagents and analytical tools, L.A.M.-G., V.M.R.-V. and J.J.V.M.; performed the data analysis, F.A.R.-M.; wrote the manuscript, E.M.-R., A.Z.-R. and R.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was from Tecnologico Nacional de México, no. 14094.22-P.
Data Availability Statement
Not applicable.
Acknowledgments
We thank CONACYT for granting a scholarship to Adalberto Zenteno Rojas and Daniel Castañeda Valbuena for technical assistance. We thank Clara I. Rincón for reading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, J.K.; Gautam, R.K.; Nanekar, S.V.; Weber, R.; Singh, B.K.; Singh, S.K.; Juwarkar, A.A. Advances and perspective in bioremediation of polychlorinated biphenyl-contaminated soils. Environ. Sci. Pollut. Res. Int. 2018, 25, 16355–16375. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Teng, Y.; Luo, Y.; Li, X.; Sun, X.; Li, Z.; Liu, W.; Christie, P. Potential for biodegradation of polychlorinated biphenyls (PCBs) by Sinorhizobium meliloti. J. Hazard. Mater. 2011, 186, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Passatore, L.; Rossetti, S.; Juwarkar, A.A.; Massacci, A. Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge and research perspectives. J. Hazard. Mater. 2014, 278, 189–202. [Google Scholar] [CrossRef]
- Huo, S.; Li, C.; Xi, B.; Yu, Z.; Yeager, K.M.; Wu, F. Historical record of polychlorinated biphenyls (PCBs) and special occurrence of PCB 209 in a shallow fresh-water lake from eastern China. Chemosphere 2017, 184, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Piazza, R.; Ruiz-Fernández, A.C.; Frignani, M.; Zangrando, R.; Bellucci, L.G.; Moret, I.; Páez-Osuna, F. PCBs and PAHs in surficial sediments from aquatic environments of Mexico City and the coastal states of Sonora, Sinaloa, Oaxaca and Veracruz (Mexico). Environ. Geol. 2008, 54, 1537–1545. [Google Scholar] [CrossRef]
- Zenteno-Rojas, A.; Martínez-Romero, E.; Castañeda-Valbuena, D.; Rincón-Molina, C.I.; Ruiz-Valdiviezo, V.M.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Vences-Guzmán, M.A.; Rincón-Rosales, R. Structure and diversity of native bacterial communities in soils contaminated with polychlorinated biphenyls. AMB Express 2020, 10, 124. [Google Scholar] [CrossRef]
- Luo, W.; D’Angelo, E.M.; Coyne, M. Plant secondary metabolites, biphenyl, and hydroxypropyl-beta-cyclodextrin effects on aerobic polychlorinated biphenyl removal and microbial community structure in soils. Soil Biol. Biochem. 2007, 39, 735–743. [Google Scholar] [CrossRef]
- Pathiraja, G.; Egodawatta, P.; Goonetilleke, A.; Téo, V.S.J. Solubilization and degradation of polychlorinated biphenyls (PCBs) by naturally occurring facultative anaerobic bacteria. Sci. Total Environ. 2019, 651, 2197–2207. [Google Scholar] [CrossRef]
- Khalid, F.; Hashmi, M.Z.; Jamil, N.; Abdul, Q.; Muhammad, I.A. Microbial and enzymatic degradation of PCBs from e-waste-contaminated sites: A review. Environ. Sci. Pollut. Res. 2021, 28, 10474–10487. [Google Scholar] [CrossRef]
- Xiang, Y.; Xing, Z.; Liu, J.; Wei, Q.; Xing, H. Recent advances in the biodegradation of polychlorinated biphenyls. World J. Microbiol. Biotechnol. 2020, 36, 145. [Google Scholar] [CrossRef]
- Egorova, D.O.; Demakov, V.A.; Plotnikova, E.G. Bioaugmentation of a polychlorobiphenyl contaminated soil with two aerobic bacterial strains. J. Hazard. Mater. 2013, 261, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-González, E.; Guevara-García, M.A.; García-Mena, J.; Ovando-Medina, V.M. Microbial diversity assessment of polychlorinated biphenyl–contaminated soils and the biostimulation and bioaugmentation processes. Environ. Monit. Assess. 2019, 191, 118. [Google Scholar] [CrossRef] [PubMed]
- Horváthová, H.; Lászlová, K.; Dercová, K. Bioremediation of PCB contaminated shallow river sediments: The efficacy of biodegradation using individual bacterial strains and their consortia. Chemosphere 2018, 193, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Steliga, T.; Wojtowicz, K.; Kapusta, P.; Brzeszcz, J. Assessment of Biodegradation Efficiency of Polychlorinated Biphenyls (PCBs) and Petroleum Hydrocarbons (TPH) in Soil Using Three Individual Bacterial Strains and Their Mixed Culture. Molecules 2020, 25, 709. [Google Scholar] [CrossRef] [Green Version]
- Zenteno-Rojas, A.; Martínez-Romero, E.; Rincón-Molina, C.I.; Ruiz-Valdiviezo, V.M.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Rincón-Rosales, R. Removal of High Concentrations Decachlorobiphenyl of Earthworm Eisenia fetida and its Symbiotic Bacteria in a Vermicomposting System. Water Air Soil Pollut. 2019, 230, 116. [Google Scholar] [CrossRef]
- Sánchez-Pérez, B.N.; Zenteno-Rojas, A.; Rincón-Molina, C.I.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A.; Vences-Guzmán, M.A.; Villalobos-Maldonado, J.J.; Rincón-Rosales, R. Rhizosphere and Endophytic Bacteria Associated to Ocimum basilicum L. with Decaclorobiphenyl Removal Potential. Water Air Soil Pollut. 2020, 231, 134. [Google Scholar] [CrossRef]
- Gómez, L.M.A.; Zenteno-Rojas, A.; Martinez-Romero, E.; Rincón-Molina, C.I.; Vences-Guzmán, M.A.; Ruíz-Valdiviezo, V.M.; Rincón-Molina, F.A.; Manzano-Gómez, L.A.; Rincón-Rosales, R. Biodegradation and Bioaccumulation of Decachlorobiphenyl (DCB) by Native Strain Pseudomonas extremaustralis ADA-5. Water Air Soil Pollut. 2021, 232, 192. [Google Scholar]
- Del Puerto, C.A.; Iglesias, E.; Morales, T.; Baños, N.; Nocedo, M.D.; Carnota, G.; Martínez, R. Organización y manejo de la colección de cepas de referencia del Instituto Finlay. Vaccimonitor 2009, 18, 20–24. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC Interna: Gaithersburg, MD, USA, 1996; Volume 1. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America Inc.; American Society of Agronomy Inc.: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- USEPA. United States Environmental Protection Agency (USEPA). Method 8270D. Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). Available online: http://www.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/8270d.pdf (accessed on 13 October 2013).
- Villalobos-Maldonado, J.J.; Meza-Gordillo, R.; Mancilla-Margalli, N.A.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Vázquez-Villegas, P.; Gutiérrez-Miceli, F.A.; Ruiz-Valdiviezo, V.M. Removal of decachlorobiphenyl in vermicomposting process amended with rabbit manure and peat moss. Water Air Soil Pollut. 2015, 226, 159. [Google Scholar] [CrossRef]
- Vergani, L.; Mapelli, F.; Suman, J.; Cajthaml, T.; Uhlik, O.; Borin, S. Novel PCB-degrading Rhodococcus strains able to promote plant growth for assisted rhizoremediation of historically polluted soils. PLoS ONE 2019, 14, e0221253. [Google Scholar] [CrossRef] [Green Version]
- Leewis, M.C.; Uhlik, O.; Leigh, M. Synergistic Processing of Biphenyl and Benzoate: Carbon Flow Through the Bacterial Community in Polychlorinated-Biphenyl-Contaminated Soil. Sci. Rep. 2016, 6, 22145. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Golyshin, P.; Timmis, K. Novel maltotriose esters enhance biodegradation of Aroclor 1242 by Burkholderia cepacia LB400. World J. Microbiol. Biotechnol. 2003, 19, 637–643. [Google Scholar] [CrossRef]
- Egorova, D.O.; Kiryanova, T.; Pyankova, A.; Ananina, L.; Plotnikova, E. Selective pressure of biphenyl/polychlorinated biphenyls on the formation of aerobic bacterial associations and their biodegradative potential. Folia Microbiol. 2021, 66, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Bako, C.M.; Mattes, T.E.; Marek, R.F.; Hornbuckle, K.C.; Schnoor, J.L. Biodegradation of PCB congeners by Paraburkholderia xenovorans LB400 in presence and absence of sediment during lab bioreactor experiments. Environ. Pollut. 2021, 271, 116364. [Google Scholar] [CrossRef]
- Chen, S.C.; Budhraja, R.; Adrian, L.; Calebrese, F.; Stryhanyuk, H.; Musat, N.; Richnow, H.H.; Duan, G.L.; Zhu, Y.G.; Musat, F. Novel clades of soil biphenyl degraders revealed by integrating isotope probing, multi-omics, and single-cell analyses. ISME J. 2021, 15, 3508–3521. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Microbial transformation and degradation of polychlorinated biphenyls. Environ. Pollut. 2008, 155, 1–12. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Mohseni-Bandpi, A.; Esrafili, A.; Nasseri, S.; Ashmagh, F.R.; Jorfi, S.; Ja’fari, M. Effectiveness of biostimulation through nutrient content on the bioremediation of phenanthrene contaminated soil. J. Environ. Health Sci. Eng. 2014, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Chun, C.L.; Payne, R.B.; Sowers, K.R.; May, H.D. Electrical stimulation of microbial PCB degradation in sediment. Water Res. 2013, 47, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.S.; Khare, K. Biodegradation of 4-chlorobiphenyl by using induced cells and cell extract of Burkholderia xenovorans. Bioremediation J. 2017, 21, 109–118. [Google Scholar] [CrossRef]
- Bedard, D.L. A case study for microbial biodegradation: Anaerobic bacterial reductive dechlorination of polychlorinated biphenyls-from sediment to defined medium. Annu. Rev. Microbiol. 2008, 62, 253–270. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Acevedo, F.; González, M.; Seeger, M. Mineralization of PCBs by the genetically modified strain Cupriavidus necator JMS34 and its application for bioremediation of PCBs in soil. Appl. Microbiol. Biotechnol. 2010, 87, 1543–1554. [Google Scholar] [CrossRef]
- Su, X.; Li, S.; Cai, J.; Xiao, Y.; Tao, L.; Hashmi, M.Z.; Lin, H.; Chen, J.; Mei, R.; Sun, F. Aerobic degradation of 3,3′,4,4′-tetrachlorobiphenyl by a resuscitated strain Castellaniella sp. SPC4: Kinetics model and pathway for biodegradation. Sci. Total Environ. 2019, 688, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.; Reich, P.; Jeffries, T.; Singh, B. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Mera, E.; García-Paredes, J.D.; Corrales-Paternina, A.; Torregroza-Espinosa, A.C. Variability of Nitrogen Mineralization from Organic Matter in Agricultural Soils in the North of Colombia. Chiang Mai Univ. J. Nat. Sci. 2021, 20, e2021073. [Google Scholar]
- Gorovtsov, A.V.; Sazykin, I.S.; Sazykina, M.A. The influence of heavy metals, polyaromatic hydrocarbons, and polychlorinated biphenyls pollution on the development of antibiotic resistance in soils. Environ. Sci. Pollut. Res. 2018, 25, 9283–9292. [Google Scholar] [CrossRef] [PubMed]
- Méndez, V.; Fuentes, S.; Morgante, V.; Hernández, M.; González, M.; Moore, E.; Seeger, M. Novel hydrocarbonoclastic metal-tolerant Acinetobacter and Pseudomonas strains from Aconcagua river oil-polluted soil. J. Soil Sci. Plant Nutr. 2017, 17, 1074–1087. [Google Scholar] [CrossRef] [Green Version]
- Vaishnavi, J.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Selvi, A.; Srinivasan, P.; Govarthanan, M. Biosurfactant mediated bioelectrokinetic remediation of diesel contaminated environment. Chemosphere 2021, 264, 128337. [Google Scholar] [CrossRef]
- Polak, L.; Demšar, L.; Kirinčič, S.; Kozolc, B.; Polak, T. Degradation of PCBs in liquid media: Effects of commercial meat starters. J. Food Sci. Technol. 2016, 65, 1087–1092. [Google Scholar] [CrossRef]
- Chang-Hyun, C.; Jaeyoon, L.; Bong-Gun, K.; Sung-Kuk, K.; Jong-Soo, C. Staphylococcus sp. KW-07 contains nahH gene encoding catechol 2,3-dioxygenase for phenanthrene degradation and a test in soil microcosm. Int. Biodeterior Biodegrad. 2011, 65, 198–203. [Google Scholar] [CrossRef]
- Eddouaouda, K.; Mnif, S.; Badis, A.; Younes, S.B.; Cherif, S.; Ferhat, S.; Mhiri, N.; Chamkha, M.; Sayadi, S. Characterization of a novel biosurfactant produced by Staphylococcus sp. strain 1E with potential application on hydrocarbon bioremediation. J. Basic Microbiol. 2012, 4, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Naeim, A.H.; Baharlouei, J.; Ataabadi, M. Biochemical tests to determine the biodegradability potential of bacterial strains in PAH polluted sites. World J. Microbiol. Biotechnol. 2020, 36, 181. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Sharifi, H.; Ghobadi, N.Z.; Yaghmaei, S. Biodegradation of Polychlorinated Biphenyls by Lysinibacillus macrolides and Bacillus firmus Isolated from Contaminated Soil. Int. J. Eng. 2019, 32, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Masika, W.S.; Moonsamy, G.; Mandree, P.; Ramchuran, S.; Lalloo, R.; Kudanga, T. Biodegradation of petroleum hydrocarbon waste using consortia of Bacillus sp. Bioremediat. J. 2020, 25, 72–79. [Google Scholar] [CrossRef]
- Shimura, M.; Mukerjee-Dhar, G.; Kimbara, K.; Nagato, H.; Kiyohara, H.; Hatta, T. Isolation and characterization of a thermophilic Bacillus sp. JF8 capable of degrading polychlorinated biphenyls and naphthalene. FEMS Microbiol. Lett. 1999, 178, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Pan, L.; Zhu, L. Formation of hydroxylated and methoxylated polychlorinated biphenyls by Bacillus subtilis: New insights into microbial metabolism. Sci. Total Environ. 2018, 613–614, 54–61. [Google Scholar] [CrossRef]
- Leaes, F.L.; Daniel, A.P.; Mello, G.B.; Battisti, V.; Bogusz, S., Jr.; Emanuelli, T.; Fries, L.L.M.; Costabeber, I. Degradation of polychlorinated biphenyls (PCBs) by Staphylococcus xylosus in liquid media and meat mixture. Food Chem. Toxicol. 2006, 44, 847–854. [Google Scholar] [CrossRef]
- Jing, R.; Fusi, S.; Kjellerup, B.V. Remediation of Polychlorinated Biphenyls (PCBs) in Contaminated Soils and Sediment: State of Knowledge and Perspectives. Front. Environ. Sci. 2018, 6, 2296–2665X. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).