Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Identifying the Research Question
2.2. Identifying the Relevant Studies
2.3. Selecting the Studies According to Inclusion Criteria
2.4. Charting and Interpreting Data
2.5. Collating, Summarising, and Reporting Results
3. Results
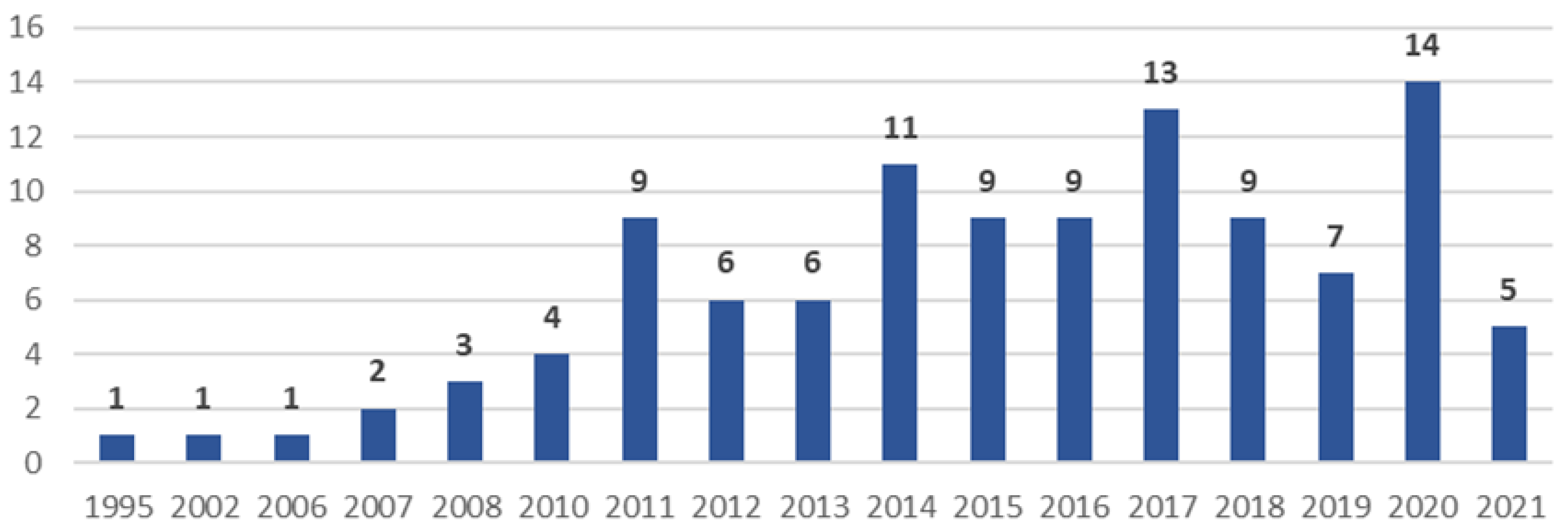
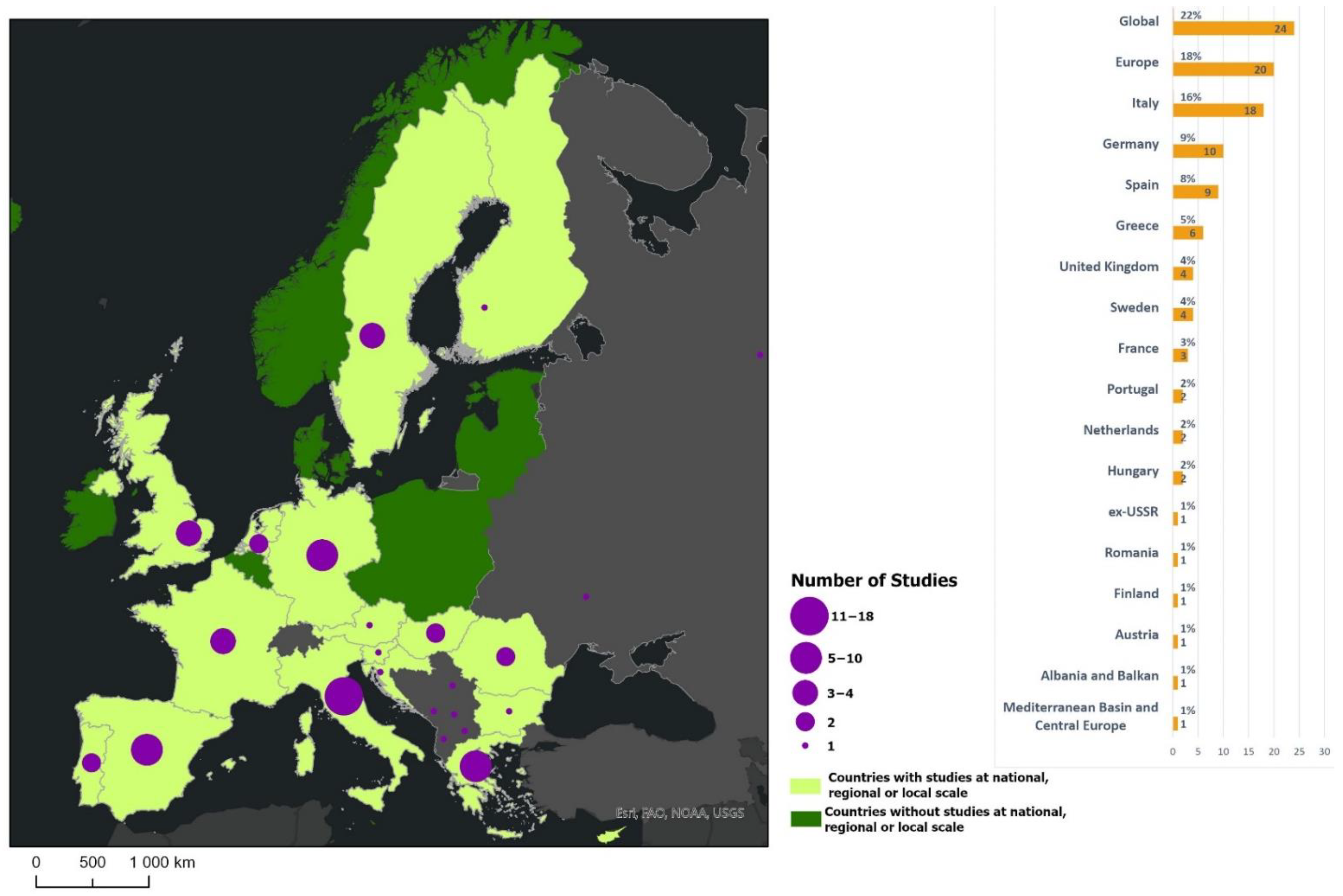
3.1. General Characteristics
3.2. Studied Vectors and Infections/Diseases
3.3. Data Used, Type of Data, Geographical Extent, and Spatial Scale
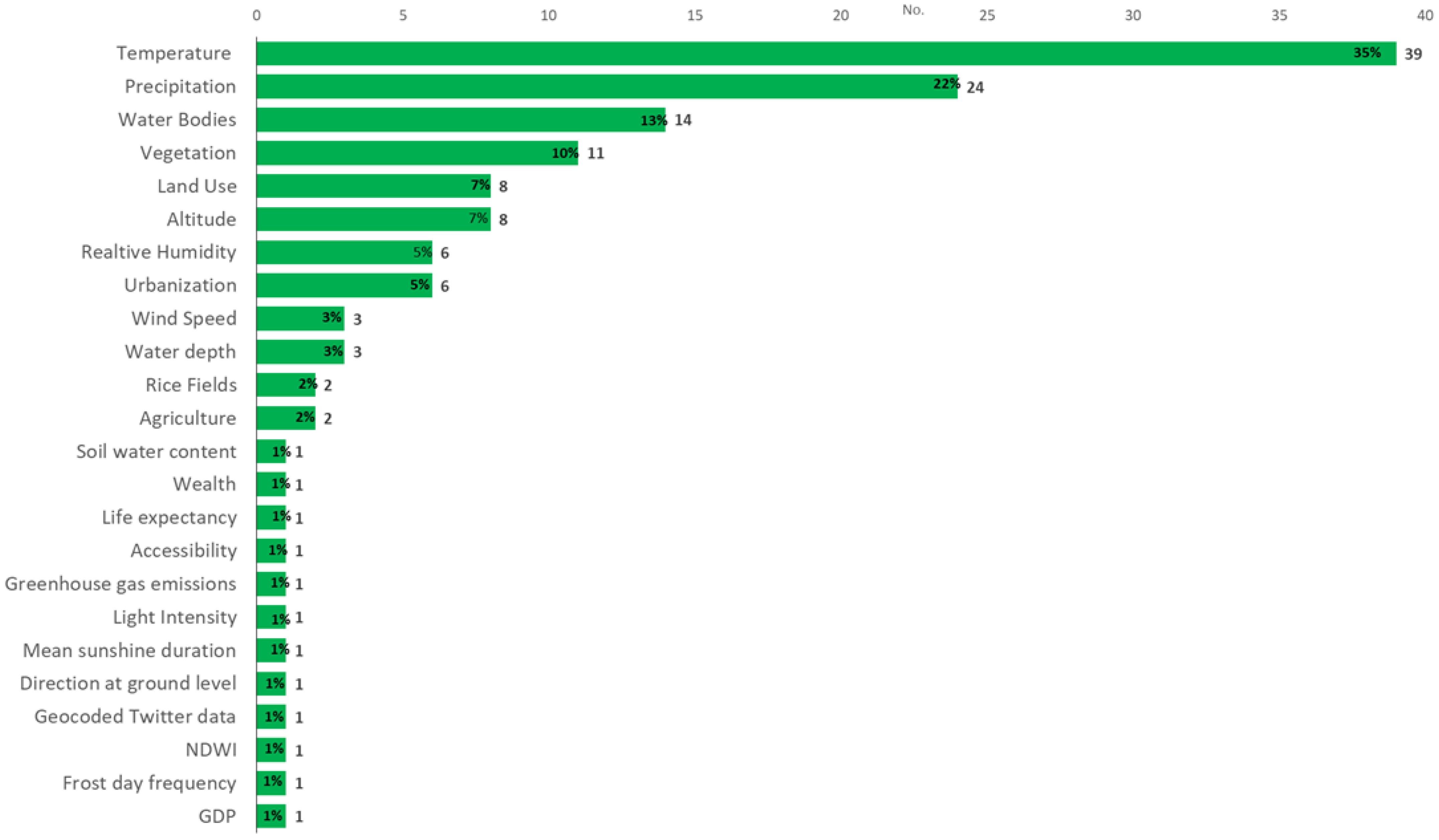
3.4. Studied Biotic and Abiotic Factors
3.5. Spatial Analysis Methods
3.6. Disease Mapping
3.7. Clusters, Clustering, and Surveillance
3.8. Geographic Correlation Studies
4. Discussion
4.1. Strengths and Limitations
4.2. Evidence Gaps and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- INSA. Doenças Associadas a Artrópodes Vetores e Roedores; Núncio, M.S., Alves, M.J., Eds.; Instituto Nacional de Saúde Doutor Ricardo Jorge, IP-Departamento de Doenças Infeciosas—Centro de Estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac: Lisboa, Portugal, 2019. [Google Scholar]
- INSA. Rede de Vigilância de Vetores—REVIVE. 2020. Available online: http://www.insa.min-saude.pt/category/areas-de-atuacao/doencas-infeciosas/revive-rede-de-vigilancia-de-vetores/ (accessed on 5 July 2021).
- Beckham, J.D.; Tyler, K.L. Arbovirus Infections (Minneap Minn). Contin. Lifelong Learn. Neurol. 2015, 21, 1599–1611. [Google Scholar] [CrossRef]
- WHO. Vector-Borne Diseases. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 10 July 2021).
- Floore, T.G. Mosquito Larval Control Practices: Past and Present. J. Am. Mosq. Control Assoc. 2006, 22, 527–533. [Google Scholar] [CrossRef]
- WHO. A Global Brief on Vector-Borne Diseases. 2014, p. 56. Available online: https://apps.who.int/iris/handle/10665/111008. (accessed on 7 July 2021).
- Almeida, A.P.G.D. Os mosquitos em Portugal século XX. Acta Med. Port. 2011, 24, 961–974. [Google Scholar]
- Rossi, G.; Karki, S.; Smith, R.L.; Brown, W.M.; Ruiz, M.O. The spread of mosquito-borne viruses in modern times: A spatio-temporal analysis of dengue and chikungunya. Spat. Spatio-Temporal Epidemiol. 2018, 26, 113–125. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Mosquito-Borne Diseases: An Emerging Threat; ECDC: Stockholm, Sweden, 2016. [Google Scholar]
- European Centre for Disease Prevention and Control. Organisation of Vector Surveillance and Control in Europe; ECDC: Stockholm, Sweden, 2021. [Google Scholar] [CrossRef]
- Balcan, D.; Colizza, V.; Gonçalves, B.; Hu, H.; Ramasco, J.J.; Vespignani, A. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc. Natl. Acad. Sci. USA 2009, 106, 21484–21489. [Google Scholar] [CrossRef]
- Malone, J.B.; Bergquist, R.; Martins, M.; Luvall, J.C. Use of Geospatial Surveillance and Response Systems for Vector-Borne Diseases in the Elimination Phase. Trop. Med. Infect. Dis. 2019, 4, 15. [Google Scholar] [CrossRef]
- Freitas, M.I.C.; Cunha, L. Cartografia da vulnerabilidade socioambiental: Convergências e divergências a partir de algumas experiências em Portugal e no Brasil. URBE-Rev. Bras. De Gestão Urbana 2013, 5, 15–31. [Google Scholar] [CrossRef]
- Beard, R.; Wentz, E.; Scotch, M. A systematic review of spatial decision support systems in public health informatics supporting the identification of high risk areas for zoonotic disease outbreaks. Int. J. Health Geogr. 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Whiteman, A.; Casas, I.; Delmelle, E. Space-time clusters and co-occurrence of chikungunya and dengue fever in Colombia from 2015 to 2016. Acta Trop. 2018, 185, 77–85. [Google Scholar] [CrossRef]
- Eisen, L.; Lozano-Fuentes, S. Use of Mapping and Spatial and Space-Time Modeling Approaches in Operational Control of Aedes aegypti and Dengue. PLoS Negl. Trop. Dis. 2009, 3, e411. [Google Scholar] [CrossRef]
- De Oliveira, M.A.; Ribeiro, H.; Castillo-Salgado, C. Geospatial analysis applied to epidemiological studies of dengue: A systematic review. Rev. Bras. De Epidemiol. 2013, 16, 907–917. [Google Scholar] [CrossRef][Green Version]
- Aswi, A.; Cramb, S.; Moraga, P.; Mengersen, K. Bayesian spatial and spatio-temporal approaches to modelling dengue fever: A systematic review. Epidemiol. Infect. 2018, 147, e33. [Google Scholar] [CrossRef] [PubMed]
- Louis, V.R.; Phalkey, R.; Horstick, O.; Ratanawong, P.; Wilder-Smith, A.; Tozan, Y.; Dambach, P. Modeling tools for dengue risk mapping—A systematic review. Int. J. Health Geogr. 2014, 13, 50. [Google Scholar] [CrossRef]
- Odhiambo, J.N.; Kalinda, C.; Macharia, P.M.; Snow, R.W.; Sartorius, B. Spatial and spatio-temporal methods for mapping malaria risk: A systematic review. BMJ Glob. Health 2020, 5, e002919. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- The EndNote Team. EndNote; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Porta, M. A Dictionary of Epidemiology, 5th ed.; Oxford University Press (OUP): New York, NY, USA, 2008. [Google Scholar]
- Elliott, P.; Wartenberg, D. Spatial Epidemiology: Current Approaches and Future Challenges. Environ. Health Perspect. 2004, 112, 998–1006. [Google Scholar] [CrossRef]
- Andreo, V.; Izquierdo-Verdiguier, E.; Zurita-Milla, R.; Rosà, R.; Rizzoli, A.; Papa, A. Identifying Favorable Spatio-Temporal Conditions for West Nile Virus Outbreaks by Co-Clustering of Modis LST Indices Time Series. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 4670–4673. [Google Scholar]
- Caputo, B.; Russo, G.; Manica, M.; Vairo, F.; Poletti, P.; Guzzetta, G.; Merler, S.; Scagnolari, C.; Solimini, A. A comparative analysis of the 2007 and 2017 Italian chikungunya outbreaks and implication for public health response. PLoS Negl. Trop. Dis. 2020, 14, e0008159. [Google Scholar] [CrossRef]
- Bouzid, M.; Colón-González, F.J.; Lung, T.; Lake, I.R.; Hunter, P.R. Climate change and the emergence of vector-borne diseases in Europe: Case study of dengue fever. BMC Public Health 2014, 14, 781. [Google Scholar] [CrossRef]
- Manica, M.; Filipponi, F.; D’Alessandro, A.; Screti, A.; Neteler, M.; Rosà, R.; Solimini, A.; della Torre, A.; Caputo, B. Spatial and Temporal Hot Spots of Aedes albopictus Abundance inside and outside a South European Metropolitan Area. PLoS Negl. Trop. Dis. 2016, 10, e0004758. [Google Scholar] [CrossRef] [PubMed]
- Pergantas, P.; Tsatsaris, A.; Malesios, C.; Kriparakou, G.; Demiris, N.; Tselentis, Y. A spatial predictive model for malaria resurgence in central Greece integrating entomological, environmental and social data. PLoS ONE 2017, 12, e0178836. [Google Scholar] [CrossRef]
- Chen, T.T.; Ljungqvist, F.C.; Castenbrandt, H.; Hildebrandt, F.; Ingholt, M.M.; Hesson, J.C.; Ankarklev, J.; Seftigen, K.; Linderholm, H.W. The spatiotemporal distribution of historical malaria cases in Sweden: A climatic perspective. Malar. J. 2021, 20, 212. [Google Scholar] [CrossRef]
- Tatem, A.J.; Noor, A.M.; Von Hagen, C.; Di Gregorio, A.; Hay, S. High Resolution Population Maps for Low Income Nations: Combining Land Cover and Census in East Africa. PLoS ONE 2007, 2, e1298. [Google Scholar] [CrossRef]
- Gething, P.W.; Van Boeckel, T.P.; Smith, D.L.; Guerra, C.A.; Patil, A.P.; Snow, R.W.; Hay, S.I. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasites Vectors 2011, 4, 92. [Google Scholar] [CrossRef]
- Bauer, N.; Kenyeres, Z.; Tóth, S.; Sáringer-Kenyeres, T.; Sáringer, G. Connections between the habitat pattern and the pattern of the mosquito larval assemblages. Biologia 2011, 66, 877–885. [Google Scholar] [CrossRef]
- Albieri, A.; Carrieri, M.; Angelini, P.; Baldacchini, F.; Venturelli, C.; Mascali Zeo, S.; Bellini, R. Quantitative monitoring of Aedes albopictus in Emilia-Romagna, Northern Italy: Cluster investigation and geostatistical analysis. Bull. Insectology 2010, 63, 209–216. [Google Scholar]
- Attaway, D.F.; Waters, N.M.; Geraghty, E.M.; Jacobsen, K.H. Zika virus: Endemic and epidemic ranges of Aedes mosquito transmission. J. Infect. Public Health 2016, 10, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Marcantonio, M.; Manica, M.; Marini, G.; Zorer, R.; Delucchi, L.; Arnoldi, D.; Montarsi, F.; Capelli, G.; Rizzoli, A.; et al. Mapping of Aedes albopictus Abundance at a Local Scale in Italy. Remote Sens. 2017, 9, 749. [Google Scholar] [CrossRef]
- Battle, K.E.; Karhunen, M.S.; Bhatt, S.; Gething, P.W.; Howes, R.E.; Golding, N.; Van Boeckel, T.P.; Messina, J.P.; Shanks, G.D.; Smith, D.L.; et al. Geographical variation in Plasmodium vivax relapse. Malar. J. 2014, 13, 144. [Google Scholar] [CrossRef]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Caputo, B.; Manica, M.; Filipponi, F.; Blangiardo, M.; Cobre, P.; Delucchi, L.; De Marco, C.M.; Iesu, L.; Morano, P.; Petrella, V.; et al. ZanzaMapp: A Scalable Citizen Science Tool to Monitor Perception of Mosquito Abundance and Nuisance in Italy and Beyond. Int. J. Environ. Res. Public Health 2020, 17, 7872. [Google Scholar] [CrossRef]
- Cibulskis, R.E.; Aregawi, M.; Williams, R.; Otten, M.; Dye, C. Worldwide Incidence of Malaria in 2009: Estimates, Time Trends, and a Critique of Methods. PLoS Med. 2011, 8, e1001142. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Candeloro, L.; Ippoliti, C.; Monaco, F.; De Massis, F.; Bruno, R.; Di Sabatino, D.; Danzetta, M.L.; Benjelloun, A.; Belkadi, B.; et al. Spatio-Temporal Identification of Areas Suitable for West Nile Disease in the Mediterranean Basin and Central Europe. PLoS ONE 2015, 10, e0146024. [Google Scholar] [CrossRef] [PubMed]
- Durand, B.; Tran, A.; Balança, G.; Chevalier, V. Geographic variations of the bird-borne structural risk of West Nile virus circulation in Europe. PLoS ONE 2017, 12, e0185962. [Google Scholar] [CrossRef]
- Esser, H.J.; Liefting, Y.; Ibáñez-Justicia, A.; van der Jeugd, H.; van Turnhout, C.A.M.; Stroo, A.; Reusken, C.B.E.M.; Koopmans, M.P.G.; de Boer, W.F. Spatial risk analysis for the introduction and circulation of six arboviruses in the Netherlands. Parasites Vectors 2020, 13, 464. [Google Scholar] [CrossRef]
- Gomes, E.; Capinha, C.; Rocha, J.; Sousa, C. Mapping Risk of Malaria Transmission in Mainland Portugal Using a Mathematical Modelling Approach. PLoS ONE 2016, 11, e0164788. [Google Scholar] [CrossRef]
- Holy, M.; Schmidt, G.; Schröder, W. Potential malaria outbreak in Germany due to climate warming: Risk modelling based on temperature measurements and regional climate models. Environ. Sci. Pollut. Res. 2010, 18, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Justicia, A.; Cianci, D. Modelling the spatial distribution of the nuisance mosquito species Anopheles plumbeus (Diptera: Culicidae) in the Netherlands. Parasites Vectors 2015, 8, 258. [Google Scholar] [CrossRef]
- Kerkow, A.; Wieland, R.; Früh, L.; Hölker, F.; Jeschke, J.M.; Werner, D.; Kampen, H. Can data from native mosquitoes support determining invasive species habitats? Modelling the climatic niche of Aedes japonicus japonicus (Diptera, Culicidae) in Germany. Parasitol. Res. 2019, 119, 31–42. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Nsoesie, E.O.; Kraemer, M.U.; Golding, N.; Pigott, D.M.; Brady, O.J.; Moyes, C.; Johansson, M.; Gething, P.; Velayudhan, R.; Khan, K.; et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Eurosurveillance 2016, 21, 30234. [Google Scholar] [CrossRef]
- Rogers, D.J.; Suk, J.E.; Semenza, J.C. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014, 129, 1–14. [Google Scholar] [CrossRef]
- Samy, A.M.; Thomas, S.M.; Wahed, A.A.; Cohoon, K.P.; Peterson, A.T. Mapping the global geographic potential of Zika virus spread. Mem. Inst. Oswaldo Cruz 2016, 111, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, F.; Martínez-López, B.; Sánchez-Vizcaíno, J.M. Identification of suitable areas for the occurrence of Rift Valley fever outbreaks in Spain using a multiple criteria decision framework. Veter. Microbiol. 2013, 165, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, A.; Amela, C.; Fernández-Carrión, E.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M.; Sierra-Moros, M.J. Risk mapping of West Nile virus circulation in Spain, 2015. Acta Trop. 2017, 169, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Schröder, W.; Pesch, R.; Schmidt, G. Statistical classification of terrestrial and marine ecosystems for environmental planning. Landsc. Online 2007, 2, 1–22. [Google Scholar] [CrossRef]
- Schröder, W.; Schmidt, G. Spatial modelling of the potential temperature-dependent transmission of vector-associated diseases in the face of climate change: Main results and recommendations from a pilot study in Lower Saxony (Germany). Parasitol. Res. 2008, 103, 55–63. [Google Scholar] [CrossRef]
- Schröder, W.; Schmidt, G. Mapping the potential temperature-dependent tertian malaria transmission within the ecoregions of Lower Saxony (Germany). Int. J. Med. Microbiol. 2008, 298, 38–49. [Google Scholar] [CrossRef]
- Simons, R.R.L.; Croft, S.; Rees, E.; Tearne, O.; Arnold, M.E.; Johnson, N. Using species distribution models to predict potential hot-spots for Rift Valley Fever establishment in the United Kingdom. PLoS ONE 2019, 14, e0225250. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef]
- Tran, A.; Ippoliti, C.; Balenghien, T.; Conte, A.; Gely, M.; Calistri, P.; Goffredo, M.; Baldet, T.; Chevalier, V. A Geographical Information System-Based Multicriteria Evaluation to Map Areas at Risk for Rift Valley Fever Vector-Borne Transmission in Italy. Transbound. Emerg. Dis. 2013, 60, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Ponçon, N.; Toty, C.; Linard, C.; Guis, H.; Ferré, J.-B.; Seen, D.L.; Roger, F.; De La Rocque, S.; Fontenille, D.; et al. Using remote sensing to map larval and adult populations of Anopheles hyrcanus (Diptera: Culicidae) a potential malaria vector in Southern France. Int. J. Health Geogr. 2008, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Sudre, B.; Paz, S.; Rossi, M.; Desbrosse, A.; Chevalier, V.; Semenza, J.C. Environmental predictors of West Nile fever risk in Europe. Int. J. Health Geogr. 2014, 13, 26. [Google Scholar] [CrossRef]
- Bisanzio, D.; Giacobini, M.; Bertolotti, L.; Mosca, A.; Balbo, L.; Kitron, U.; Vazquez-Prokopec, G.M. Spatio-temporal patterns of distribution of West Nile virus vectors in eastern Piedmont Region, Italy. Parasites Vectors 2011, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef]
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial Capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Quam, M.; Wilder-Smith, A.; Stenlund, H.; Ebi, K.; Massad, E.; Rocklöv, J. Climate Change and Aedes Vectors: 21st Century Projections for Dengue Transmission in Europe. eBioMedicine 2016, 7, 267–277. [Google Scholar] [CrossRef]
- Messina, J.P.; Kraemer, M.U.; Brady, O.J.; Pigott, D.M.; Shearer, F.M.; Weiss, D.J.; Golding, N.; Ruktanonchai, C.W.; Gething, P.W.; Cohn, E.; et al. Mapping global environmental suitability for Zika virus. eLife 2016, 5, e15272. [Google Scholar] [CrossRef]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2018, 13, e0210122. [Google Scholar] [CrossRef]
- Thomas, S.M.; Tjaden, N.B.; Frank, C.; Jaeschke, A.; Zipfel, L.; Wagner-Wiening, C.; Faber, M.; Beierkuhnlein, C.; Stark, K. Areas with High Hazard Potential for Autochthonous Transmission of Aedes albopictus-Associated Arboviruses in Germany. Int. J. Environ. Res. Public Health 2018, 15, 1270. [Google Scholar] [CrossRef]
- Battle, K.E.; Lucas, T.C.D.; Nguyen, M.; Howes, R.E.; Nandi, A.K.; Twohig, K.A.; Pfeffer, D.A.; Cameron, E.; Rao, P.C.; Casey, D.; et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–2017: A spatial and temporal modelling study. Lancet 2019, 394, 332–343. [Google Scholar] [CrossRef]
- Ippoliti, C.; Candeloro, L.; Gilbert, M.; Goffredo, M.; Mancini, G.; Curci, G.; Falasca, S.; Tora, S.; Di Lorenzo, A.; Quaglia, M.; et al. Defining ecological regions in Italy based on a multivariate clustering approach: A first step towards a targeted vector borne disease surveillance. PLoS ONE 2019, 14, e0219072. [Google Scholar] [CrossRef] [PubMed]
- Valiakos, G.; Papaspyropoulos, K.; Giannakopoulos, A.; Birtsas, P.; Tsiodras, S.; Hutchings, M.R.; Spyrou, V.; Pervanidou, D.; Athanasiou, L.V.; Papadopoulos, N.; et al. Use of Wild Bird Surveillance, Human Case Data and GIS Spatial Analysis for Predicting Spatial Distributions of West Nile Virus in Greece. PLoS ONE 2014, 9, e96935. [Google Scholar] [CrossRef]
- Broderick, C.; Friend, P.; Smith, V.; Blaze, M.; Gothard, P.; Chiodini, P.L.; Whitty, C.J. Geographical concentration of falciparum malaria treated in the UK and delay to treatment with artesunate in severe cases: An observational study. BMJ Open 2012, 2, e001854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durand, B.; Lecollinet, S.; Beck, C.; Martínez-López, B.; Balenghien, T.; Chevalier, V. Identification of Hotspots in the European Union for the Introduction of Four Zoonotic Arboviroses by Live Animal Trade. PLoS ONE 2013, 8, e70000. [Google Scholar] [CrossRef]
- García-Carrasco, J.-M.; Muñoz, A.-R.; Olivero, J.; Segura, M.; Real, R. Predicting the spatio-temporal spread of West Nile virus in Europe. PLoS Negl. Trop. Dis. 2021, 15, e0009022. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.M.; Fajardo, D.; Waller, S.T.; Wang, O.; Sarkar, S. A Predictive Spatial Model to Quantify the Risk of Air-Travel-Associated Dengue Importation into the United States and Europe. J. Trop. Med. 2012, 2012, 103679. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Vairo, F.; Mammone, A.; Lanini, S.; Poletti, P.; Manica, M.; Rosa, R.; Caputo, B.; Solimini, A.; Della Torre, A.; et al. Spatial modes for transmission of chikungunya virus during a large chikungunya outbreak in Italy: A modeling analysis. BMC Med. 2020, 18, 226. [Google Scholar] [CrossRef]
- Millet, J.-P.; Montalvo, T.; Bueno-Marí, R.; Romero, I.M.; Uribe, A.P.; Fernández, I.; Camprubí, E.; Del Baño, L.; Peracho, V.; Figuerola, J.; et al. Imported Zika Virus in a European City: How to Prevent Local Transmission? Front. Microbiol. 2017, 8, 1319. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Aguilar-Alba, M.; Vetter, M.; García-Barrón, L.; Morales, J. Spatiotemporal Distribution of Malaria in Spain in a Global Change Context. Atmosphere 2020, 11, 346. [Google Scholar] [CrossRef]
- Tatem, A.J.; Jia, P.; Ordanovich, D.; Falkner, M.; Huang, Z.; Howes, R.; Hay, S.I.; Gething, P.W.; Smith, D.L. The geography of imported malaria to non-endemic countries: A meta-analysis of nationally reported statistics. Lancet Infect. Dis. 2016, 17, 98–107. [Google Scholar] [CrossRef]
- Sainz-Elipe, S.; Latorre, J.M.; Escosa, R.; Masià, M.; Fuentes, M.V.; Mas-Coma, S.; Bargues, M.D. Malaria resurgence risk in southern Europe: Climate assessment in an historically endemic area of rice fields at the Mediterranean shore of Spain. Malar. J. 2010, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Thomas, S.M.; Suk, J.E.; Sudre, B.; Hess, A.; Tjaden, N.B.; Beierkuhnlein, C.; Semenza, J.C. Climate change effects on Chikungunya transmission in Europe: Geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 2013, 12, 51. [Google Scholar] [CrossRef]
- Desvars-Larrive, A.; Liu, X.; Hjertqvist, M.; Sjöstedt, A.; Johansson, A.; Rydén, P. High-risk regions and outbreak modelling of tularemia in humans. Epidemiol. Infect. 2016, 145, 482–490. [Google Scholar] [CrossRef]
- Sousa, A.; García-Barrón, L.; Vetter, M.; Morales, J. The Historical Distribution of Main Malaria Foci in Spain as Related to Water Bodies. Int. J. Environ. Res. Public Health 2014, 11, 7896–7917. [Google Scholar] [CrossRef]
- Sudre, B.; Rossi, M.; Van Bortel, W.; Danis, K.; Baka, A.; Vakalis, N.; Semenza, J.C. Mapping Environmental Suitability for Malaria Transmission, Greece. Emerg. Infect. Dis. 2013, 19, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Schulte, C.; Behrens, R.; Grobusch, M.P.; Coulaud, J.P.; Bisoffi, Z.; Matteelli, A.; Clerinx, J.; Corachan, M.; Puente, S.; et al. Imported Falciparum Malaria in Europe: Sentinel Surveillance Data from the European Network on Surveillance of Imported Infectious Diseases. Clin. Infect. Dis. 2002, 34, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, F.; Roiz, D.; De Valk, H.; Noël, H.; L’Ambert, G.; Franke, F.; Paty, M.-C.; Guinard, A.; Desenclos, J.-C.; Roche, B. From importation to autochthonous transmission: Drivers of chikungunya and dengue emergence in a temperate area. PLoS Negl. Trop. Dis. 2020, 14, e0008320. [Google Scholar] [CrossRef] [PubMed]
- Rocklöv, J.; Quam, M.B.; Sudre, B.; German, M.; Kraemer, M.U.; Brady, O.; Bogoch, I.I.; Liu-Helmersson, J.; Wilder-Smith, A.; Semenza, J.C.; et al. Assessing Seasonal Risks for the Introduction and Mosquito-borne Spread of Zika Virus in Europe. eBioMedicine 2016, 9, 250–256. [Google Scholar] [CrossRef]
- Salami, D.; Capinha, C.; Martins, M.D.R.O.; Sousa, C.A. Dengue importation into Europe: A network connectivity-based approach. PLoS ONE 2020, 15, e0230274. [Google Scholar] [CrossRef]
- Semenza, J.C.; Sudre, B.; Miniota, J.; Rossi, M.; Hu, W.; Kossowsky, D.; Suk, J.E.; Van Bortel, W.; Khan, K. International Dispersal of Dengue through Air Travel: Importation Risk for Europe. PLoS Negl. Trop. Dis. 2014, 8, e3278. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Calzolari, M.; Bonilauri, P.; Bellini, R.; Albieri, A.; Defilippo, F.; Maioli, G.; Galletti, G.; Gelati, A.; Barbieri, I.; Tamba, M.; et al. Evidence of Simultaneous Circulation of West Nile and Usutu Viruses in Mosquitoes Sampled in Emilia-Romagna Region (Italy) in 2009. PLoS ONE 2010, 5, e14324. [Google Scholar] [CrossRef]
- Calzolari, M.; Pautasso, A.; Montarsi, F.; Albieri, A.; Bellini, R.; Bonilauri, P.; Defilippo, F.; Lelli, D.; Moreno, A.; Chiari, M.; et al. West Nile Virus Surveillance in 2013 via Mosquito Screening in Northern Italy and the Influence of Weather on Virus Circulation. PLoS ONE 2015, 10, e0140915. [Google Scholar] [CrossRef]
- Golding, N.; Nunn, M.A.; Purse, B.V. Identifying biotic interactions which drive the spatial distribution of a mosquito community. Parasites Vectors 2015, 8, 367. [Google Scholar] [CrossRef]
- Manica, M.; Riello, S.; Scagnolari, C.; Caputo, B. Spatio-Temporal Distribution of Aedes Albopictus and Culex Pipiens along an Urban-Natural Gradient in the Ventotene Island, Italy. Int. J. Environ. Res. Public Health 2020, 17, 8300. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ruiz, L.; Megía-Palma, R.; Reguera, S.; Ruiz, S.; Zamora-Camacho, F.J.; Figuerola, J.; Moreno-Rueda, G. Opposed elevational variation in prevalence and intensity of endoparasites and their vectors in a lizard. Curr. Zool. 2018, 64, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dörge, D.D.; Cunze, S.; Schleifenbaum, H.; Zaenker, S.; Klimpel, S. An investigation of hibernating members from the Culex pipiens complex (Diptera, Culicidae) in subterranean habitats of central Germany. Sci. Rep. 2020, 10, 10276. [Google Scholar] [CrossRef]
- Marini, F.; Caputo, B.; Pombi, M.; Travaglio, M.; Montarsi, F.; Drago, A.; Rosà, R.; Manica, M.; Della Torre, A. Estimating Spatio-Temporal Dynamics of Aedes Albopictus Dispersal to Guide Control Interventions in Case of Exotic Arboviruses in Temperate Regions. Sci. Rep. 2019, 9, 10281. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Neteler, M.; Castellani, C.; Arnoldi, D.; Rizzoli, A. Climatic Factors Driving Invasion of the Tiger Mosquito (Aedes albopictus) into New Areas of Trentino, Northern Italy. PLoS ONE 2011, 6, e14800. [Google Scholar] [CrossRef] [PubMed]
- Tisseuil, C.; Velo, E.; Bino, S.; Kadriaj, P.; Mersini, K.; Shukullari, A.; Simaku, A.; Rogozi, E.; Caputo, B.; Ducheyne, E.; et al. Forecasting the spatial and seasonal dynamic of Aedes albopictus oviposition activity in Albania and Balkan countries. PLoS Negl. Trop. Dis. 2018, 12, e0006236. [Google Scholar] [CrossRef]
- Kartashev, V.; Afonin, A.; González-Miguel, J.; Sepúlveda, R.; Simón, L.; Morchón, R.; Simón, F. Regional Warming and Emerging Vector-Borne Zoonotic Dirofilariosis in the Russian Federation, Ukraine, and Other Post-Soviet States from 1981 to 2011 and Projection by 2030. BioMed Res. Int. 2014, 2014, 858936. [Google Scholar] [CrossRef]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosà, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the Environmental Conditions Favouring West Nile Virus Outbreaks in Europe. PLoS ONE 2015, 10, e0121158. [Google Scholar] [CrossRef]
- Moirano, G.; Richiardi, L.; Calzolari, M.; Merletti, F.; Maule, M. Recent rapid changes in the spatio-temporal distribution of West Nile Neuro-invasive Disease in Italy. Zoonoses Public Health 2019, 67, 54–61. [Google Scholar] [CrossRef]
- Candeloro, L.; Ippoliti, C.; Iapaolo, F.; Monaco, F.; Morelli, D.; Cuccu, R.; Fronte, P.; Calderara, S.; Vincenzi, S.; Porrello, A.; et al. Predicting WNV Circulation in Italy Using Earth Observation Data and Extreme Gradient Boosting Model. Remote Sens. 2020, 12, 3064. [Google Scholar] [CrossRef]
- Rotejanaprasert, C.; Lawson, A.; Rossow, H.; Sane, J.; Huitu, O.; Henttonen, H.; Vilas, V.J.D.R. Towards integrated surveillance of zoonoses: Spatiotemporal joint modeling of rodent population data and human tularemia cases in Finland. BMC Med. Res. Methodol. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Samy, A.; Elaagip, A.H.; Kenawy, M.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D. Climate Change Influences on the Global Potential Distribution of the Mosquito Culex quinquefasciatus, Vector of West Nile Virus and Lymphatic Filariasis. PLoS ONE 2016, 11, e0163863. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Mulatti, P.; Mazzucato, M.; Montarsi, F.; Ciocchetta, S.; Capelli, G.; Bonfanti, L.; Marangon, S. Retrospective space–time analysis methods to support West Nile virus surveillance activities. Epidemiol. Infect. 2014, 143, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Paz, S.; Malkinson, D.; Green, M.S.; Tsioni, G.; Papa, A.; Danis, K.; Sirbu, A.; Ceianu, C.; Katalin, K.; Ferenczi, E.; et al. Permissive Summer Temperatures of the 2010 European West Nile Fever Upsurge. PLoS ONE 2013, 8, e56398. [Google Scholar] [CrossRef]
- Stilianakis, N.I.; Syrris, V.; Petroliagkis, T.; Pärt, P.; Gewehr, S.; Kalaitzopoulou, S.; Mourelatos, S.; Baka, A.; Pervanidou, D.; Vontas, J.; et al. Identification of Climatic Factors Affecting the Epidemiology of Human West Nile Virus Infections in Northern Greece. PLoS ONE 2016, 11, e0161510. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors 2014, 7, 338. [Google Scholar] [CrossRef]
- Palmer, J.R.B.; Oltra, A.; Collantes, F.; Delgado, J.A.; Lucientes, J.; Delacour-Estrella, S.; Bengoa, M.; Eritja, R.; Bartumeus, F. Citizen science provides a reliable and scalable tool to track disease-carrying mosquitoes. Nat. Commun. 2017, 8, 916. [Google Scholar] [CrossRef]
- Dickens, B.L.; Sun, H.; Jit, M.; Cook, A.R.; Carrasco, L.R. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob. Health 2018, 3, e000801. [Google Scholar] [CrossRef]
- Maftei, C.; Bărbulescu, A.; Rugina, S.; Nastac, C.; Dumitru, I. Analysis of the Arbovirosis Potential Occurrence in Dobrogea, Romania. Water 2021, 13, 374. [Google Scholar] [CrossRef]
- Martens, W.J.; Niessen, L.W.; Rotmans, J.; Jetten, T.H.; McMichael, A.J. Potential impact of global climate change on malaria risk. Environ. Health Perspect. 1995, 103, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Campbell, H. Imported malaria in Britain: Survey of British residents travelling to areas in which malaria is endemic. BMJ 1985, 291, 1013–1014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwamura, T.; Guzman-Holst, A.; Murray, K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020, 11, 2130. [Google Scholar] [CrossRef]
- Zhao, X.; Smith, D.L.; Tatem, A.J. Exploring the spatiotemporal drivers of malaria elimination in Europe. Malar. J. 2016, 15, 122. [Google Scholar] [CrossRef]
- Zittra, C.; Vitecek, S.; Obwaller, A.G.; Rossiter, H.; Eigner, B.; Zechmeister, T.; Waringer, J.; Fuehrer, H.-P. Landscape structure affects distribution of potential disease vectors (Diptera: Culicidae). Parasites Vectors 2017, 10, 205. [Google Scholar] [CrossRef]
- Pernat, N.; Kampen, H.; Ruland, F.; Jeschke, J.M.; Werner, D. Drivers of spatio-temporal variation in mosquito submissions to the citizen science project ‘Mückenatlas’. Sci. Rep. 2021, 11, 1356. [Google Scholar] [CrossRef]
- Rocklöv, J.; Tozan, Y.; Ramadona, A.; Sewe, M.O.; Sudre, B.; Garrido, J.; Lary, C.B.D.S.; Lohr, W.; Semenza, J.C. Using Big Data to Monitor the Introduction and Spread of Chikungunya, Europe, 2017. Emerg. Infect. Dis. 2019, 25, 1041–1049. [Google Scholar] [CrossRef]
- Schröder, W.; Schmidt, G.; Bast, H.; Pesch, R.; Kiel, E. Pilot-study on GIS-based risk modelling of a climate warming induced tertian malaria outbreak in Lower Saxony (Germany). Environ. Monit. Assess. 2007, 133, 483–493. [Google Scholar] [CrossRef]
- Thomas, S.M.; Fischer, D.; Fleischmann, S.; Bittner, T.; Beierkuhnlein, C. Risk assessment of dengue virus amplification in Europe based on spatio-temporal high resolution climate change projections. Erdkunde 2011, 65, 137–150. [Google Scholar] [CrossRef]
- Desvars-Larrive, A.; Furberg, M.; Hjertqvist, M.; Vidman, L.; Sjöstedt, A.; Rydén, P.; Johansson, A.F. Epidemiology and Ecology of Tularemia in Sweden, 1984–2012. Emerg. Infect. Dis. 2015, 21, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, S.; Piakis-Chatzievagelou, N.; Mourelatos, S. Ecological mapping: The use of Geographic Information Systems (GIS) for rational mosquito control in natural wetlands. J. Environ. Prot. Ecol. 2006, 7, 617–625. [Google Scholar]
- Kenyeres, Z.; Tóth, S.; Sáringer-Kenyeres, T.; Márkus, A.; Bauer, N. Ecology-based mapping of mosquito breeding sites for area-minimized BTI treatments. Biologia 2017, 72, 204–214. [Google Scholar] [CrossRef]
- ESRI. How Create Space Time Cube Works. 2022. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/space-time-pattern-mining/learnmorecreatecube.htm (accessed on 20 March 2022).
- ESRI. Deep Learning Using the ArcGIS Image Analyst Extension. 2022. Available online: https://pro.arcgis.com/en/pro-app/2.8/help/analysis/image-analyst/deep-learning-in-arcgis-pro.htm (accessed on 22 March 2022).
- Openshaw, S.; Taylor, P. Million or so correlation coefficients: Three experiments on the modifiable areal unit problem. In Statistical Methods in Spatial Sciences; Wrigley, N., Ed.; Routledge & Kegan Paul: London, UK, 1979; pp. 127–144. [Google Scholar]
- Prata, J.; Ribeiro, A.; Rocha-Santos, T. One Health—Integrated Approach to 21st Century Challenges to Health, 1st ed.; Academic Press: London, UK, 2022. [Google Scholar] [CrossRef]
- Mendes, J.; Ribeiro, A.I.; Severo, M.; Niza-Ribeiro, J. A multilevel study of the environmental determinants of swine ascariasis in England. Prev. Veter. Med. 2017, 148, 10–20. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Krainski, E.; Carvalho, M.S.; Pina, M.D.F. The influence of socioeconomic deprivation, access to healthcare and physical environment on old-age survival in Portugal. Geospat. Health 2017, 12, 581. [Google Scholar] [CrossRef]
- Magalhães, J.P.M.; Ribeiro, A.I.; Caetano, C.P.; Machado, R.S. Community socioeconomic deprivation and SARS-CoV-2 infection risk: Findings from Portugal. Eur. J. Public Health 2021, 32, 145–150. [Google Scholar] [CrossRef]
- Whiteman, A.; Loaiza, J.R.; Yee, D.A.; Poh, K.C.; Watkins, A.S.; Lucas, K.J.; Rapp, T.J.; Kline, L.; Ahmed, A.; Chen, S.; et al. Do socioeconomic factors drive Aedes mosquito vectors and their arboviral diseases? A systematic review of dengue, chikungunya, yellow fever, and Zika Virus. One Health 2020, 11, 100188. [Google Scholar] [CrossRef]



| Method Category | Method | Number | References |
|---|---|---|---|
| Disease mapping | |||
| Risk map | 32 | [31,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] | |
| Rate map | 31 | [31,32,34,38,39,40,42,44,45,46,47,50,53,54,56,58,59,60,61,64,66,67,68,69,70,71,72,73,74,75,76] | |
| Case counts maps | 14 | [28,42,68,75,77,78,79,80,81,82,83,84,85] | |
| Temporal trend map | 10 | [32,34,43,45,59,60,66,75,86,87] | |
| Distance map | 10 | [43,50,54,64,66,78,81,82,88,89] | |
| Predictive map | 9 | [31,37,43,45,55,57,62,64,90] | |
| Travel/time map | 8 | [81,82,85,91,92,93,94,95] | |
| Suitability map | 6 | [37,43,51,56,57,96] | |
| Dot map | 3 | [88,97,98] | |
| Clusters, clustering, and surveillance | |||
| Kernel density map | 6 | [31,32,49,96,97,98] | |
| GetisOrd statistic (Hotspot analysis) | 3 | [29,31,32] | |
| Mahalanobis Distance Analysis | 3 | [43,54,77] | |
| Autocorrelation I Moran’s | 2 | [94,99] | |
| Kulldorff’s spatial scan statistic | 1 | [88] | |
| Mann-Kendall Test | 1 | [89] | |
| Ripley′s K Function | 1 | [66] | |
| Geographic correlation | |||
| Generalized linear models (GLM) | 20 | [30,38,41,48,56,61,66,88,93,94,95,100,101,102,103,104,105,106,107,108] | |
| Spatial prediction | 16 | [31,43,49,50,54,55,57,61,65,73,75,96,109,110,111,112] | |
| Ecological niche models of occurrence | 12 | [49,50,52,53,55,70,73,87,96,111,112,113] | |
| Conventional logistic | 10 | [44,46,50,57,65,80,104,114,115,116] | |
| Regression models with spatial terms | 9 | [47,57,62,64,65,81,88,95,108] | |
| Bayesian models | 8 | [41,66,75,99,110,111,117,118] | |
| Generalized Additive Models (GAM) | 7 | [29,30,41,50,74,100,103] | |
| Pearson′s Correlation | 5 | [32,35,38,106,115] | |
| Kriging estimation | 4 | [36,46,47,58] |
| Variable | Non-Significant Association | Positive Association | Negative Association |
|---|---|---|---|
| Abiotic factors | |||
| Precipitation | [80,115] | [38,46,56,87,89,98,102,103,107,119,120,121,122,123,124] | [30,32,86,96,102,104,107,125,126] |
| Temperature | [30,32,34,38,46,47,56,58,59,60,61,71,80,86,89,96,98,99,101,102,103,104,105,106,107,115,116,119,120,121,122,123,125,126,127,128,129] | [68,96,102,103,124] | |
| Wind speed | [103] | [116,126] | |
| Altitude | [56,61,101,102,130] | [80,96,105] | |
| Land Use | [30,38,46,64,80,105,107,125] | ||
| Water bodies | [30,35,38,46,56,64,89,99,105,107,126,130,131] | [80] | |
| Water depth | [99,125] | [125] | |
| NDWI (Normalized difference water index) | [107] | ||
| Vegetation | [30,35,38,61,80,86,105,107,119,131] | [30,124] | |
| Rice fields | [64,86] | ||
| Soil water content | [116] | ||
| Frost day frequency | [124] | ||
| Urbanization | [96] | [30,61,105,107] | [124] |
| Wealth | [124] | ||
| Life expectancy | [124] | ||
| Accessibility | [119] | ||
| Relative Humidity | [102] | [119,120,125] | [115,116] |
| Greenhouse gas emissions | [71] | ||
| Light Intensity | [103] | ||
| Mean sunshine duration | [125] | ||
| Direction at ground level | [103] | ||
| Geocoded Twitter data-Geolocated activity data and computed mobility patterns of users | [127] | ||
| GDP-Gross domestic product | [124] | ||
| Agriculture | [80] | [61] | |
| Biotic factors | |||
| Population Density | [107,119] | [46] | |
| Mobility | [81,94] | [94] | |
| Human Population Data/Ratio | [30,71,104,106,126] | ||
| Ditch shrimp of the genus Palaemonetes and Fish as predators | [99] | ||
| Birds | [80] | ||
| Horses | [80] | ||
| Animals in farms | [56,61,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutinho, S.; Rocha, J.; Gomes, A.; Gomes, B.; Ribeiro, A.I. Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review. Sustainability 2022, 14, 8975. https://doi.org/10.3390/su14158975
Moutinho S, Rocha J, Gomes A, Gomes B, Ribeiro AI. Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review. Sustainability. 2022; 14(15):8975. https://doi.org/10.3390/su14158975
Chicago/Turabian StyleMoutinho, Sandra, Jorge Rocha, Alberto Gomes, Bernardo Gomes, and Ana Isabel Ribeiro. 2022. "Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review" Sustainability 14, no. 15: 8975. https://doi.org/10.3390/su14158975
APA StyleMoutinho, S., Rocha, J., Gomes, A., Gomes, B., & Ribeiro, A. I. (2022). Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review. Sustainability, 14(15), 8975. https://doi.org/10.3390/su14158975








