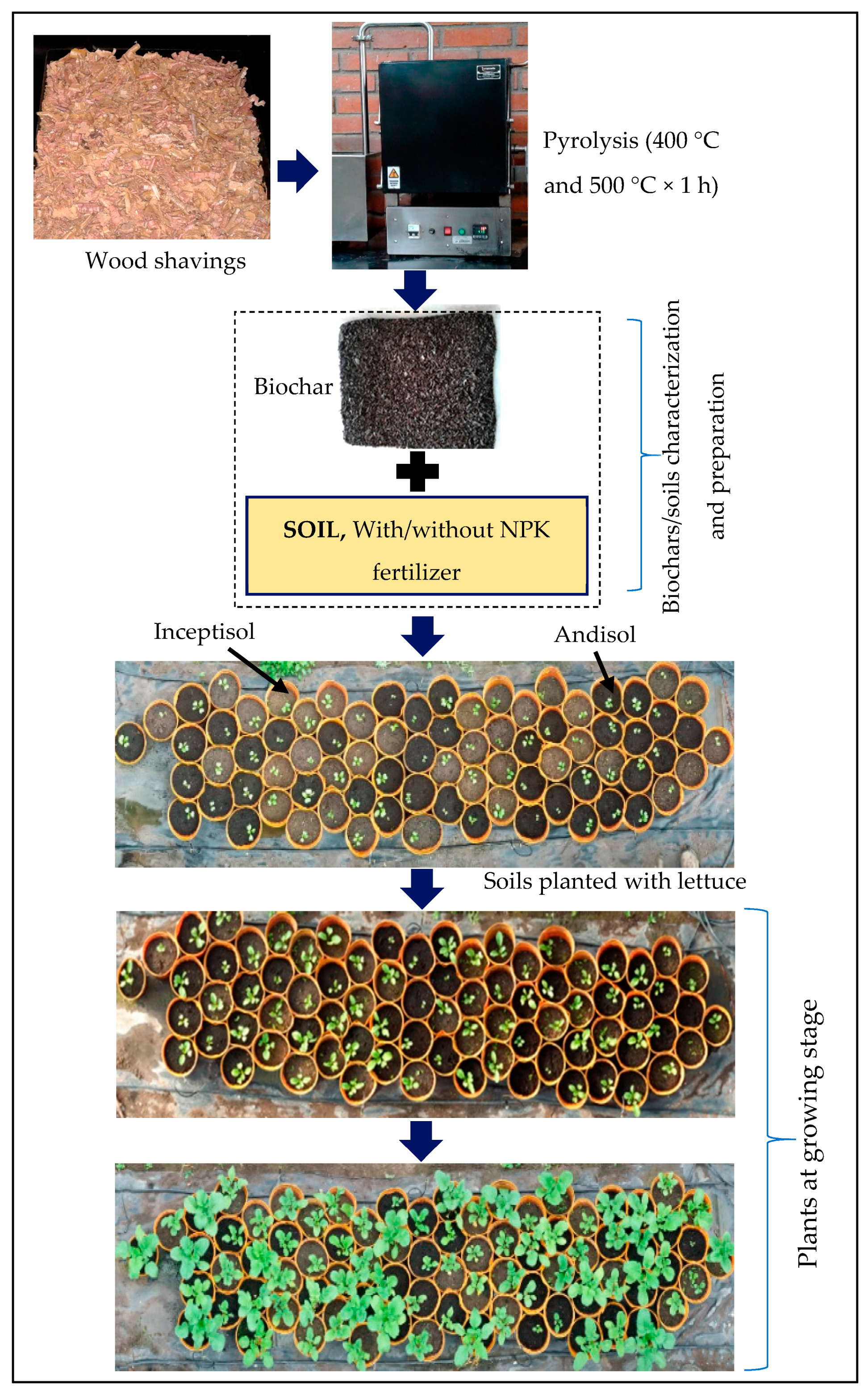
1. Introduction
Population growth has been a driving force for the expansion of urban areas worldwide, causing a reduction in available agricultural lands [
1,
2,
3]. This has affected large parts of arable lands that have been engulfed by growing urban centers in the last three decades. A decrease in available arable soil promotes deforestation for agriculture and cattle pasture use, expansion into erosion-prone (due to rugged topography and steepness) highlands [
4,
5,
6,
7] and soils with low nutrients content, which is the case of some places in the Andean highlands [
8]. Soil erosion in the Andean highlands is accompanied by difficulty to retain water. Reforestation could help to reduce erosion, but the thin soil covers in steep lands limits these efforts. Moreover, a method frequently used for augmenting the nutrients content in soil is through the addition of nitrogen-based synthetic fertilizers. However, this method for soil improvement is also contributing to further soil acidification since N-based fertilizers can increase soil acidification [
9,
10,
11]. Soils in the Andean highlands are commonly characterized by low pH (in some places below 5) [
12,
13,
14]. Reducing soil erosion and, simultaneously, increasing soil fertility, retaining moisture in the soil, and avoiding soil acidification are challenging tasks in these types of soils.
Biochar has been identified as a promising material for agricultural soil improvement [
15,
16,
17,
18] and carbon storage in biochar amended soils [
19]. For soils such as those found in parts of the Andean highlands, biochar could help to retain moisture and, thus, improve soil fertility. Multiple factors must be considered, however, when assessing soil’s response to biochar [
16,
17,
20,
21,
22]. Thus, specific studies are necessary to evaluate the properties of the Andean soils affected by biochar and determine the related impacts. The methods used to produce the soil–biochar amendment must also be considered [
23]. In Ecuador, a previous work assessed the benefits of using up to 6 t ha
−1 of biochar in soils in the Ecuadorian Southern Amazonian region, showing that biochar plus fertilizer contributed to faster growing rates of
Gmelina arborea trees [
24]. The authors used charcoal obtained from a local market, which in Ecuador is produced using rudimentary kilns [
25]. Thus, little is known about the characteristics of this biochar. Because different biochar production conditions such as temperature and time lead to biochars with diverse characteristics, different types of biochar–soil interactions can promote specific responses in soil characteristics [
16] and plants’ responses. Biochar type and dosage, as well as soil type and the corresponding interactions, impact soil fertility and performance [
18].
Although previous studies have analyzed the effect of biochar in Andisol and Inceptisol [
23,
26], only a few have analyzed the effect of biochar in these soils in the conditions of the Andean highlands. Andisols from the Ecuadorian paramos are susceptible to irreversible changes upon drying, which is expected to negatively affect its physical properties [
27]. Andisols are also characterized by a large porosity [
27] and pH below 5 [
14,
27]. Although it is expected that biochar could help to reduce such susceptibility by improving water retention capacity and, thus, helping to improve the quality of these soils, more work is necessary to better understand the dosages and interactions of biochars with these types of soils and the addition of fertilizers. The strategy for storing C in a stable manner and for long times via biochar addition to soils [
19] is of practical interest at the farmers level only if there is an agronomic value of biochar in the soils; i.e., if the fertilizer value of biochar and its positive effects on soil physical properties are confired [
26]. Farmers in the Ecuadorian Andes grow a variety of crops, among which vegetables are of great economic and ecological importance due to their market value and the technological intensity required for their production [
28,
29]. The intensity of use of the soils dedicated to vegetable production (frequent tillage, use of labile forms or organic matter, and frequent irrigation), offers an appropriate setting for developing better soil conservation strategies and selection of amendments with potential soil-transforming effects such as biochars. Therefore, more work is necessary to better understand these benefits, especially in the conditions of soils in the Andean highlands. The objective of this work was twofold: (a) to study the effect of two types of biochars on two agricultural soils commonly found in the Andean highlands (Andisol and Inceptisol) and the corresponding soil–biochar–fertilizer interactions; and, (b) to assess the response to biochar of two vegetable crops grown in succession in a simulated double-cropping system. The work aims to explore the consistency of biochar amendments at producing desired responses in Andean soils’ properties and vegetable growth (lettuce and radish) under local conditions.
4. Discussion
There exists a growing global interest on using biochar as a soil amendment due to its capacity to improve soil properties and increase crops production [
50,
51]. Still, information on biochar-amended soils also reports agriculturally unfavorable effects of biochar, in both crop development and soil properties [
52,
53]. The differing responses of soils and crops to biochar application suggest that complex interactions between the type of biochar, percent of biochar in the soil, soil properties, climate factors, and plant type exist. In this work, some of these interactions were tested by simulating a rotational cropping system using two soils commonly found in the agriculture of the Andean highlands. Biochar production conditions that could further complicate the responses of soil and crops to the amendment were also tested.
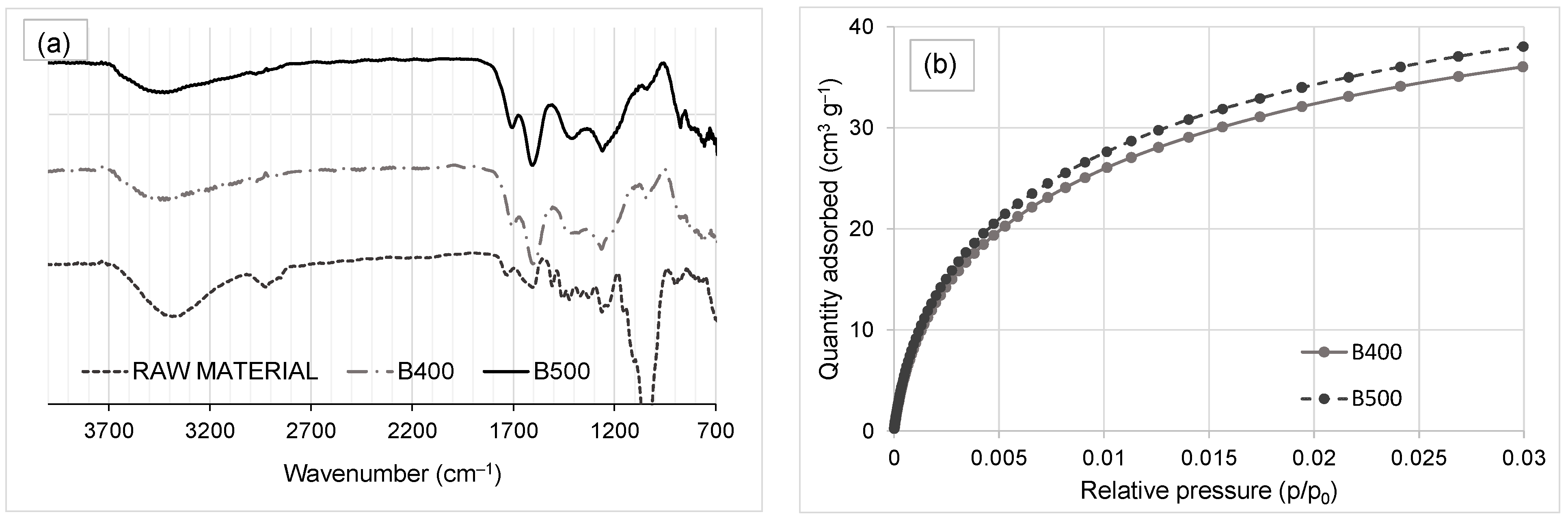
Although the sole difference between the production conditions of the two biochar types was temperature, different responses were produced by the biochar type on soil properties and growth of high rotation plants. The differences between B400 and B500 biochar types include elemental composition (particularly C and O content), degree of aromaticity, hydrophobicity, and surface area. These differences promote visible changes on the responses of the soils to the biochars. Higher hydrophilicity allows better compatibility between water and biochar. Water retention capacity is undoubtedly a factor that positively impacts soil properties and further plants’ growth. However, our result suggests that this trend should not always be expected since the type of soil also affects the results. It appears that the main interactions between the biochars (both B400 and B500) and the soil are second order, affecting the bulk density, θCC, θPMP, and total available water in the soil. As a consequence of these interactions, the biochar B500 produced more desirable changes (lower bulk density and higher total available water) in the Inceptisol whereas in the Andisol, biochar B400 produced more desirable changes. Although the response to the biochar amendment in the Inceptisol could be labeled as neutral or beneficial to hydrophysical properties, in the Andisol the effects of biochar amendment with B500 could be considered detrimental as it reduced the total available water in the soil, which could in turn affect plant growth. A possible explanation for the better performance of B400 compared to B500 is that B400 is less hydrophobic than B500.
Although biochar can increase total available water in different types of soils [
54], lack of effects on total available water have also been reported [
30,
55]. In our work, increases, no changes, or reductions in total available water depended on the combination of biochar amendment and soil type. Different effects of biochar on the physical properties of different soil types have been reported previously, suggesting that beneficial biochar effects on soil hydrophysical properties would be less pronounced in fine textured soils [
51]. Nonetheless, it has also been reported that the hydrophysical properties of silt rich soils could solely be minutely affected by biochar [
56]. In our experiment, the Inceptisol and Andisol were loam and sandy loam soils, respectively, and, thus, expected to respond rather well in terms of hydrophysical properties to the application of biochar. The mixed results in this experiment, however, make it difficult to provide general recommendations on the use of biochar to improve the hydrophysical characteristics of the tested soils.
Similar to the response of hydrophysical properties of the soils to the addition of biochar, chemical properties also showed a varied response with significant interactions between the type of biochar, soil, and whether fertilizer was added or not to the pots. In general, biochar is considered to have a liming effect on the soil, increasing its pH [
53,
57]. This effect has been reported to be dependent on the buffering capacity of the soil and the influence of added fertilizer [
50,
58,
59]. No significant effects (neither main nor interaction effects) of biochar addition were detected on the pH of either soils after the first crop cycle, suggesting that the liming capacity of the tested biochars was limited, even accounting for the rate at which the biochar was applied, which was higher than in other studies where such an effect has been reported [
57,
59]. However, a significant interaction between the biochar and soil type produced higher pH in the biochar-amended Andisol after the second crop. This result indicates a delayed liming effect that increased the buffering capacity of the soil, which is in agreement with previous observations showing that the soil response to biochar increases over time after the initial application [
51]. As expected, the addition of fertilizer reduced the pH of both soils, but this reduction was less marked in the Andisol than in the Inceptisol. Van Zwieten et al. (2010) reported similar results for an acidic Ferrosol and an alkaline Calcarosol in the biochar they produced and attributed this liming effect to Ca complexes formed from CaCO
3 present in their feedstock [
59]. The feedstock used in their study was different than the one used herein. Nevertheless, in general, biochars contain small amounts of ashes that can contribute bases to neutralize acidic soils [
53], which is also our case, since ash content in our biochars can be up to 1.75 mass% (
Table 1). Ashes in biochar are also attributed to positively impact CEC of soils.
Another property that showed a marked and contrasting response to the application of biochar in both soils was the organic matter content. Soils that received biochar had less organic matter after the first crop in the Inceptisol, whereas the opposite was observed in the Andisol. Interestingly, after the second crop, this result was reversed and the organic matter content was higher than controls in the biochar amended Inceptisol, while the opposite was identified in the biochar amended Andisol. Still, even though a statistically significant interaction between soil type and biochar addition supports this observation, the magnitude of the change in organic matter in any of the cases is negligible for any practical implication. In general, higher available water content was found in soils amended with B400 than with B500, which is a consequence of the higher hydrophilicity of B400 compared to B500. The results suggest that biochar produced at 400 °C performs better than biochar produced at 500 °C because B400, apparently, promotes a better environment for bacteria growth in the soils, in part as a consequence of more OH available groups in B400 and its better interaction with water and fertilizer.
Generally recognized as stable under soil conditions, a net increase and relatively stable organic matter content was expected in the biochar amended soils. Nevertheless, the addition of biochar could promote degradation of labile carbon [
60,
61], which could explain the decrease of soil organic matter in our results. Still, the reversal of the original effect in both soils highlights the importance of planning long term experiments and rotations to avoid confusion of transient changes with long term effects of biochar amendments in different types of soils. Biochar amendment effects on bacterial counts were not detected until the end of the second crop when the effect of biochar was strongly dependent on the type of soil, with B400 increasing counts in the Inceptisol and B500 producing no effect, whereas in the Andisol, the opposite was true. The effects of biochar on microbial abundance are mixed and suggest the presence of important interactions between the amendment, the soil type, and the group of microorganisms, since some groups of organisms seem to benefit from the introduction of biochar whereas others do not [
62]. This finding is in agreement with results reported in previous works [
21].
Biochar has been proposed as a way to increase the CEC of soils and, consequently, increase their capacity to hold nutrients [
53]. The consistent increase in Ca, Mg, Zn, Cu, Fe, and Mn concentrations in the B500-amended Andisol could be a consequence of increased cation exchange sites due to biochar addition. However, the lack of consistent response in the Inceptisol and the B400-amended Andisol are indicative of important soil-biochar interaction effects and the influence of biochar processing conditions in this interaction.
In terms of plant growth, our results show a slight increase in the weight of the crops used in the simulated rotation, with larger effects of fertilizer and soil type on the development of the plants. Such a small effect, however, is in agreement with the estimated effect of biochar applications in other crops. Jeffery et al. estimated in their meta-analysis an average increase in crop productivity by ~10% with a range from −28% to 39% change relative to the controls [
52]. In our case, the range of responses was varied, ranging from ~34% reduction in weight of radish in the biochar amended Inceptisol to ~100% increase of weight in radish in the B400 amended Inceptisol with addition of fertilizer (all values relative to the corresponding control).
5. Conclusions
Biochars produced from hardwood residues at 400 °C and 500 °C for 1 h (B400 and B500, respectively) were tested to identify the main and interaction effects of biochar type, soil type, and NPK fertilizer addition on the physical, chemical, and microbiological characteristics of the soils. Results show that biochar alone is not sufficient to promote visible benefits, although it allows better interaction of soils with fertilizers and water. Biochar produced at lower temperatures (i.e., B400) works better than biochar produced at higher temperatures (i.e., B500). The presence of oxygen functional groups and, thus, biochar’s hydrophilicity, appears to positively impact biochar properties required for soil amendment. The effect of biochar affinity to soils and water seems to play an important role for this biochar’s behavior. However, it appears that some positive effects are not possible to detect in the short term (i.e., in the order of a few weeks, as tested herein). It has also been found that the type of soil responds differently to different types of biochar additions. In the case of degraded soils such as those in some parts of the Andean highlands, the addition of biochar could help to increase the water retention capacity of the soils, a critical factor to support agriculture in steep soils in these regions. Although this study shows some positive effects of B400 and B500 biochar in Andisol and Inceptisol in the Andean highlands, further long-term research is needed for more data acquisition on biochar–soil interactions. Additional benefits of using biochar in soils (e.g., carbon sequestration and soil physical characteristics) need to be tested, preferably under local conditions, in long-term tests.