RETRACTED: Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Consortia
2.2. Selection of the Microbial Consortium
2.3. Kinetics of Microbial Growth and Removal of Textile Dyes
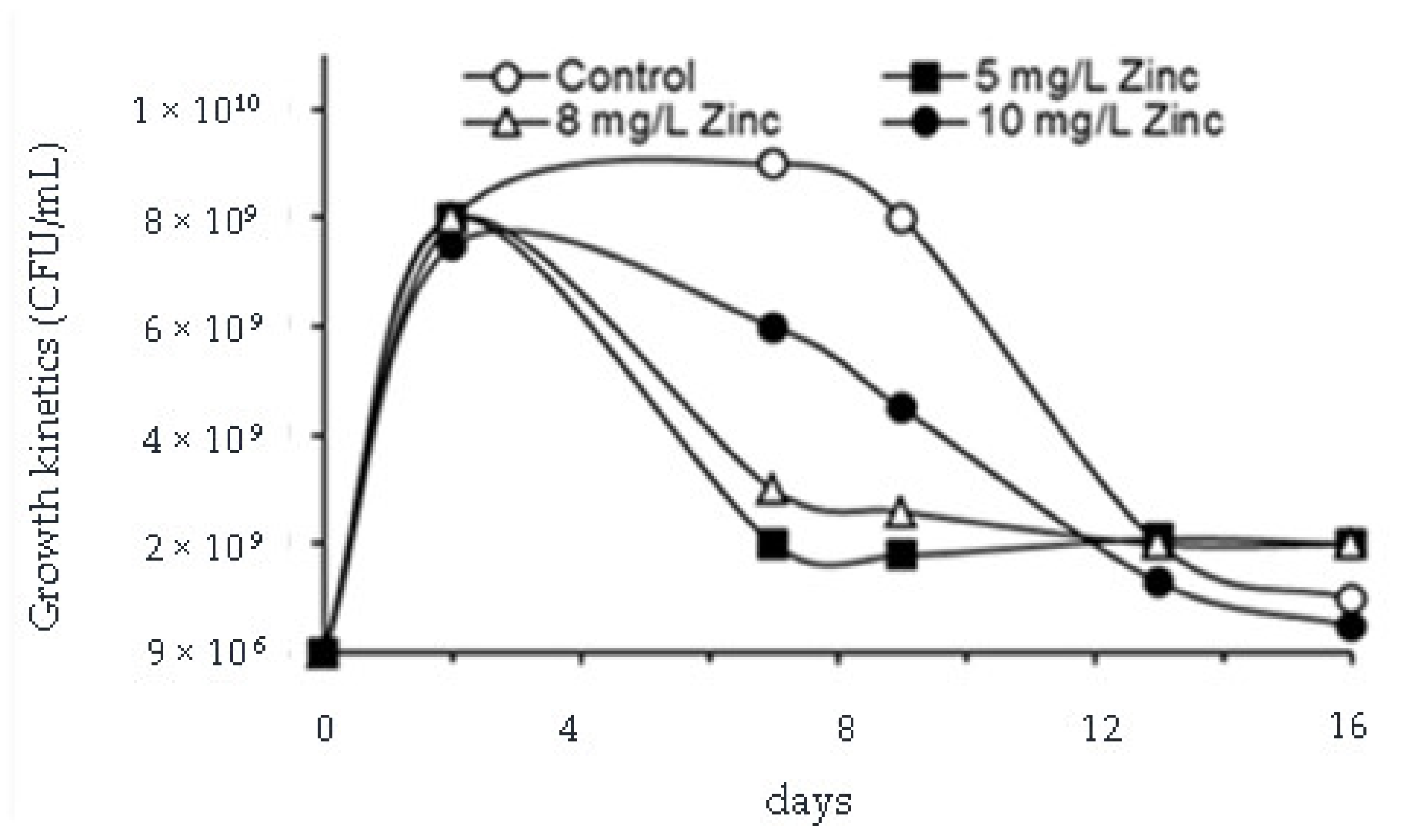
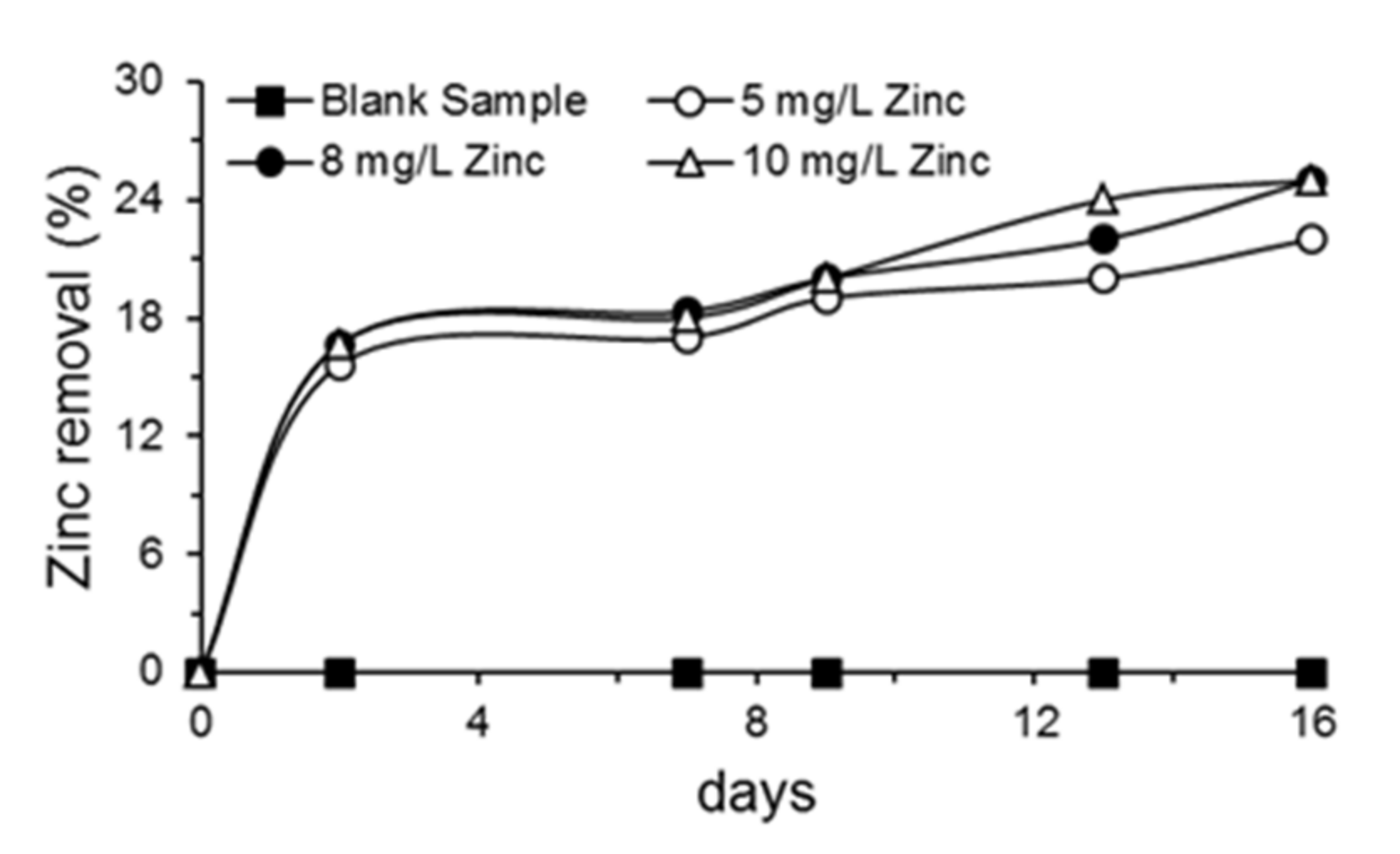
2.4. Removal of Phenol, Chromium(VI), Total Chromium, Zinc and Evaluation of Microbial Growth
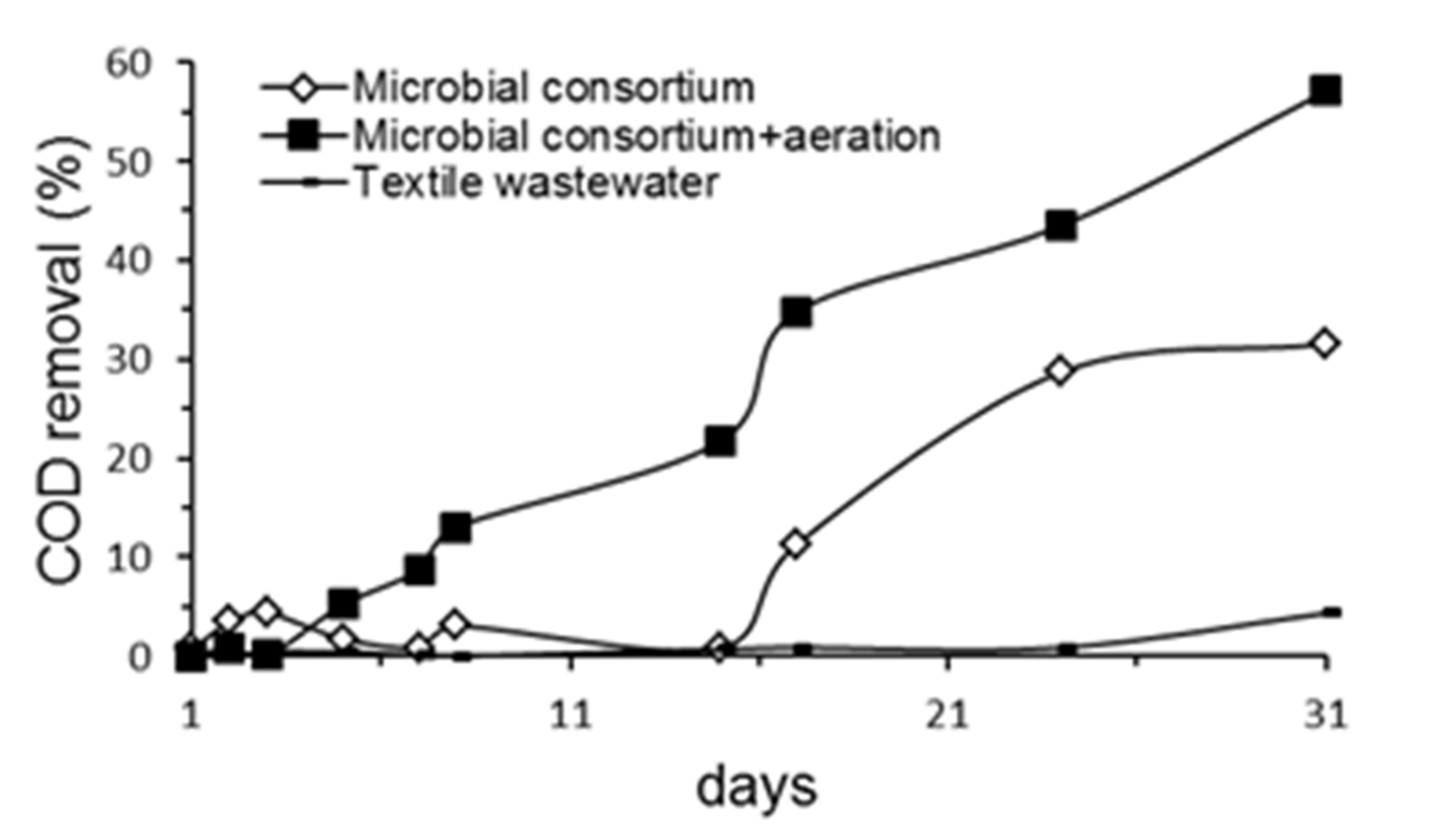
2.5. Reduction in COD in Textile Wastewater by the Microbial Consortium
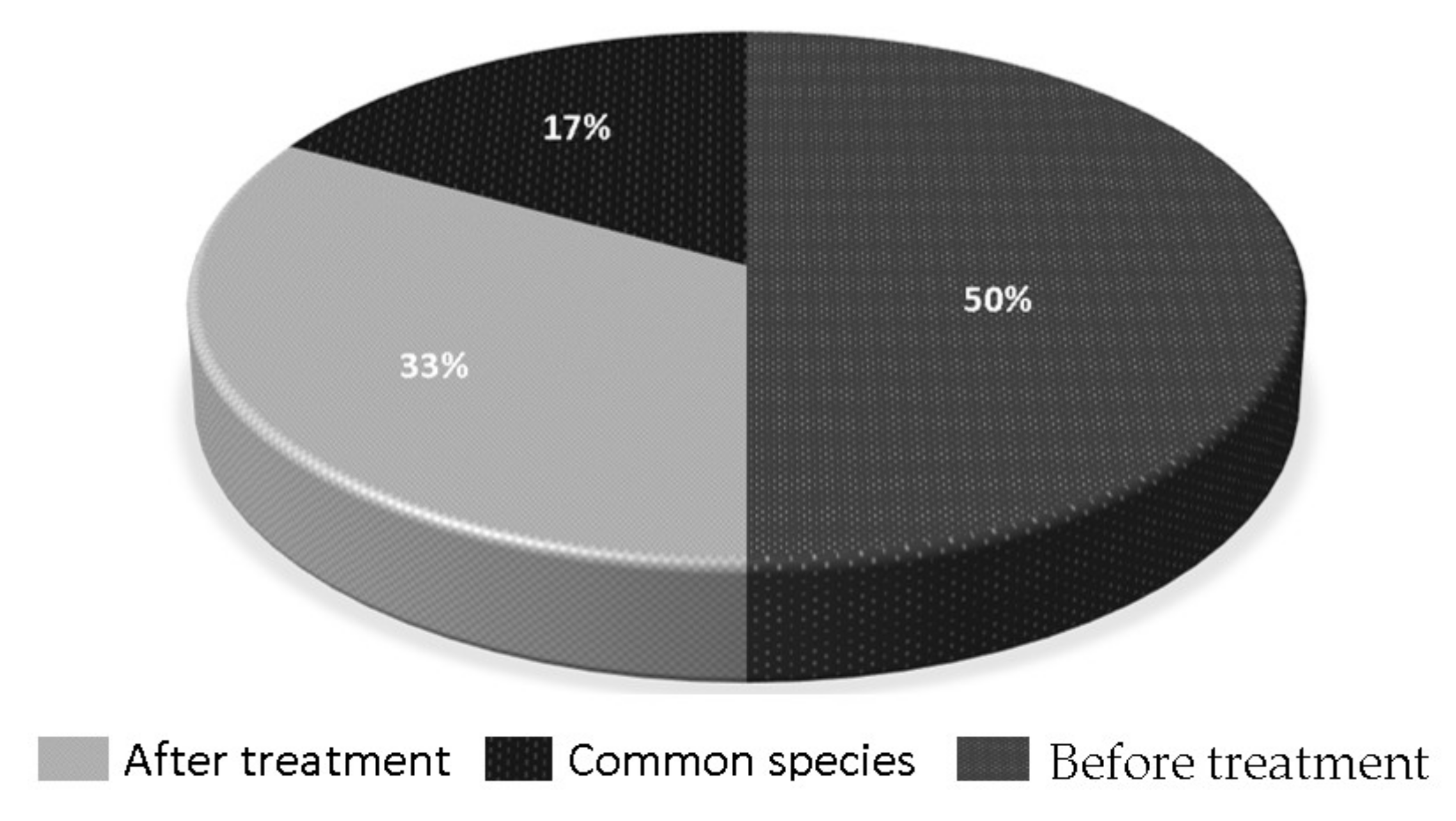
2.6. Microbiological Identification before and after Textile Wastewater Treatment
2.7. Selection of Support for Anaerobic Reactor at Laboratory Level
2.8. Statistical Data Analysis
3. Results
3.1. Selection of the Microbial Consortium
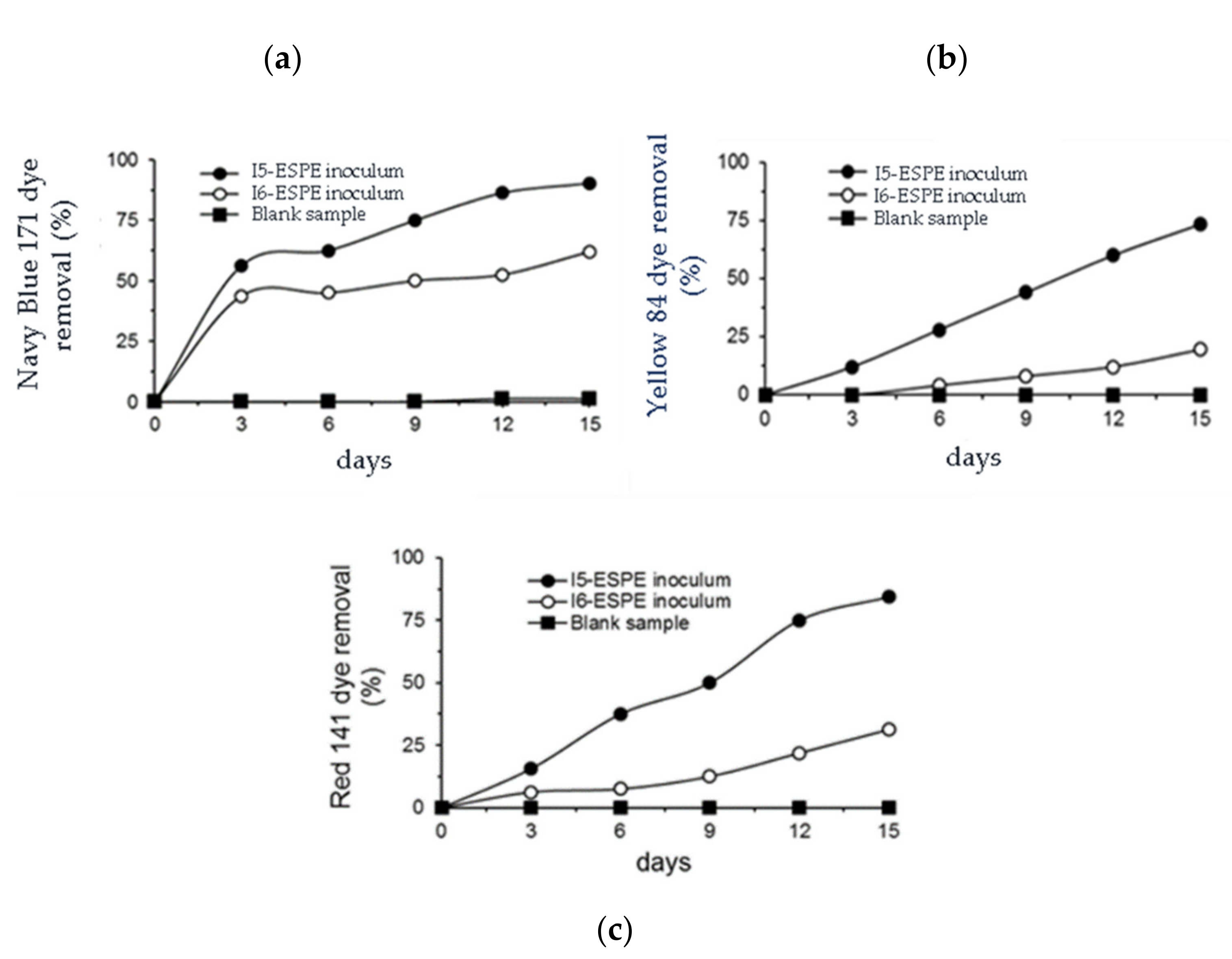
3.2. Kinetics of Microbial Growth and Removal of Textile Dyes
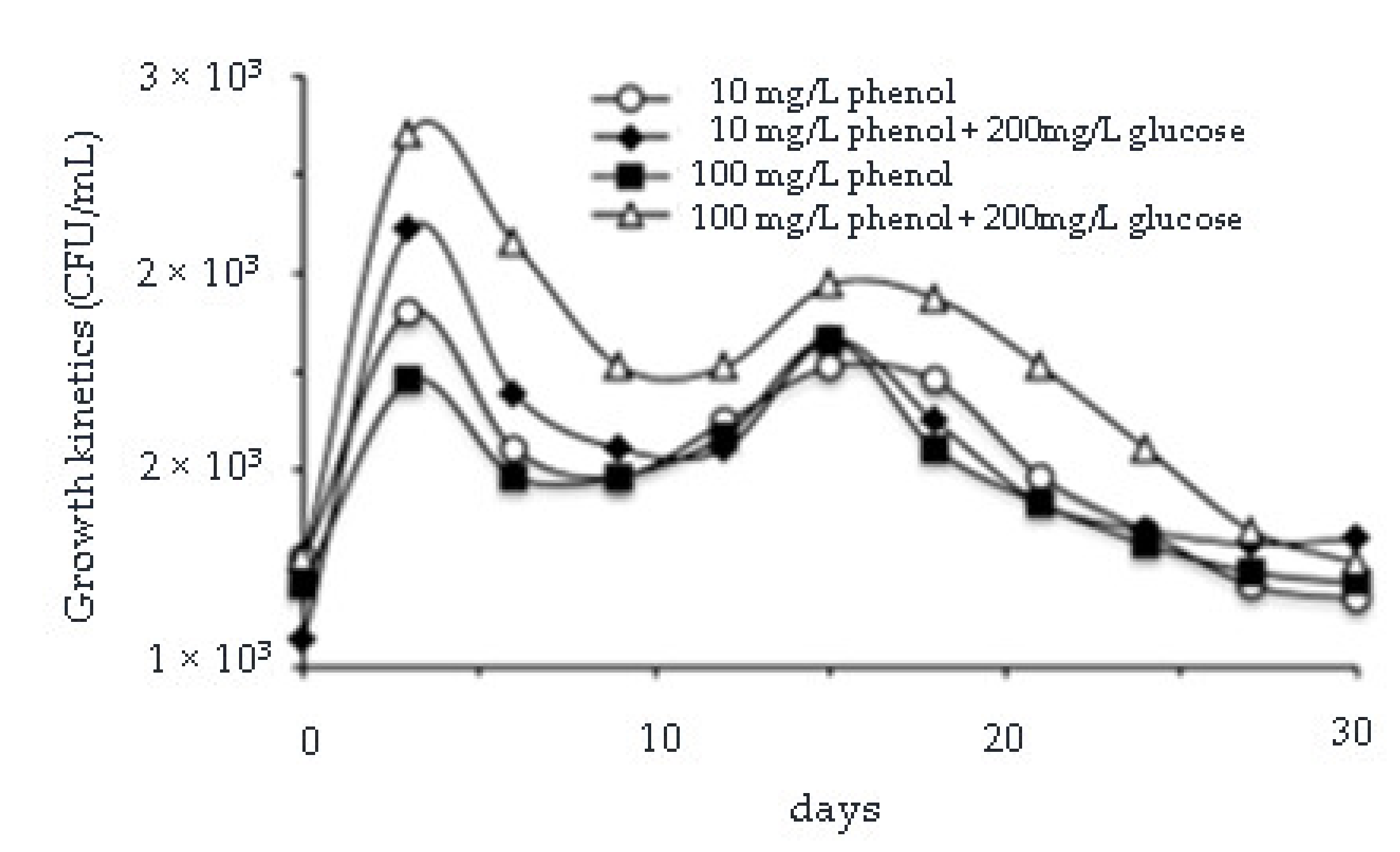
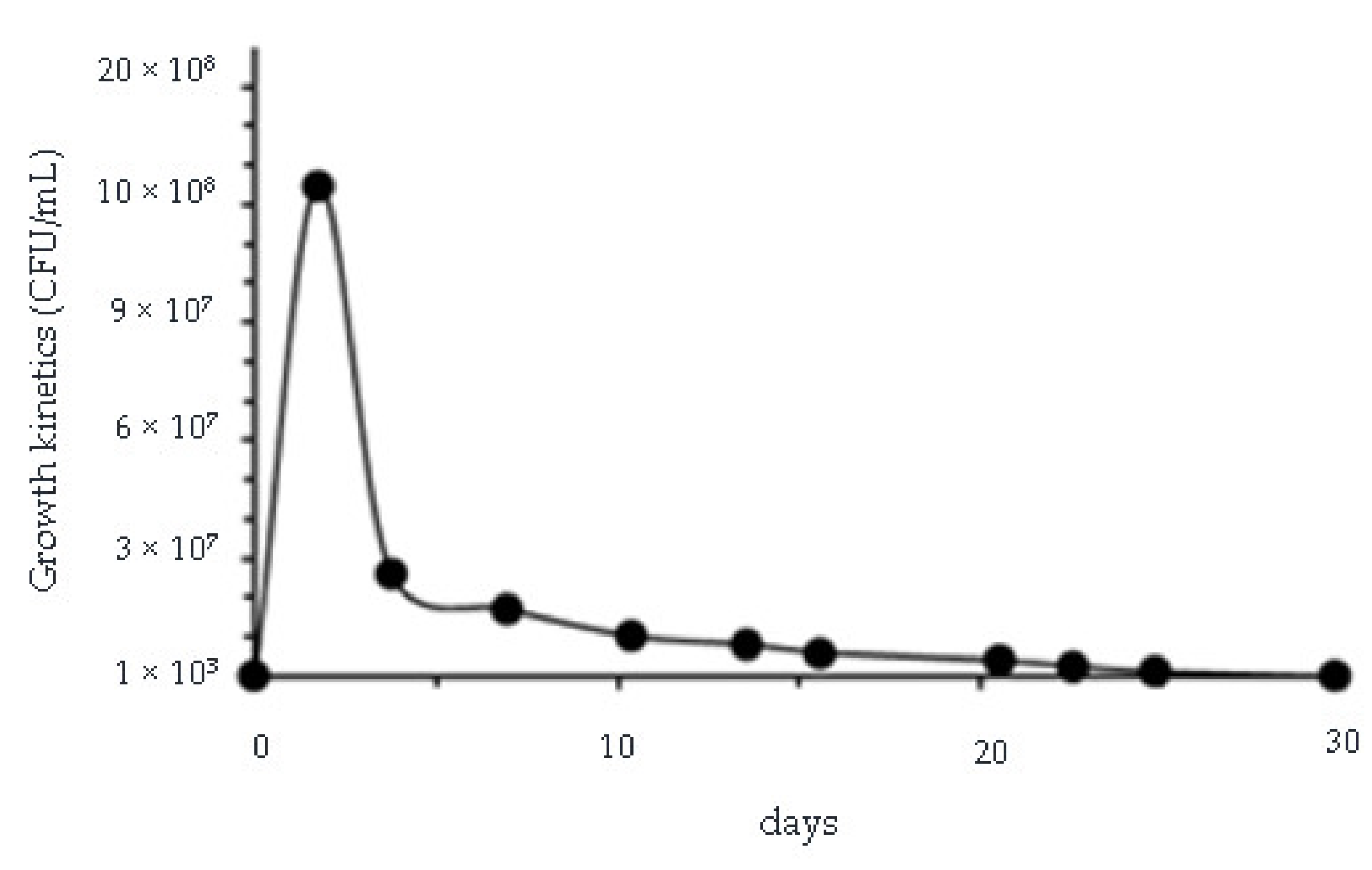
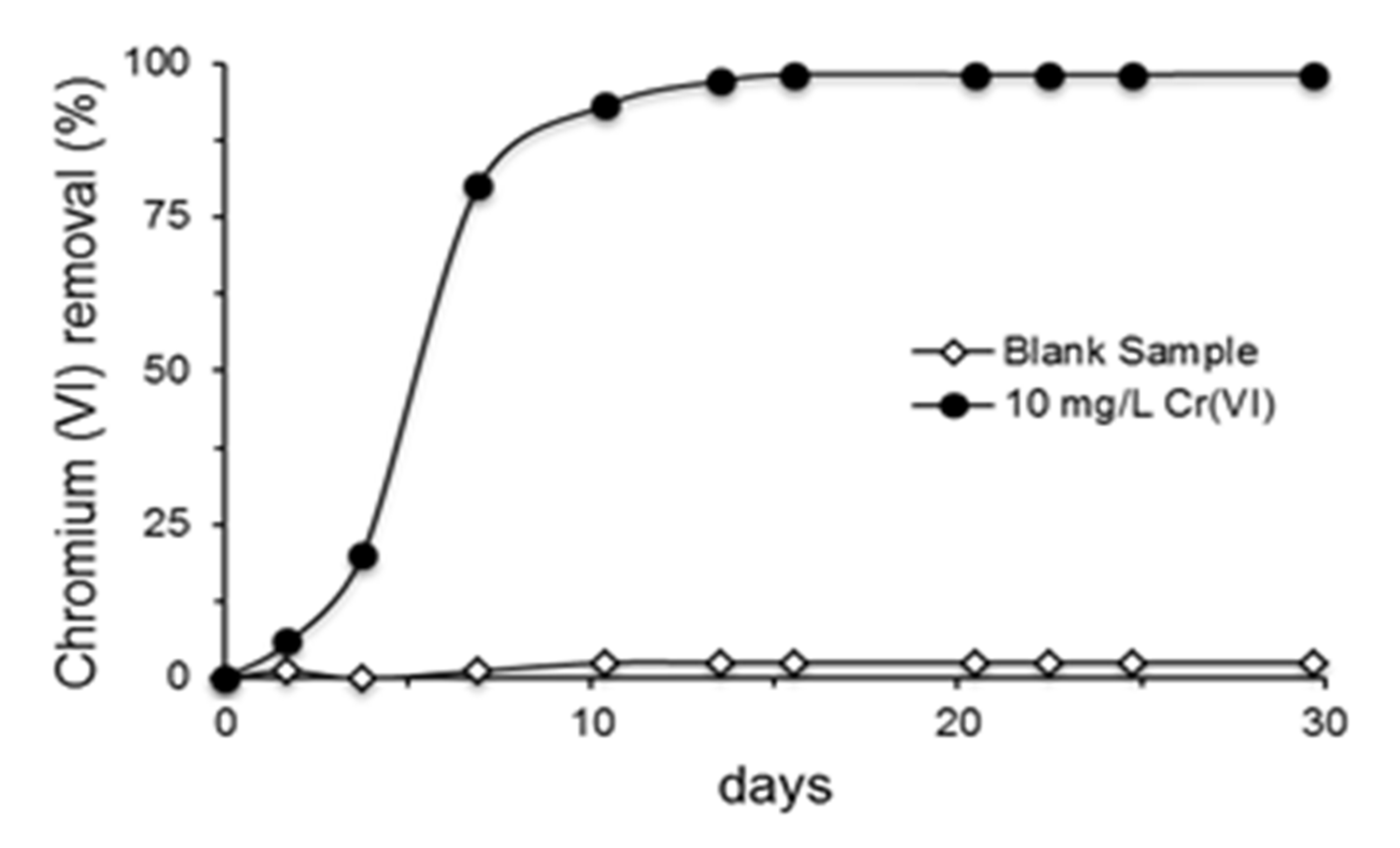
3.3. Removal of Phenol, Chromium(VI), Total Chromium and Zinc and Evaluation of Microbial Growth
3.4. Reduction in COD in Textile Wastewater by the Microbial Consortium
3.5. Microbiological Identification before and after Textile Wastewater Treatment
3.6. Selection of Support for Anaerobic Reactor at Laboratory Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pey, C. Aplicación de Procesos de Oxidación Avanzada (Fotocatálisis Solar) para Tratamiento y Reutilización de Efluentes Textiles. Ph.D. Thesis, Universitat Politécnica de Valéncia, Departamento de Ingeniería Textil y Papelera, Valéncia, Spain, 2008. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N.M. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodríguez, M.; Cabañas-Vargas, D.; Gamboa-Marrufo, M.; Domínguez-Benetton, X. Evaluation of the biosorption process with orange peels for the elimination of Lanasol Navy CE commercial dye in wastewater from the textile industry. Ingeniería 2009, 13, 39–43. [Google Scholar]
- Husseiny, S.M. Biodegradation of the Reactive and Direct Dyes using Egyptian Isolates. J. Appl. Sci. Res. 2008, 4, 599–606. [Google Scholar]
- Mansilla, H.; Lizama, C.; Gutarra, A.; Rodríguez, J. Tratamiento De Residuos Líquidos De La Industria Celulosa Y Textil; CYTED: Buenos Aires, Argentina, 2001. [Google Scholar]
- Park, J.W.; Kim, S.Y.; Noh, J.H.; Bae, Y.H.; Lee, J.W.; Maeng, S.K. A shift from chemical oxygen demand to total organic carbon for stringent industrial wastewater regulations: Utilization of organic matter characteristics. J. Environ. Manag. 2022, 305, 114412. [Google Scholar] [CrossRef]
- Talarposhti, A.M.; Donnelly, T.; Aderson, G.K. Colour removal from a simulated day wastewater using a two-phase anaerobic packed bed reactor. Water Res. 2001, 35, 425–432. [Google Scholar] [CrossRef]
- Bock, D.; Rott, U. Co-fermentation of colored concentrates from the textile processing industry. J. Environ. Sci. Health 2003, 38, 1889–1901. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Crowley, D.E. Accelerated decolorization of structurally different azo dyes by newly isolated bacterial strains. Appl. Microbiol. Biotechnol. 2008, 78, 361–369. [Google Scholar] [CrossRef]
- Supaka, N.; Juntongjin, K.; Damronglerd, S.; Delia, M.; Strehaiano, P. Microbial decolorization of reactive azo dyes in a sequential anaerobic-aerobic system. Chem. Eng. J. 2004, 99, 169–176. [Google Scholar] [CrossRef]
- Dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef]
- Hendrickx, I.; Boardman, G. Pollution Prevention Studies in the Textile Wet Processing Industry. Master’s Thesis, Virginia Politechnic Institute and State University, Blacksburg, VA, USA, 1995. [Google Scholar]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Smith, B. Pollutant Source Reduction: Part II-Chemical handling. Am. Dyest. Rep. 1989, 78, 26–32. [Google Scholar]
- Rodríguez, Z.; Boucourt, R.; Rodríguez, J.; Albelo, N.; Núñez, O.; Herrera, F. Isolation and selection of microorganisms with the ability to degrade starch. Rev. Cuba. De Cienc. Agrícola 2006, 40, 349–354. [Google Scholar]
- Rădulescu, C.; Ionită, I.; Moater, E. Monitoring and degradation of some organic pollutants from waste waters resulting from textile industry. Eurasian J. Anal. Chem. 2008, 3, 151–169. [Google Scholar]
- Takahashi, N.; Kumagai, T.; Shimizu, M.; Suzuki, T.; Ohtsuki, T. Removal of dissolved organic carbon and color from dyeing wastewater by pre-ozonation and subsequent biological treatment using test-scale plant. Ozone Sci. Eng. 2007, 29, 169–176. [Google Scholar] [CrossRef]
- Georgiou, D.; Aivasidis, A. Decoloration of textile wastewater by means of a fluidized-bed loop reactor and immovilized anaerobic bacteria. J. Hazard. Mater. 2006, 135, 372–377. [Google Scholar] [CrossRef]
- Torres, P.; Rodríguez, J.; Uribe, I. Wastewater treatment of the cassava starch extraction process in anaerobic filter: Influence of the support medium. Sci. Tech. 2003, 23, 75–80. [Google Scholar] [CrossRef]
- Pinto, J.; Chernicharo, C. Escoria de altoforno: Una nova alternativa de meio suporte para filtros anaeróbios. In Anais Do III Simposio Ítalo—Brasileiro De Ingenharia Sanitaria E Ambiental; Universidad Sao Paulo: Sao Paulo, Brasil, 1996. [Google Scholar]
- Jiang, H.-L.; Tay, J.-H.; Tay, S.T.-L. Changes in structure, activity and metabolism of aerobic granules as a microbial response to high phenol loading. Appl. Microbiol. Biotechnol. 2004, 63, 602–608. [Google Scholar] [CrossRef]
- Rivas, C.; Mota, M. Temas De Bacteriología Y Virología Médica; FEFMUR: Montevideo, Uruguay, 2006. [Google Scholar]
- Moosvi, S.; Keharia, H.; Madamwar, D. Decolourization of textile dye Reactive Violet 5 by a newly isolated bacterial consortium RVM 11.1. World J. Microbiol. Biotechnol. 2005, 21, 667–672. [Google Scholar] [CrossRef]
- Bell, J.; Buckley, C.A. Treatment of a textile dye in the anaerobic baffled reactor. Water SA 2003, 29, 129–134. [Google Scholar] [CrossRef]
- Russ, R.; Rau, J.; Stolz, A. The function of citoplasmic flavin reductases in the reduction of Azo Dyes by bacteria. Appl. Environ. Microbiol. 2000, 66, 1429–1434. [Google Scholar] [CrossRef]
- Harrelkas, F.; Paulo, A.; Alves, M.M.; EI Khadir, L.; Zahraa, O.; Pons, M.N.; van der Zee, F.P. Photocatalytic and combined anaerobic-photocatalytic treatment of textile dyes. Chemosphere 2008, 72, 1816–1822. [Google Scholar] [CrossRef]
- Lob, K.C.; Tar, C. Effect of Additional Carbon Sources on Biodegradation of Phenol. Bull. Env. Contam. Toxicol. 2000, 64, 756–763. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, C.; Xia, W.; Zeng, G.; Zhou, M.; Liu, Y.Y.; Ke, J.; Huang, C. Simultaneous removal of Cr(VI) and phenol in consortium culture of Bacillus sp. and Pseudomonas putida Migula (CCTCC AB2019). Trans. Nonferrous Met. Soc. China 2008, 18, 1014–1020. [Google Scholar] [CrossRef]
- Chirwa, E.; Wang, Y.-T. Simultaneous chromium (VI) reduction and phenol degradation in an anaerobic consortium of bacteria. Water Res. 2000, 34, 2376–2384. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Zeng, G.; Li, X.; Gao, H. Cr(VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem. 2006, 41, 1981–1986. [Google Scholar] [CrossRef]
- Chowdhury, S.; Thakur, A.; Chaudhuri, R. Novel microbial consortium for laboratory scale lead removal from city effluent. J. Environ. Sci. Technol. 2010, 4, 41–54. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Xu, W.; Zeng, G.; Aibibu, N.; Xu, L.; Beibei, C. Simultaneous Cr(VI) reduction and phenol in pure cultures of Pseudomonas aeruginosa CCTCC AB91095. Bioresour. Technol. 2009, 100, 5079–5084. [Google Scholar] [CrossRef]
- Ambujom, S. Studies on composition and stability of a large membered bacterial consortium degrading phenol. Microbiol. Res. 2001, 156, 293–302. [Google Scholar] [CrossRef]
- Clesceri, L.S. Standard Methods of Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; Volumes 3500-Cr CHROMIUM. [Google Scholar]
- Lokeshwari, N.; Keshava, J. Biosorption of heavy metal (chromium) using biomass. Glob. J. Environ. Res. 2009, 3, 29–35. [Google Scholar]
- Lima, S.A.A.; Pontes, P.M.; Silva, R.F.G.; Hofer, E. Utilization of phenol in the presence of heavy metals by metal-tolerant nonfermentative Gram-negative bacteria isolated from wastewater. Rev. Latinoam. Microbiol. 2007, 49, 68–73. [Google Scholar]
- Quintero, L.; Cardona, S. Evaluation of the biological treatment for indigo color removal of industrial textile waste water by a microbial fluidized bed consortium. Rev. Gestión Y Ambiente 2011, 14, 105–113. [Google Scholar] [CrossRef]
- APHA. Standard Methods of Examination of Water and Wastewater, 16th ed.; AWWA & WEF: Washington, DC, USA, 1998. [Google Scholar]
- Khadijah, O.; Lee, K.K.; Mohd, F. Isolation, screening and development of local bacterial consortia with azo dyes decolourising capability. Malays. J. Microbiol. 2009, 5, 25–32. [Google Scholar] [CrossRef]
- Carrillo, E.; Ruíz, A.; Yeomans, H. Isolation, identification and evaluation of a mixed culture of microorganisms with the capacity to degrade DDT. Rev. Int. Contam. Ambient. 2004, 20, 69–75. [Google Scholar]
- Martínez, G.; Perurena, M.; Núñez, J.; Fernández, C.; Bandera, F. Aislamiento, identificación y tipificación de levaduras en pacientes VIH positivos con candidiasis oral. Rev. Cuba. Med. Trop. 1997, 49, 174–180. [Google Scholar]
- Murray, P.; Rosenthal, K.; Pfaller, M. Microbiología Médica; Elsevier: Barcelona, España, 2009. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology; Williams & Wilkins: Baltimore, MA, USA, 2000. [Google Scholar]
- BioMérieux. API. Available online: http://www.biomerieux-usa.com (accessed on 5 February 2022).
- Bourbie, T.; Zinszner, B. Hydraulic and acoustic properties as an function of porosity in Fontainebleau sandstone. J. Geophys. Res. 1985, 90, 11524–11532. [Google Scholar] [CrossRef]
- Muñoz, D. Diseño e Implementación de Una Planta Piloto para Remoción de DQO de Aguas Residuales de la Industria Textil, Utilizando el Inóculo Microbiano Nativo I5-ESPE, Tésis de Grado; Universidad de las Fuerzas Armasdas ESPE: Sangolquí, Ecuador, 2011. [Google Scholar]
- Lozano, W. Calidad Fisicoquímica del Agua: Métodos Simplificados para Muestreo y Análisis; Universidad Piloto de Colombia: Bogotá, Colombia, 2013. [Google Scholar]
- Martí, N. Phosphorus Precipitation in Anaerobic Digestion Process; Universal-Publishers: Boca Ratón, FL, USA, 2006. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of Levene’s test of equality of variances on statistical theory and practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Mathews, K. Theory & methods: A weighted least squares approach to Levene’s test of homogeneity of variance. Aust. N. Z. J. Stat. 2000, 42, 81–100. [Google Scholar]
- Hoaglin, D.C.; Welsch, R.E. The hat matrix in regression and ANOVA. Am. Stat. 1978, 32, 17–22. [Google Scholar]
- Keenan, D.M. A Tukey nonadditivity type test for time series nonlinearity. Biometrika 1985, 72, 39–44. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Newman-Keuls Test and Tukey Test. Encyclopedia of Research Design; Sage: Thousand Oaks, CA, USA, 2010; pp. 1–11. [Google Scholar]
- King, W.C., Jr.; Miles, E.W.; Day, D.D. A test and refinement of the equity sensitivity construct. J. Organ. Behav. 1993, 14, 301–317. [Google Scholar] [CrossRef]
- Permanasari, A.E.; Rambli, D.R.A.; Dominic, P.D.D. Forecasting method selection using ANOVA and Duncan multiple range tests on time series dataset. In Proceedings of the 2010 International Symposium on Information Technology, Miyazaki, Japan, 23–25 June 2010; Volume 2, pp. 941–945. [Google Scholar]
- Diaconis, P.; Efron, B. Testing for independence in a two-way table: New interpretations of the chi-square statistic. Ann. Stat. 1985, 13, 845–874. [Google Scholar] [CrossRef]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, M. On Fisher′s test of significance in harmonic analysis. Geophys. J. Int. 1971, 23, 373–377. [Google Scholar] [CrossRef]
- Odén, A.; Wedel, H. Arguments for Fisher’s permutation test. Ann. Stat. 1975, 3, 518–520. [Google Scholar] [CrossRef]
- Jurgensen, C.E. Table for determining phi coefficients. Psychometrika 1947, 12, 17–29. [Google Scholar] [CrossRef]
- Ozer, D.J. Correlation and the coefficient of determination. Psychol. Bull. 1985, 97, 307. [Google Scholar] [CrossRef]
- Anderson, T.W. A modification of the sequential probability ratio test to reduce the sample size. Ann. Math. Stat. 1960, 31, 165–197. [Google Scholar] [CrossRef]
- Huber, P.J. A robust version of the probability ratio test. Ann. Math. Stat. 1965, 36, 1753–1758. [Google Scholar] [CrossRef]
- Real, R. Tables of significant values of Jaccard’s index of similarity. Miscel·Lania Zool. 1999, 22, 29–40. [Google Scholar]
- Ivchenko, G.I.; Honov, S.A. On the jaccard similarity test. J. Math. Sci. 1998, 88, 789–794. [Google Scholar] [CrossRef]
- Boesch, D.F. Application of Numerical Classification in Ecological Investigations of Water Pollution; Environmental Protection Agency, Office of Research and Development; Environmental Research Laboratory: Corvallis, OR, USA, 1977.
- Morales, F.; Melgosa, R. Treatment of azo Red Direct dye 23 by anaerobic/aerobic sequenced sequenced reactors. Inf. Tecnol. 2009, 20, 73–82. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Stolz, A. Basis and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 2001, 56, 69–80. [Google Scholar] [CrossRef]
- Gerginova, M.; Dimova, N.; Ivanova, D.; Alexieva, Z. Studies on biodegradation of aromatic pollutants by Trichosroron cutaneum yeast strain. Bioremediation Soils Contam. Aromat. Compd. 2007, 76, 7–74. [Google Scholar] [CrossRef]
- Muñoz, J.; Pérez, B.; Esteban, M.; de la Escalera, S.D.; Gómez, M.; Martínez, M.; González, M. Growth of moderately halophilic bacteria isolated from sea water using phenol as the sole carbon source. Folia Microbiol 2001, 46, 297–302. [Google Scholar] [CrossRef]
- Brady, J.M.; Tobin, J.M. Binding of hard and soft metal ions to Rhizopus arrhizus biomass. Enzime Microb. Technol. 1995, 17, 791–796. [Google Scholar] [CrossRef]
- López, A.; Lázaro, N.; Priego, J.M.; Marqués, A. Effect of pH on the biosorption of nickel and other heavy metals by Pseudomonas fluorescens 4F39. Ind. Microbiol. Biotechnol. 2000, 24, 146–151. [Google Scholar] [CrossRef]
- Ochie, V.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. Clean-J. 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Carmona, M.E.R.; da Silva, M.A.; Ferreira Leite, S.G. Biosorption of chromium using factorial experimental desing. Process Biochem. 2005, 40, 779–788. [Google Scholar] [CrossRef]
- Zahoor, A.; Rehman, A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009, 21, 814–820. [Google Scholar] [CrossRef]
- Garza, M. Isolation of Microorganisms with High Capacity to Tolerate and Remove Pb(II), Cr(VI), Cd(II), Cu(II), Zn(II) y Ni(II). Doctoral Thesis, Universidad de la Habana, La Habana, Cuba, 2005. [Google Scholar]
- Nweke, C.; Okolo, J.; Nwanyanwu, C.; Alisi, C. Response of planktonic bacteria of New Calabar River to zinc stress. Afr. J. Biotechnol. 2006, 5, 653–658. [Google Scholar]
- Nweke, C. Kinetics of zinc toxicity to environmentalbacterial isolates. Ambiente Água 2009, 4, 23–24. [Google Scholar] [CrossRef]
- Dolla, A.; Fournier, M.; Dermoun, Z. Oxygen defense in sulfate-reducing bacteria. J. Biotechnol. 2006, 126, 87–100. [Google Scholar] [CrossRef]
- Kjeldsen, K.; Joulian, C.; Ingvorsen, K. Oxygen tolerance of Sulfate-Reducing Bacteria in Activated Sludge. Environ. Sci. Technol. 2004, 38, 2038–2043. [Google Scholar] [CrossRef]
- Sigalevich, P.; Meshorer, E.; Helman, Y.; Cohen, Y. Transition from anaerobic to aerobic growth conditions for the sulfate-reducing bacterium Desulfovibrio oxyclinae results in flocculation. Appl. Environ. Microbiol. 2000, 66, 5005–5012. [Google Scholar] [CrossRef]
- Butani, N.; Jobanputra, J.; Bhatiya, P.; Patel, R. Recent biological technologies for textile effluent treatment. Int. Res. J. Biol. Sci. 2013, 2, 77–82. [Google Scholar]
- Blümel, S.; Knackmuss, H.J.; Stolz, A. Molecular cloning and characterization of the gene coding for the aerobic azoreductase from Xenophilus azovorans KF46F. Appl. Environ. Microbiol. 2002, 68, 3948–3955. [Google Scholar] [CrossRef]
- Ramya, M.; Anusha, B.; Kalavathy, S. Decolorization and biodegradation of indigo carmine by a textile soil isolate Peaniacillus larvae. Biodegradation 2008, 19, 283–291. [Google Scholar] [CrossRef]
- Snellinx, Z.; Taghavi, S.; Vangronsveld, J.; van der Lelie, D. Microbial consortia that degrade 2,4-DNT by interspecies metabolism: Isolation and characterization. Biodegradation 2003, 14, 19–29. [Google Scholar] [CrossRef]
- Knobelsdorf, J. Biological Elimination of Nutrients in An ARU of Low Organic Load through the VIP Process; Universidad Politécnica de Catalunya: Barcelona, España, 2005. [Google Scholar]
- Pereira, L.; Coelho, A.; Viegas, C.; Correia, M.; Robalo, M.; Martins, L. Enzimatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J. Biotechnol. 2009, 139, 68–77. [Google Scholar] [CrossRef]
- Praveen, G.; Bhat, K. Decolorization of azo dye red 3BN by bacteria. Int. Res. J. Biol. Sci. 2012, 1, 46–52. [Google Scholar]
- Barakat, O.; Darwesh, M.; Sedik, Z.; Moawad, H.; Abd, W. Evidence of biodegradation of reactive red textile azo dye in anoxic/aerobic bioremediation system. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2009, 4, 85–90. [Google Scholar]
- Ziagova, M.G.; Liakopoulou-Kyriakides, M. Comparative studies on the degradation of three aromatic compounds by Pseudomonas sp. and Staphylococcus xylosus. J. Environ. Sci. Health Part A 2010, 45, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewater: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Saravanan, P.; Pakshirajan, K.; Saha, P. Kinetics of phenol and m-cresol biodegradation by an indigenous mixed microbial culture isolated from a sewage treatment plant. J. Environ. Sci. 2008, 20, 1508–1513. [Google Scholar] [CrossRef]
- Işik, M. Efficiency of simulated textile wastewater decolorization process based on the methanogenic activity of upflow anaerobic sludge blanket reactor in salt inhibition condition. Enzim. Microb. Technol. 2004, 35, 399–404. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Oztekin, R. Decolorization of textile dyestuff Reactive Orange 16 in fed-batch reactor under anaerobic condition. Enzym. Microb. Technol. 2003, 33, 231–235. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Anaerobic/aerobic treatment of a simulated textile wastewater. Sep. Purif. Technol. 2008, 60, 64–72. [Google Scholar] [CrossRef]
- Brás, R.; Gomes, A.; Ferra, M.I.A.; Pinheiro, H.M.; Gonçalves, I.C. Monoazo and diazo dye decolourisation studies in a methanogenic UASB reactor. J. Biotechnol. 2005, 115, 57–66. [Google Scholar] [CrossRef]
- Rittman, B.; McCarty, P. Biotecnología del Medio Ambiente, Principios y Aplicaciones; McGraw-Hill: Madrid, España, 2010. [Google Scholar]
- Lapo, B.; Romero, H.; Martínez, O.; García, C.; Lemus, M. CODs removal of domestic wastewater by solid plastic wastes materials: Influence of organic loading rate. Int. J. Appl. Environ. Sci. 2018, 13, 595–604. [Google Scholar]
- Espinoza, K.; Fernandez, C.; Perez, J.; Benalcazar, D.; Romero, D.; Lapo, B. Support materials of fixed biofilm based on solid plastic wastes for domestic wastewater treatment. Rev. Técnica De La Fac. De Ing. Univ. Del Zulia 2019, 42, 67–75. [Google Scholar] [CrossRef]
- Bermudez, J.; Canovas, M.; Manjon, A.; Iborra, J.; Howell, J. Anaerobic Digestion; Unidad Gráfica de Murcia: Murcia, España, 2001. [Google Scholar]
- López, C.; Moreira, M.; Feijoo, G.; Lema, M. Technologies for the treatment of effluents from textile industries. Afinidad 2006, 64, 561–573. [Google Scholar]
- Ramalho, R. Tratamiento de Aguas Residuales; Reverté: Madrid, España, 2003. [Google Scholar]
- Alvarado, A. Evaluation of Waste Materials as a Filter MEDIUM in Anaerobic Upflow Filters. Master’s Thesis, Technologycal Institute of Costa Rica, San Jose, Costa Rica, 2011. [Google Scholar]
- Batero, Y.; Cruz, E. Evaluation of Anaerobic upflow Filters with Guadua Support Medium for the Removal of Organic Matter from a Synthetic Wastewater. Master’s Thesis, Universidad Tecnológica de Pereira, Pereira, Colombia, 2007. [Google Scholar]
- Sattler, M. Anaerobic Processes for Waste Treatment and Energy Generation; Sunill Kumar: Washington, DC, USA, 2011. [Google Scholar]
- Ponce-Arguello, M.; Abad-Sarango, V.; Crisanto-Perrazo, T.; Toulkeridis, T. Removal of METH through Tertiary or Advanced Treatment in a WWTP. Water 2022, 14, 1807. [Google Scholar] [CrossRef]
- Dueñas-Muñoz, D.; Guevara, O.; Oviedo, G.-R.; Crisanto-Perrazo, T.; Toulkeridis, T. Sustainable Treatment Techniques for Emerging Pollutants—The Case of Personal Hygiene Products. Appl. Sci. 2022, 12, 6330. [Google Scholar] [CrossRef]
- Crisanto-Perrazo, T.; Guayasamín-Vergara, J.; Mayorga-Llerena, E.; Sinde-Gonzalez, I.; Vizuete-Freire, D.; Toulkeridis, T.; Flores Gomez, G.; Fierro-Naranjo, G. Determination of Empirical Environmental Indices for the Location of Cemeteries—An Innovative Proposal for Worldwide Use. Sustainability 2022, 14, 6284. [Google Scholar] [CrossRef]
- Poma, P.; Usca, M.; Polanco, M.; Toulkeridis, T.; Mestanza-Ramón, C. Estimation of Biogas Generated in Two Landfills in South-Central Ecuador. Atmosphere 2021, 12, 1365. [Google Scholar] [CrossRef]
- Flores Gómez, G.; Crisanto-Perrazo, T.; Toulkeridis, T.; Fierro-Naranjo, G.; Guevara-García, P.; Mayorga-Llerena, E.; Vizuete-Freire, D.; Salazar, E.; Sinde-Gonzalez, I. Proposal of an Initial Environmental Management and Land Use for Critical Cemeteries in Central Ecuador. Sustainability 2022, 14, 1577. [Google Scholar] [CrossRef]



| Material | Porosity (%) | Density (g/mL) | Specific Weight (N/m3) |
|---|---|---|---|
| Common stone | 44.24 | 1.81 | 17,738 |
| Plastic | 13.96 | 1.26 | 12,348 |
| Coconut shell | 83.06 | 1.03 | 10,084 |
| Material | Dye Removal (%) | COD Reduction (mg/L) | Growth Kinetics (CFU/mL) | VSS (mg/L) | TSS (mg/L) |
|---|---|---|---|---|---|
| Common stone | 34.48 | 674.05 | 3.9 × 107 | 2498.09 | 281.0 |
| Plastic | 32.42 | 540.66 | 2.1 × 107 | 2284.36 | 282.82 |
| Coconut shell | 45.92 | 500.66 | 1.6 × 107 | 2545.46 | 282.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-R, M.; Layedra-Almeida, A.P.; Torres, Y.; Toulkeridis, T. RETRACTED: Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry. Sustainability 2022, 14, 8889. https://doi.org/10.3390/su14148889
Heredia-R M, Layedra-Almeida AP, Torres Y, Toulkeridis T. RETRACTED: Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry. Sustainability. 2022; 14(14):8889. https://doi.org/10.3390/su14148889
Chicago/Turabian StyleHeredia-R, Marco, Andrea Paola Layedra-Almeida, Yenny Torres, and Theofilos Toulkeridis. 2022. "RETRACTED: Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry" Sustainability 14, no. 14: 8889. https://doi.org/10.3390/su14148889
APA StyleHeredia-R, M., Layedra-Almeida, A. P., Torres, Y., & Toulkeridis, T. (2022). RETRACTED: Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry. Sustainability, 14(14), 8889. https://doi.org/10.3390/su14148889






