Climate Change Impact on the Habitat Suitability of Pseudotsuga menziesii Mirb. Franco in Mexico: An Approach for Its Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Records of Presence
2.2. Current and Future Climatic Variables
2.3. Topographic Variables
2.4. Environmental Variable Selection and Model Calibration
2.5. Current Habitat Suitability Model
2.6. Future Habitat Suitability Model and Conservation Zones
2.7. Validation of Habitat Suitability Models
2.8. Contribution of the Environmental Variables
3. Results
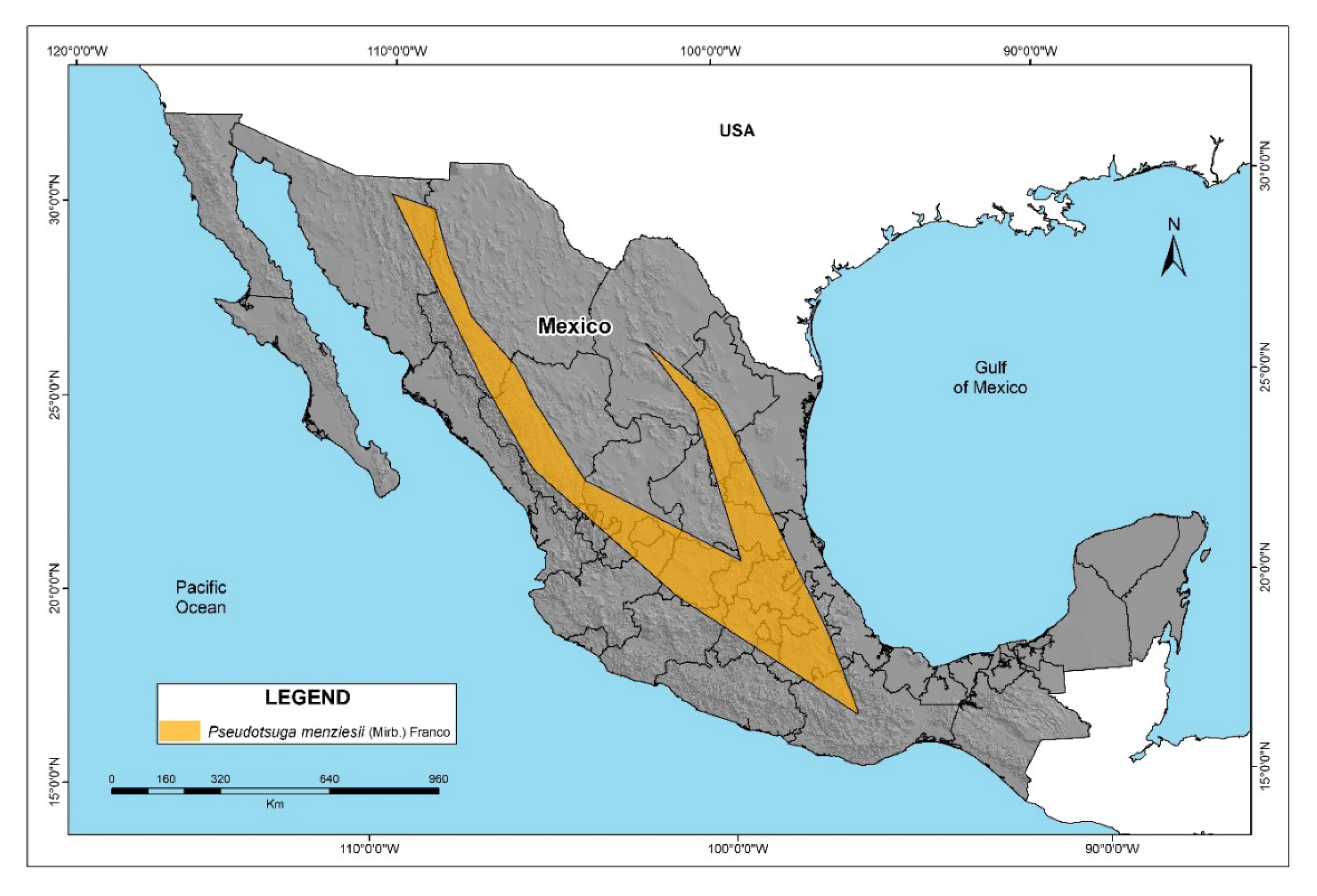
3.1. Model of the Current Habitat Suitability of the P. menziesii
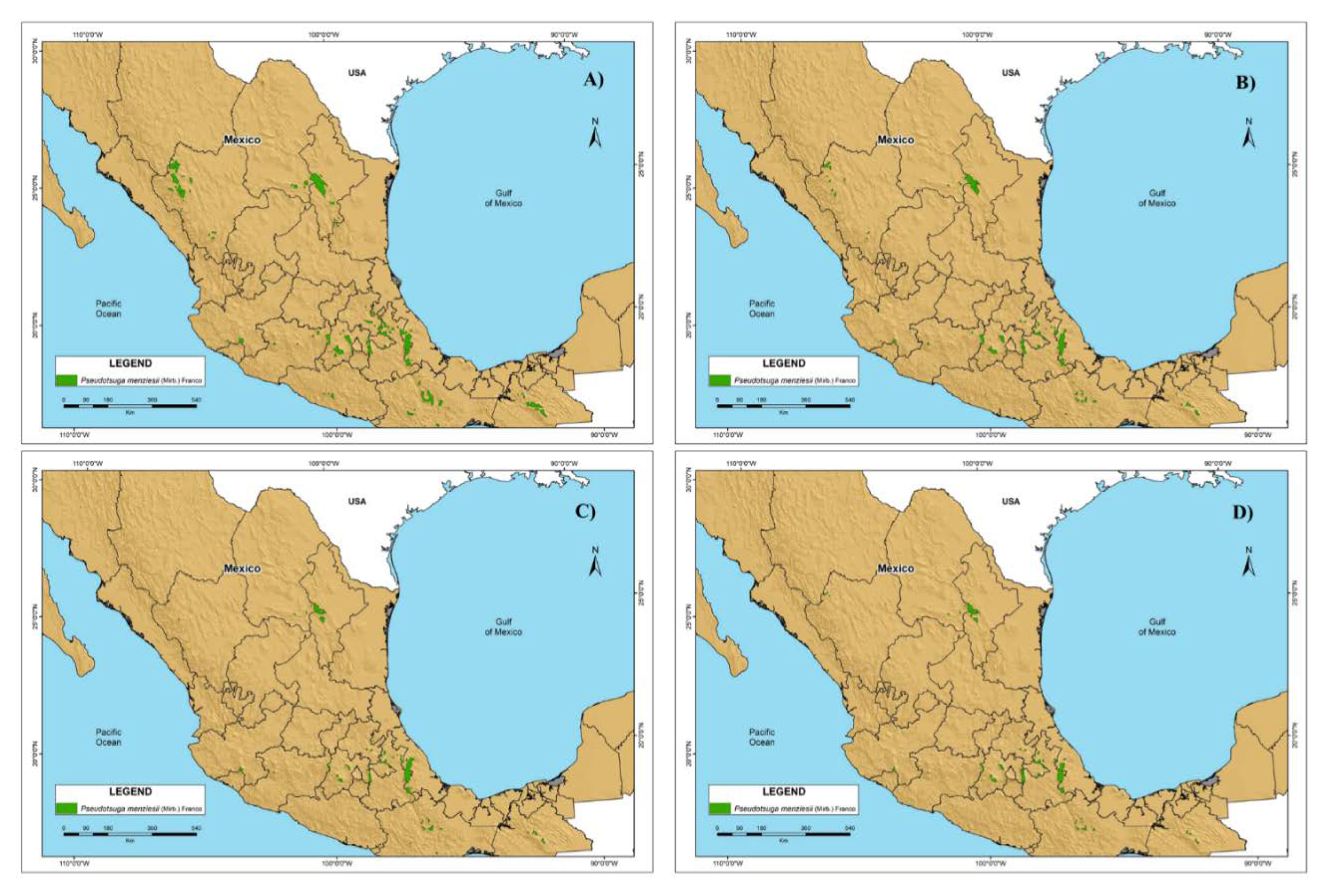
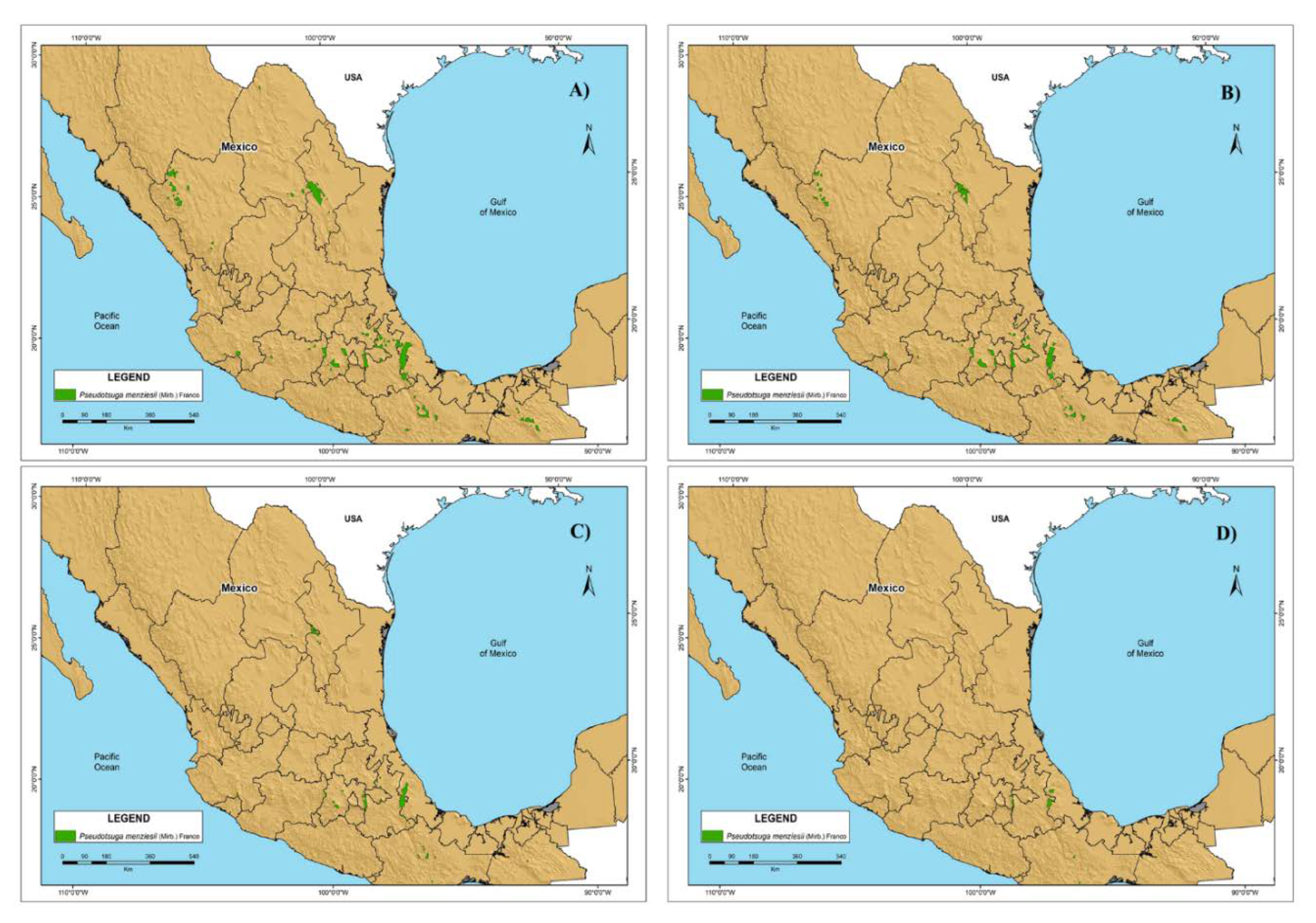
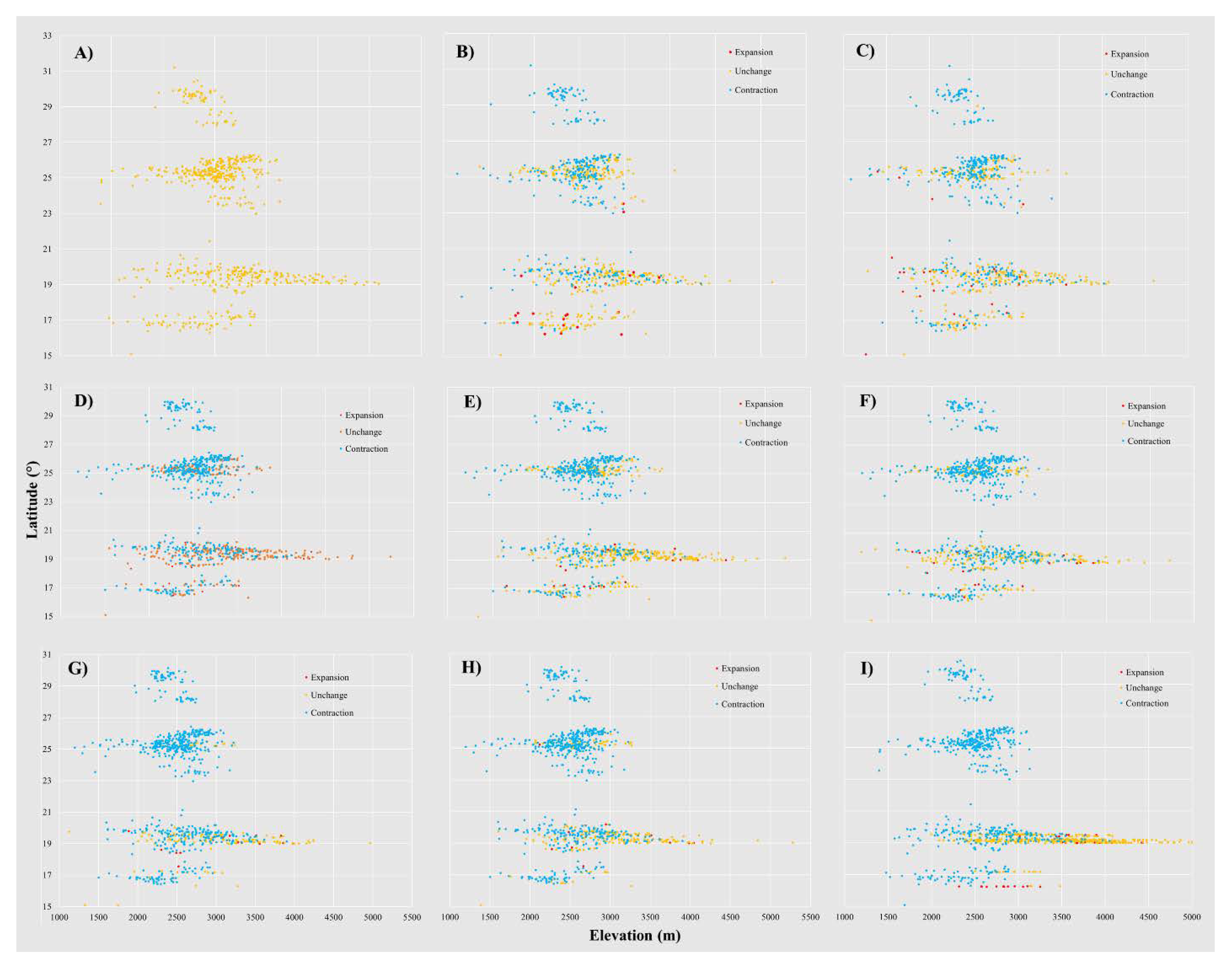
3.2. Habitat Suitability Model under the Climate Change Scenarios
3.3. Relevant Variables in the Habitat Suitability of Current and Future Climate
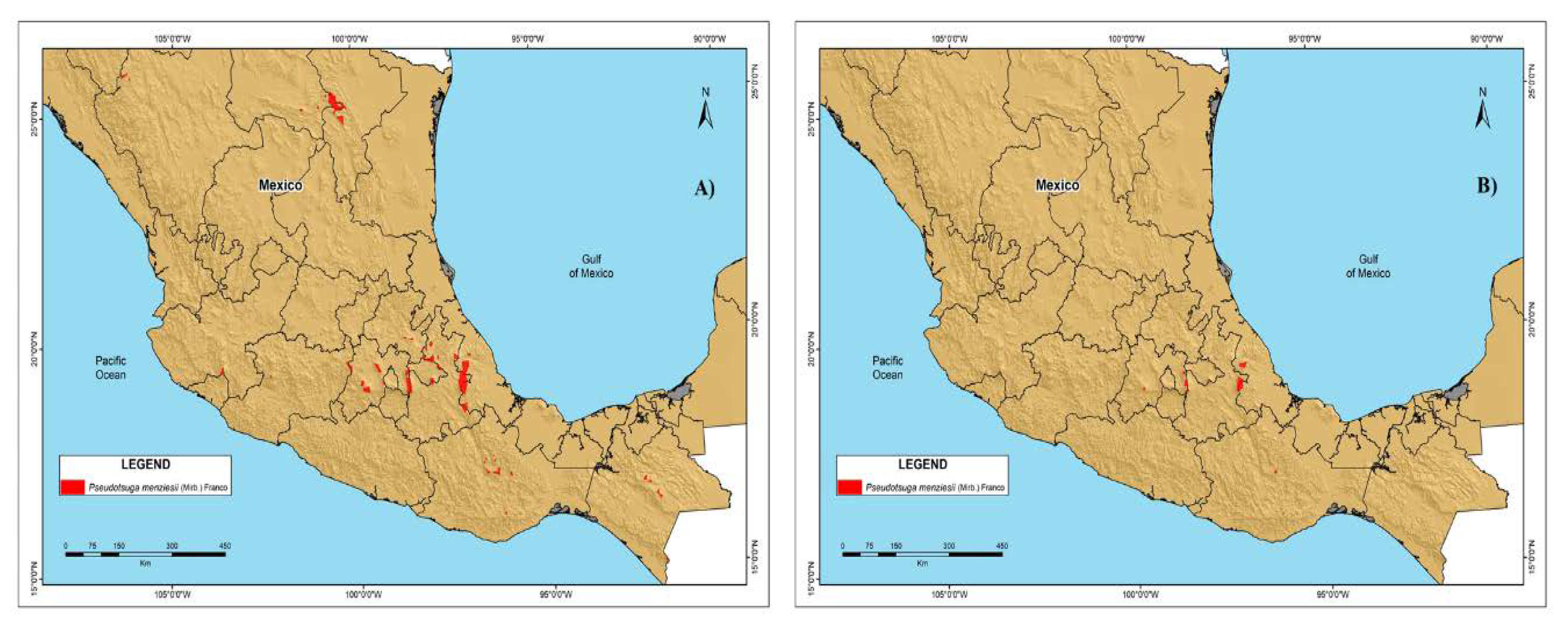
3.4. Future P. menziesii area in México
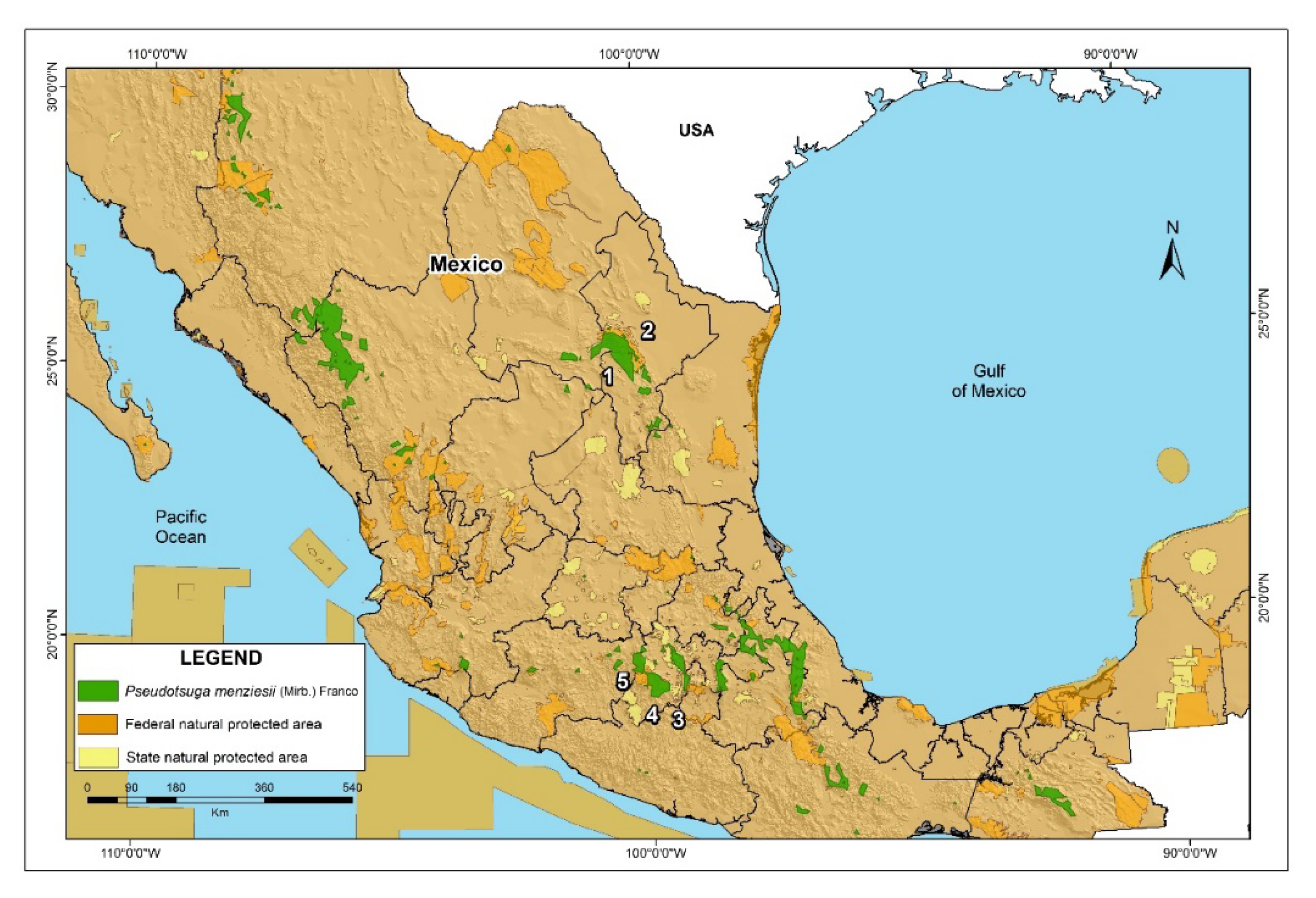
3.5. Suggested P. menziesii Conservation Areas in México
4. Discussion
4.1. Spatial P. menziesii Model
4.2. Relevant Environmental Variables
4.3. Climate Change Scenarios
4.4. Preservation Areas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CONAFOR. Bosques, Cambio Climático y REDD+ en México, 2nd ed.; Guía Básica: Zapopan, Mexico, 2013; pp. 15–17.
- Solís-Sánchez, J.; Blanco-García, A.; Sáenz-Romero, C.; Méndez-Toribio, M.; López-Toledo, L. El cambio climático y las coníferas mexicanas. Biodiversitas 2018, 141, 7–10. [Google Scholar]
- INEGI. Capa Vectorial de Uso de Suelo y Vegetación Serie VI. Aguascalientes, Aguascalientes, Mexico. 2017. Available online: https://www.inegi.org.mx/temas/usosuelo/#Descargas (accessed on 5 December 2021).
- Guerra-De la Cruz, V.; López-Domínguez, J.C.; López-Upton, J.; Bautista-Sampayo, C.; Hernández-García, L. Estructura sil-vícola de poblaciones de Pseudotsuga menziesii (Mirb.) Franco en Tlaxcala y Puebla. Rev. Mex. Cien. For. 2012, 3, 73–86. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11322012000500007&lng=es&tlng=es (accessed on 14 November 2021).
- Martínez, M. Las Pináceas Mexicanas, 3rd ed.; Instituto de Biología, Universidad Autónoma de México: México City, Mexico, 1963; p. 400. [Google Scholar]
- Clair, J.B.; Mandel, N.L.; Vance-Borland, K.W. Genecology of Douglas-fir in Western Oregon and Washington. Ann. Bot. 2005, 96, 1199–1214. [Google Scholar] [CrossRef]
- Reyes-Hernández, V.J.; Vargas-Hernández, J.J.; López-Upton, J.; Vaquera-Huerta, H. Similitud fenotípica de poblaciones mexi-canas de Pseudotsuga Carr. México. Agrociencia 2006, 40, 545–556. Available online: https://www.agrociencia-colpos.mx/index.php/agrociencia/article/view/487/487 (accessed on 11 November 2021).
- Reyes-Hernández, V.J.; Vargas, J.J.; López, J.; Vaquera, H. Variación morfológica y anatómica en poblaciones mexicanas de Pseudotsuga (Pinaceae). Acta Botánica Mex. 2005, 70, 47–67. [Google Scholar] [CrossRef][Green Version]
- DOF. Norma Oficial Mexicana NOM-059-ECOL-2001. SEMARNAT. Protección Ambiental de Especies Nativas de México de Flora y Fauna Silvestres Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies En riesgo. Secr. Medio Ambiente Recur. Nat. 2002. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5173091&fecha=30/12/2010#gsc.tab=0 (accessed on 14 October 2021).
- Domínguez, A. Análisis histórico-ecológico de los bosques de Pseudotsuga menziesii en México. INIFAP-CIR Golfo Centro. Folleto Técnico No 23. 1994. 43p. Available online: https://scholar.google.com/scholar?hl=es&as_sdt=0%2C5&q=An%C3%A1lisis+hist%C3%B3rico+ecol%C3%B3gico+de+los+bosques+de+Pseudotsuga+en+M%C3%A9xico&btnG (accessed on 17 December 2021).
- Del Castillo, R.F.; Pérez-de la Rosa, J.A.; Vargas-Amado, G.; Rivera-García, R. Coníferas. Biodiversidad de Oaxaca; Insti-tuto de Biología-UNAM-Fondo Oaxaqueño para la Conservación de la Naturaleza-World Wildlife Fund: México City, Mexico, 2004; p. 19. [Google Scholar]
- Rzedowski, J. Vegetación de México. Primera Edicion Digital; Comisión Nacional Para el Conocimiento y Uso de la Biodiver-sidad: México City, Mexico, 2006; p. 504. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species distributions areas. Biod. Infor. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeo. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Martínez-Méndez, N.; Aguirre-Planter, E.; Eguiarte, L.E.; Jaramillo-Correa, J.P. Modelado de nicho ecológico de las especies del género Abies (Pinaceae) en México: Algunas implicaciones taxonómicas y para la conservación [Ecological niche modeling of species of the genus Abies (Pinaceae) in Mexico: Some taxonomic and conservation implications]. Bot. Sci. 2016, 94, 5–24. [Google Scholar] [CrossRef]
- Caballero, M.; Lozano-Garcia, S.; Vázquez-Selem, L.; Ortega, B. Evidencias del cambio climático y ambiental en registros glaciales y en cuencas lacustres del centro de México durante el último máximo glacial. Bol. Soc. Geo. Méx. 2010, 62, 359–377. [Google Scholar] [CrossRef]
- Manzanilla-Quiñones, U.; Aguirre-Calderón, O.A.; Jiménez-Pérez, J.; Treviño-Garza, E.J.; Yerena-Yamallel, J.I. Distribución actual y futura del bosque subalpino de Pinus hartwegii Lindl. En el Eje Neovolcánico Transversal. Mad. Bos. 2019, 25, e2521804. [Google Scholar] [CrossRef]
- Svensson, A.; Andersen, K.; Bigler, M.; Clausen, B.; Dahl-Jensen, D.; Davies, M.; Johnsen, J.; Muscheler, R.; Parrenin, F.; Ras-mussen, O.; et al. A 60,000 year Greenland stratigraphic ice core chronology. Clim. Past. 2008, 4, 47–57. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Araújo, M.B. Consequences of climate change on the Tree of Life in Eu-rope. Nature 2011, 448, 550–552. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Rehfeldt, G.E.; Crookston, N.L.; Pierre, D.; St-Amant, R.; Beaulieu, J.; Richardson, B. Contemporary and projected Spline Climate surfaces for Mexico and their use in understanding Climate-plant relationships. Clim. Chan. 2010, 102, 595–623. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on the Impacts of Global Warming of 1.5 °C Relative to Pre-Industrial Levels and Corresponding Trajectories That Global Greenhouse Gas Emission Should Follow, in the Context of Strengthening the Global Response to the Threat Climate Change, Sustainable Development and Efforts to Eradicate Poverty; OMMPNUMA, IPCC: Geneva, Switzerland, 2019; p. 32. Available online: http://www.ipcc.ch/site/assets/uploads/sites/2/2019/09/IPCC-Special-Report-1.5-SPM_es.pdf (accessed on 8 January 2022).
- IPCC. Resumen Para Responsables de Políticas. Contribución del Grupo de trabajo II al Quinto Informe de Evaluación del Panel Intergubernamental de Expertos sobre el Cambio Climático; Impactos, adaptación y vulnerabilidad; En Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastran-drea, M.D., Billir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambio Climático: Ginebra, Switzerland, 2014. [Google Scholar]
- Cruz-Cárdenas, G.; López-Mata, L.; Silva, J.T.; Bernal-Santana, N.; Estrada-Godoy, F.; López-Sandoval, J.A. Potential distribu-tion model of Pinaceae species under climate change scenarios in Michoacán. Rev. Chap. Ser. Cie. For. Amb. 2016, 22, 135–148. [Google Scholar] [CrossRef]
- Hernández-Ruíz, J.; Herrera-Cabrera, B.E.; Delgado-Alvarado, A.; Salazar-Rojas, V.M.; Bustamante-González, A.; Cam-pos-Contreras, J.E.; Ramírez-Juárez, J. Distribución potencial y características geográficas de poblaciones silvestres de Vanilla planifolia (Orchidaceae) en Oaxaca, México. Rev. Biol. Trop. 2016, 64, 235–246. [Google Scholar] [CrossRef]
- Martínez-Sifuentes, A.R.; Villanueva-Díaz, J.; Crisantos, E.; Stahle, D. Current and future spatial modeling of habitat suitabil-ity of the Mexican baldcypress (Taxodium mucronatum Ten.): A proposal for conservation in Mexico. Bot. Sci. 2021, 99, 752–770. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Rehfeldt, G.E.; Ortega-Rodríguez, J.M.; Marín-Togo, M.C.; Madrigal-Sánchez, X. Pinus leiophylla suitable habitat for 1961–1990 and future climate. Bot. Sci. 2015, 93, 709–718. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Mod. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- García-Aranda, M.A.; Cantú-Ayala, C.; Estrada-Castillón, E.; Pando-Moreno, M.; Moreno-Ralamantes, A. Distribución actual y potencial de Taxus globosa (Taxaceae) en México. J. Bot. Res. Inst. Tex. 2012, 6, 587–598. Available online: https://www.researchgate.net/publication/246548792_DISTRIBUCION_ACTUAL_Y_POTENCIAL_DE_TAXUS_GLOBOSA_TAXACEAE_EN_MEXICO (accessed on 11 February 2022).
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. 2018. Available online: https://www.gbif.org/occurrence/download/0033021-200221144449610 (accessed on 5 April 2021). [CrossRef]
- CONAFOR. Inventario Nacional Forestal y de Suelos 2013–2014. Regist. Pseudotsuga Menziesii. 2014. Available online: https://www.inegi.org.mx/rnm/index.php/catalog/390 (accessed on 12 December 2021).
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; Valdez-Leal, J.D.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruíz, C.; Rubio-Rocha, Y. Distribución potencial histórica y contemporánea de la familia Psittacidae en México. Rev. Mex. Biod. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Vijay, B.; Narayani, B.; Soberón, J.; Falconi, M. Ntbox: From Getting Biodiversity Data to Evaluating Species Distributions Models in a Friendly GUI Environment. R Package Version 0.2.5.4. 2019. Available online: https://github.com/luismurao/ntbox (accessed on 9 May 2021).
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Inter-comparison Project Phase-6 (CMIP-6) experimental design and organization. Geosci. Model. Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- INEGI. Continuo de Elevaciones Mexicano 3.0. Aguascalientes, Aguascalientes, México. 2013. Available online: https://www.inegi.org.mx/app/geo2/elevacionesmex/ (accessed on 14 June 2021).
- ESRI. Software ArcGIS Version 10.3. 2014. Available online: https://www.arcgis.com/index.html (accessed on 5 February 2021).
- Fitz-Maurice, B.; Sotomayor, M.; Fitz-Maurice, W.A.; Hernández, H.; Smith, M. Astrophytum Coahuilense (Bonete de Obispo); Distribución Conocida; Catálogo de Metadatos Geográficos/Comisión Nacional para el Conocimiento y Uso de la Biodi-Versidad (CONABIO) México. 2013. Available online: http://www.conabio.gob.mx/informacion/gis (accessed on 8 September 2021).
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. App. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 6 March 2021).
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche mod-els. Met. Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Leh-mann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T. Introducción a la Biogeografía, 1st ed.; Universidad Nacional Autónoma de México: México City, Mexico, 2016; ISBN 968-36-9463-2. [Google Scholar]
- Martínez-Sifuentes, A.R.; Villanueva-Díaz, J.; Manzanilla-Quiñones, U.; Becerra-López, J.L.; Hernández-Herrera, J.A.; Estrada-Ávalos, J.; Velázquez-Pérez, A. Spatial modeling of the ecological niche of Pinus greggii Engelm. (Pinaceae): A species conservation proposal in Mexico under climate change scenarios. iForest 2020, 13, 426–434. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Peterson, A.T.; Nakazawa, Y. Environmental data sets matter in ecological niche modelling: An example with Solenopsis invicta and Solenopsis richteri. Glob. Ecol. Biogeo. 2008, 17, 135–144. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R. Estimating optimal complexity for ecological niche models: A Jacknife approach for species with small simple sices. Ecol. Mod. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Barve, N. Tool for Partial ROC, Ver 1.0.; Biodiversity Institute: Lawrence, KS, USA, 2008. [Google Scholar]
- Ramos-Dorantes, D.B.; Villaseñor, J.L.; Ortiz, E.; Gernandt, D.S. Biodiversity, distribution, and conservation status of Pinaceae in Puebla, Mexico. Rev. Mex. Biod. 2017, 88, 215–223. [Google Scholar] [CrossRef]
- Domínguez, A.; Vargas, J.J.; López, J.; Ramírez, P.; Guízar, E. Aspectos ecológicos de Pseudotsuga menziesii en el ejido de Barranca, Pinal de Amoles; Querétaro. In Anales del Instituto de Biología; Serie Botánica, julio–diciembre, núm. 002; Univer-sidad Nacional Autónoma de México: México City, Mexico, 2004; Volume 75, p. 203. [Google Scholar]
- Dominguez, F.A. Estudio Ecológico de Pseudotsuga menziesii (Mirb) Franco, en la Región de Huayacocotla, Ver; Tesis. Universidad Autónoma Chapingo: Texcoco, Mexico, 1986. [Google Scholar]
- Vázquez, H.T. Caracterización ecológica de una localidad de Pseudotsuga menziesii (Mirb.) Franco var. glauca (Beissn.) Franco, en el estado de Puebla. Tesis de Licenciatura. División de Ciencias Forestales, Universidad Autónoma Chapingo. Chapingo, Edo. de México. 2004. Available online: https://1library.co/document/dzxvk6dy-conservacion-caracterizacion-pseudotsuga-menziesii-mirb-franco-centro-mexico.html (accessed on 8 October 2021).
- Villagómez-Loza, M.A.; Bello-González, M.A. Pseudotsuga menziesii (Mirb.) Franco var. glauca (Beissn.) Franco: Nuevo registro para Guanajuato. Rev. Mex. Cien. For. 2015, 6, 66–73. [Google Scholar] [CrossRef]
- Debreczy, Z.; Rácz, I. New species and varieties of conifers from Mexico. Phytologia 1995, 78, 217–243. [Google Scholar] [CrossRef]
- Rzedowski, J.; Vela, L.; Madrigal, Y.X. Algunas consideraciones acerca de la dinámica de los bosques de coníferas en México. Cien. For. 1977, 1, 15–35. [Google Scholar]
- Aceves-Rangel, L.D.; Méndez-González, J.; García- Aranda, M.A.; Nájera-Luna, J.A. Potential distribution of 20 pine species in Mexico. Agrociencia 2018, 52, 1043–1057. Available online: http://www.colpos.mx/agrocien/Bimestral/2018/octnov/art-9.pdf (accessed on 5 November 2021).
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwelad, E.; Juenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- SEMARNAP. NOM-ECOL-054-1994. Diario Oficial. 1994. Available online: https://www.dof.gob.mx/nota_detalle_popup.php?codigo=662124 (accessed on 9 September 2021).
- García, E. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). 1998. Available online: http://www.conabio.gob.mx/informacion/metadata/gis/clima1mgw.xml?_xsl=/db/metadata/xsl/fgdc_html.xsl&_indent=no (accessed on 12 July 2021).
- Villanueva-Díaz, J.; Constante-García, V.; Cerano-Paredes, J.; Martínez-Sifuentes, A.R.; Stahle, D.W.; Estrada-Ávalos, J. Fenología y crecimiento radial del sabino (Taxodium mucronatum Ten.) en el río San Pedro Mezquital, Durango. Gómez Palacio, Durango, México: Centro Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Folleto técnico 27. 2013. Available online: http://cenid-raspa.inifap.gob.mx/demo/modulo/Folletos%20tecnicos/2013/27_fenologia%20y%20crecimiento%20radial%20del%20sabino%20(%20TAXODIUM%20MUCRONATUM%20TEN).pdf (accessed on 9 December 2021).
- Girardin, M.P.; Guo, X.J.; Bernier, P.Y.; Raulier, F.; Gauthier, S. Changes in growth of pristine boreal North American forests from 1950 to 2005 driven by land-scape demographics and species traits. Biogeosciences 2012, 9, 2523–2536. [Google Scholar] [CrossRef]
- Medhaug, I.; Stolpe, M.B.; Fischer, E.M.; Knutti, R. Reconciling controversies about the “global warming hiatus”. Nature 2017, 545, 41–47. [Google Scholar] [CrossRef]
- Abram, N.; Gattuso, J.P.; Prakash, A.; Cheng, L.; Chidichimo, M.P.; Crate, S.; Enomoto, H.; Garschagen, M.; Gruber, N.; Har-per, S.; et al. Framing and Context of the Report. In The Ocean and Cryosphere in a Changing Climate. A Special Report of the Intergovernmental Panel on Climate Change; Pörter, H.O., Roberts, D.C., Masson-Delmonte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019; pp. 73–131. Available online: https://www.ipcc.ch/site/assets/upload/sites/3/2019/12/SROCC_FullReport_FINAL.pdf (accessed on 11 December 2021).
- Gennaretti, F.; Arseneault, D.; Nicault, A.; Perreault, L.; Begin, Y. Volcano-induced regime shifts in millennial tree-ring chro-nologies from northeastern North America. Proc. Natl. Acad. Sci. USA 2014, 111, 10077–10082. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ramos, J.; Reynoso, R.; Hernández-Ramos, A.; García-Cuevas, X.; Hernández-Máximo, E.; Cob-Uicab, J.V.; Su-mano-López, D. Distribución histórica, actual y futura de Cedrela odorata en México. Act. Bot. Mex. 2018, 124, 117–134. [Google Scholar] [CrossRef]
- Turner, J.; Mitchell, S.J. The effect of short day treatments on containerized Douglas-fir morphology, physiology and phenology. New For. 2003, 26, 279–295. [Google Scholar] [CrossRef]
- Reynoso-Santos, R.; Pérez-Hernández, M.J.; López-Báez, W.; Hernández-Ramoz, J.; Muñoz-Flores, H.J.; Cob-Uicab, J.V.; Rey-noso-Santos, M.D. El nicho ecológico como herramienta para predecir áreas potenciales de dos especies de pino. Rev. Mex. Cien. For. 2018, 9, 47–70. [Google Scholar] [CrossRef]
- Villanueva-Díaz, J.; Stahle, D.; Luckman, B.; Cerano-Paredes, J.; Therrell, M.; Cleaveland, K.; Cornejo-Oviedo, E. Winter-spring precipitation reconstructions from tree rings for northeast Mexico. Clim. Chan. 2007, 83, 117–131. [Google Scholar] [CrossRef]
- Sanhueza, A.; Bourke, M.; Grosse, H.; Chacón, I.; Alvarez, P. Cultivo de Pino de Oregon; Programa de Diversificación Forestal; Corporación Nacional Forestal: Santiago, Chile, 1998; p. 106. [Google Scholar]
- Shafer, S.L.; Bartlein, P.J.; Thompson, R.S. Potential changes in the distributions of western North America tree and shrub taxa under future climate scenarios. Ecosystems 2001, 4, 200–215. [Google Scholar] [CrossRef]
- Soberón, J.; Miller, C. Evolución de los nichos ecológicos. Misc. Mat. 2009, 49, 83–99. Available online: https://miscelaneamatematica.org/download/tbl_articulos.pdf2.bd85f1f79716195b.343930362e706466.pdf (accessed on 12 February 2022).
- Aguirre-Gutiérrez, J.; Duivenvoorden, J.F. Can we expect to protect threatened species in protected areas? A case study of the genus Pinus in Mexico. Rev. Mex. Biod. 2010, 81, 875–882. [Google Scholar]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Modeling the climate suitability of tea Camellia sinensis (L.) O. Kuntze in Sri Lanka in response to current and future climate change scenarios. Agric. For. Meteorol. 2019, 272, 102–117. [Google Scholar] [CrossRef]
| Variable | Description |
|---|---|
| BIO1 | Annual average temperature |
| BIO2 | Diurnal average range |
| BIO3 | Isotherms |
| BIO4 | Seasonal temperature |
| BIO5 | Maximum temperature of the hottest month |
| BIO6 | Minimum temperature of the coldest month |
| BIO7 | Annual temperature range |
| BIO8 | Average temperature of the most humid trimester |
| BIO9 | Average temperature of the driest trimester |
| BIO10 | Average temperature of the hottest trimester |
| BIO11 | Average temperature of the coldest trimester |
| BIO12 | Annual precipitation |
| BIO13 | Precipitation of the most humid month |
| BIO14 | Precipitation of the driest month |
| BIO15 | Seasonality (Coefficient of variation) |
| BIO16 | Precipitation of the most humid trimester |
| BIO17 | Precipitation of the driest trimester |
| BIO18 | Precipitation of the hottest trimester |
| BIO19 | Precipitation of the coldest trimester |
| Model | AUC (2030) | AUC (2050) | AUC (2070) | AUC (2090) |
|---|---|---|---|---|
| CNRM-CM6 (SSP 245) | 0.902–0.907 T 0.764–0.936 V | 0.906–0.907 T 0.865–0.906 V | 0.900–0.906 T 0.774–0.905 V | 0.903–0.907 T 0.796–0.911V |
| CNRM-CM6 (SSP 585) | 0.901–0.906 T 0.715–0.907 V | 0.898–0.906 T 0.790–0.910 V | 0.901–0.906 T 0.795–0.901 V | 0.902–0.906 T 0.851–0.908 V |
| Model | Partial ROC | Standard Error | Z Test |
|---|---|---|---|
| CNRM-CM6 (SSP 245) (2030) | 1.92 | 0.06 | <0.01 |
| CNRM-CM6 (SSP 585) (2030) | 1.91 | 0.06 | <0.01 |
| CNRM-CM6 (SSP 245) (2050) | 1.92 | 0.05 | <0.01 |
| CNRM-CM6 (SSP 585) (2050) | 1.90 | 0.05 | <0.01 |
| CNRM-CM6 (SSP 245) (2070) | 1.89 | 0.06 | <0.01 |
| CNRM-CM6 (SSP 585) (2070) | 1.88 | 0.06 | <0.01 |
| CNRM-CM6 (SSP 245) (2090) | 1.90 | 0.06 | <0.01 |
| CNRM-CM6 (SSP 585) (2090) | 1.91 | 0.06 | <0.01 |
| Model | Variables | Total Contribution (%) |
|---|---|---|
| Current | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Average temperature of the coldest trimester, Average temperature of the most humid trimester and Precipitation of the driest month | 91.1 |
| CNRM-CM6 (SSP 245) (2030) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest month, Precipitation of the driest trimester and Average temperature of the coldest trimester | 95.8 |
| CNRM-CM6 (SSP 585) (2030) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest month, Precipitation of the driest trimester and Average temperature of the most humid trimester | 95.8 |
| CNRM-CM6 (SSP 245) (2050) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest month, Average temperature of the coldest trimester and Average temperature of the most humid trimester | 95.5 |
| CNRM-CM6 (SSP 585) (2050) | Maximum temperature of the hottest month, Average temperature of the coldest trimester, Precipitation of the driest trimester, Precipitation of the coldest trimester and Precipitation of the driest month | 93.5 |
| CNRM-CM6 (SSP 245) (2070) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest month, Precipitation of the driest trimester and Average temperature of the most humid trimester | 95.2 |
| CNRM-CM6 (SSP 585) (2070) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest trimester, Average temperature of the coldest trimester and Average temperature of the most humid trimester | 94.8 |
| CNRM-CM6 (SSP 245) (2090) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest month, Average temperature of the coldest trimester and Orientation | 97.9 |
| CNRM-CM6 (SSP 585) (2090) | Maximum temperature of the hottest month, Precipitation of the coldest trimester, Precipitation of the driest trimester, Average temperature of the coldest trimester and Precipitation of the driest month | 97.2 |
| Model | Area (km2) |
|---|---|
| CNRM-CM6 (SSP 245) (2030) | 15,856.49 |
| CNRM-CM6 (SSP 585) (2030) | 13,091.17 |
| CNRM-CM6 (SSP 245) (2050) | 7940.77 |
| CNRM-CM6 (SSP 585) (2050) | 8662.28 |
| CNRM-CM6 (SSP 245) (2070) | 5511.16 |
| CNRM-CM6 (SSP 585) (2070) | 3103.58 |
| CNRM-CM6 (SSP 245) (2090) | 5199.82 |
| CNRM-CM6 (SSP 585) (2090) | 913.73 |
| Natural Protected Area | SSP 245 Scenario Area (km2) | SSP 585 Scenario Area (km2) |
|---|---|---|
| Bajo Río San Juan | 547.46 | - |
| Iztaccihuatl-Popocatépetl | 297.50 | 157.06 |
| Cumbres de Monterrey | 246.16 | - |
| Nevado de Toluca | 226.82 | 22.91 |
| Parque Otomí-Mexica | 185.04 | - |
| Pico de Orizaba | 179.54 | 174.32 |
| Cofre de Perote | - | 76.96 |
| Montaña Malinche | - | 11.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sifuentes, A.R.; Hernández-Herrera, J.A.; Valenzuela-Núñez, L.M.; Briceño-Contreras, E.A.; Manzanilla-Quiñones, U.; Gastélum-Arellánez, A.; Trucíos-Caciano, R.; López Calderón, M.J. Climate Change Impact on the Habitat Suitability of Pseudotsuga menziesii Mirb. Franco in Mexico: An Approach for Its Conservation. Sustainability 2022, 14, 8888. https://doi.org/10.3390/su14148888
Martínez-Sifuentes AR, Hernández-Herrera JA, Valenzuela-Núñez LM, Briceño-Contreras EA, Manzanilla-Quiñones U, Gastélum-Arellánez A, Trucíos-Caciano R, López Calderón MJ. Climate Change Impact on the Habitat Suitability of Pseudotsuga menziesii Mirb. Franco in Mexico: An Approach for Its Conservation. Sustainability. 2022; 14(14):8888. https://doi.org/10.3390/su14148888
Chicago/Turabian StyleMartínez-Sifuentes, Aldo Rafael, José Antonio Hernández-Herrera, Luis Manuel Valenzuela-Núñez, Edwin Amir Briceño-Contreras, Ulises Manzanilla-Quiñones, Argel Gastélum-Arellánez, Ramón Trucíos-Caciano, and Magali Jeaneth López Calderón. 2022. "Climate Change Impact on the Habitat Suitability of Pseudotsuga menziesii Mirb. Franco in Mexico: An Approach for Its Conservation" Sustainability 14, no. 14: 8888. https://doi.org/10.3390/su14148888
APA StyleMartínez-Sifuentes, A. R., Hernández-Herrera, J. A., Valenzuela-Núñez, L. M., Briceño-Contreras, E. A., Manzanilla-Quiñones, U., Gastélum-Arellánez, A., Trucíos-Caciano, R., & López Calderón, M. J. (2022). Climate Change Impact on the Habitat Suitability of Pseudotsuga menziesii Mirb. Franco in Mexico: An Approach for Its Conservation. Sustainability, 14(14), 8888. https://doi.org/10.3390/su14148888







