Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Sample Collection
2.3.2. Soil Physicochemical Analysis
2.3.3. DNA Extraction
2.3.4. PCR Amplification
2.3.5. Library Construction and MiSeq Sequencing
2.4. Data Analysis
3. Results
3.1. Changes in Physical and Chemical Properties of Reclaimed Water under Different Irrigation Levels
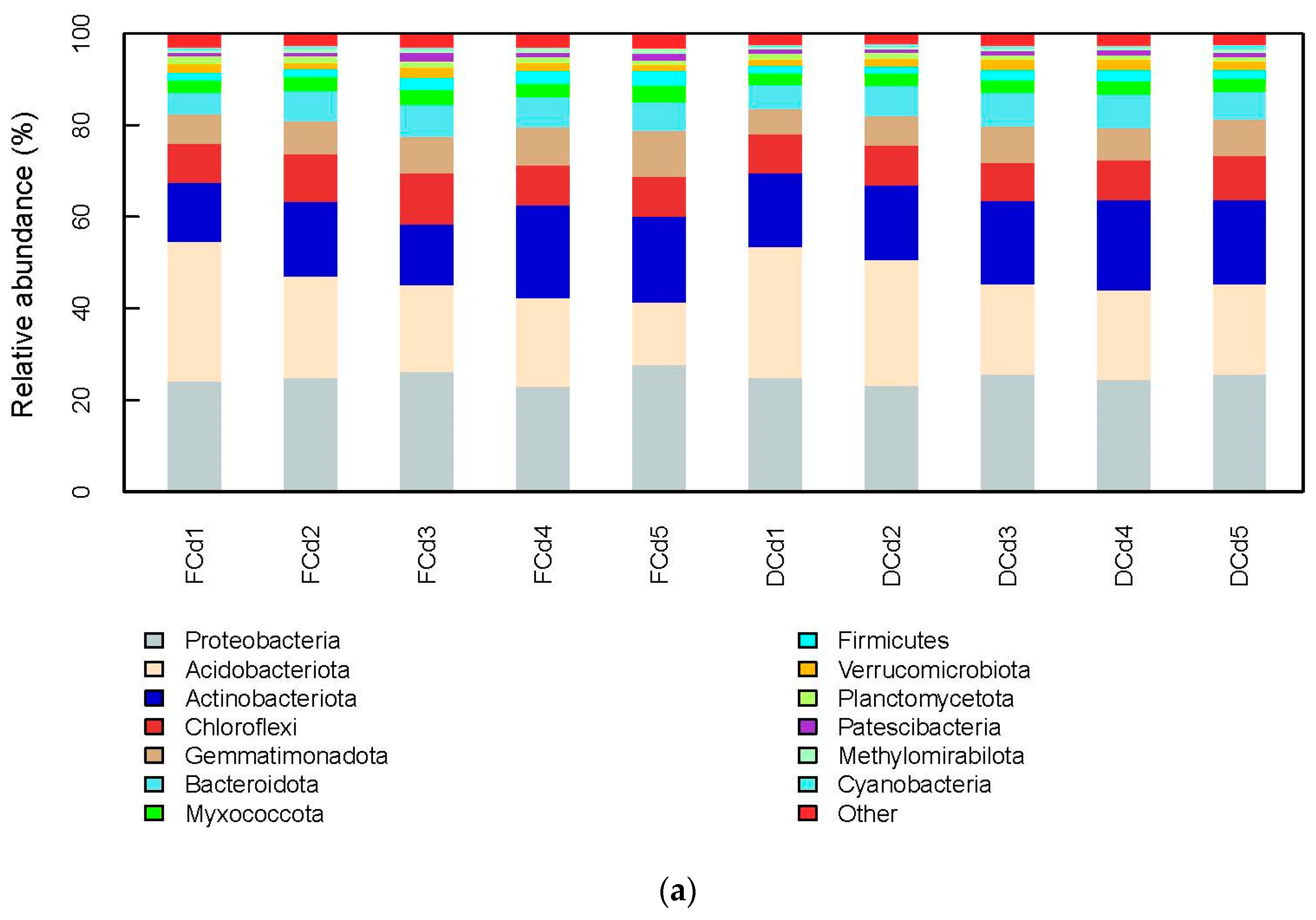
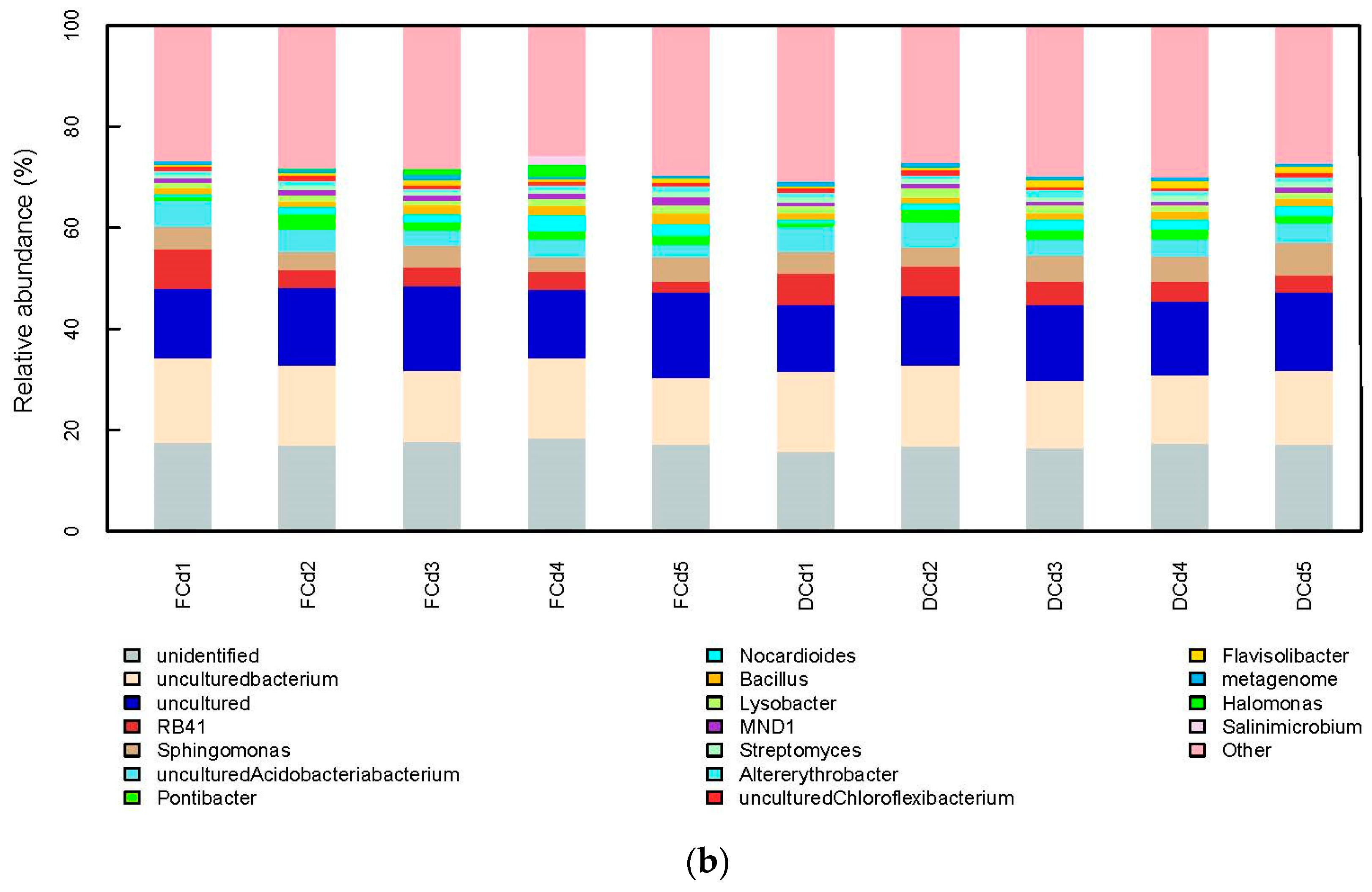
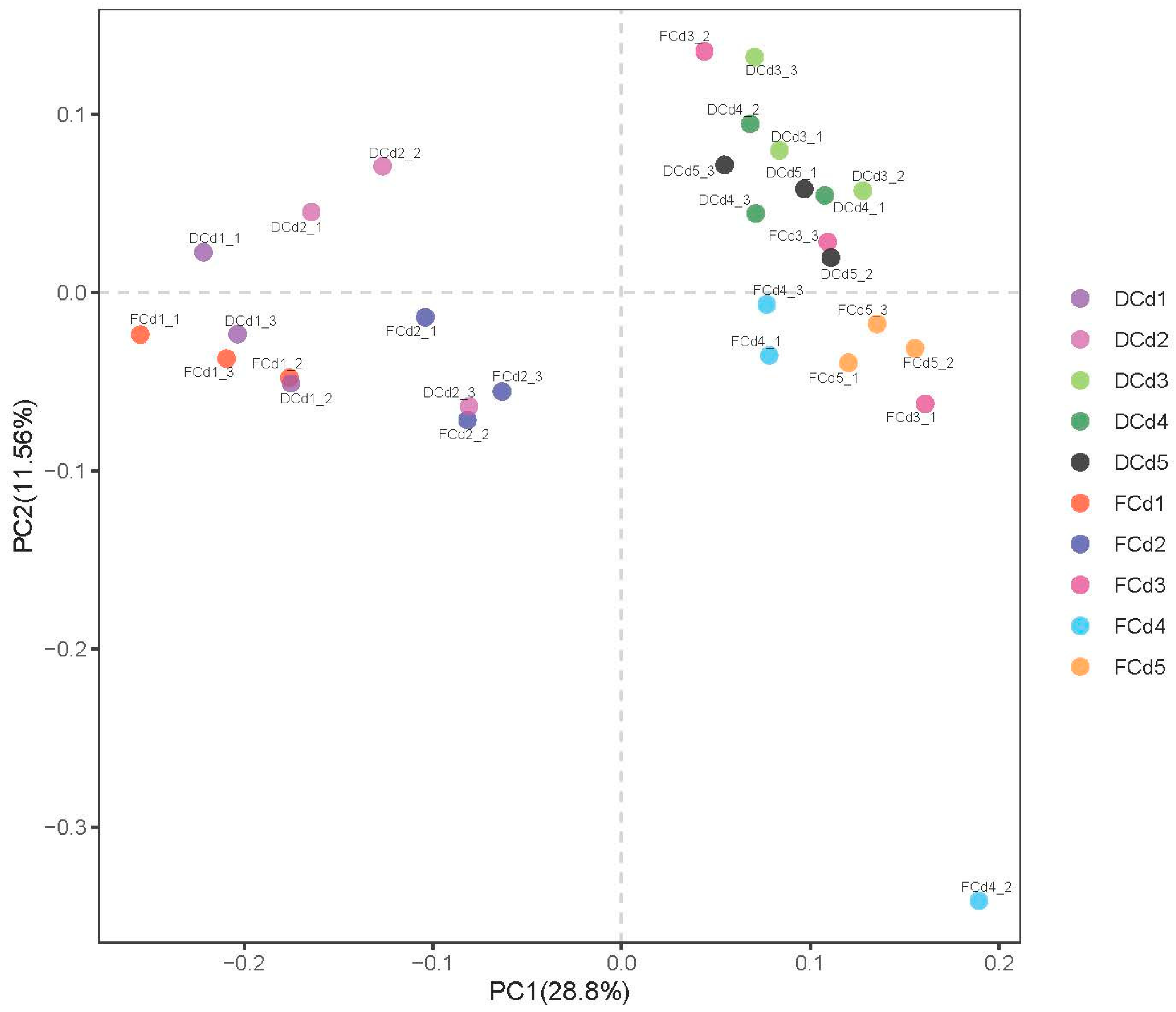
3.2. Analysis of Bacterial Community Structure and Diversity
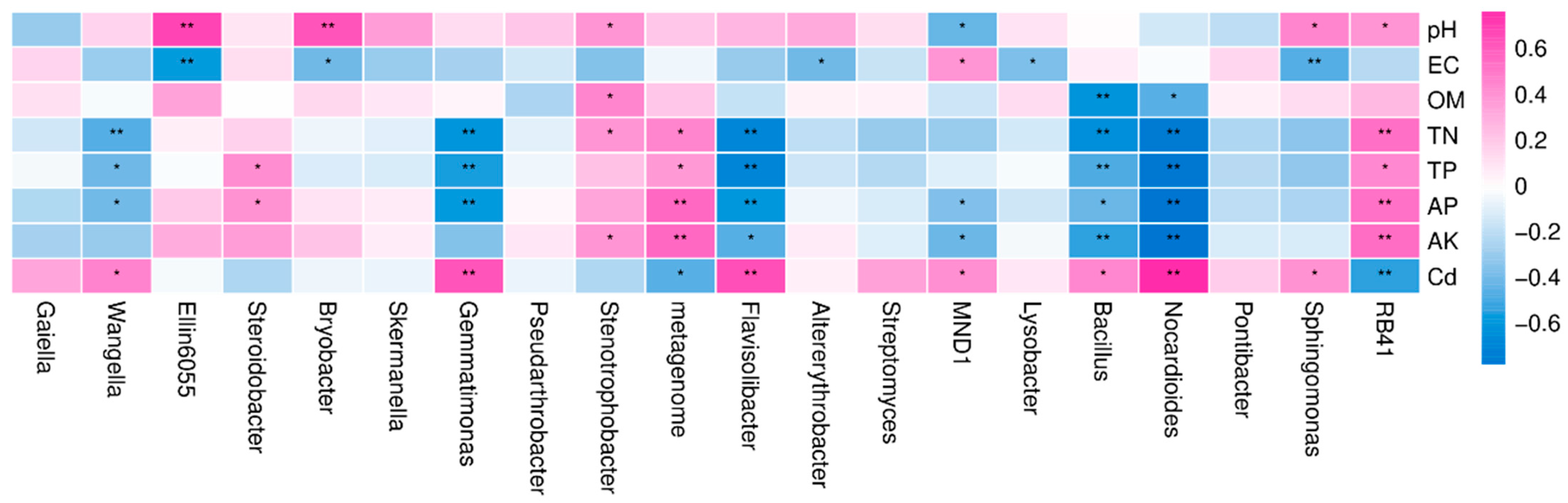
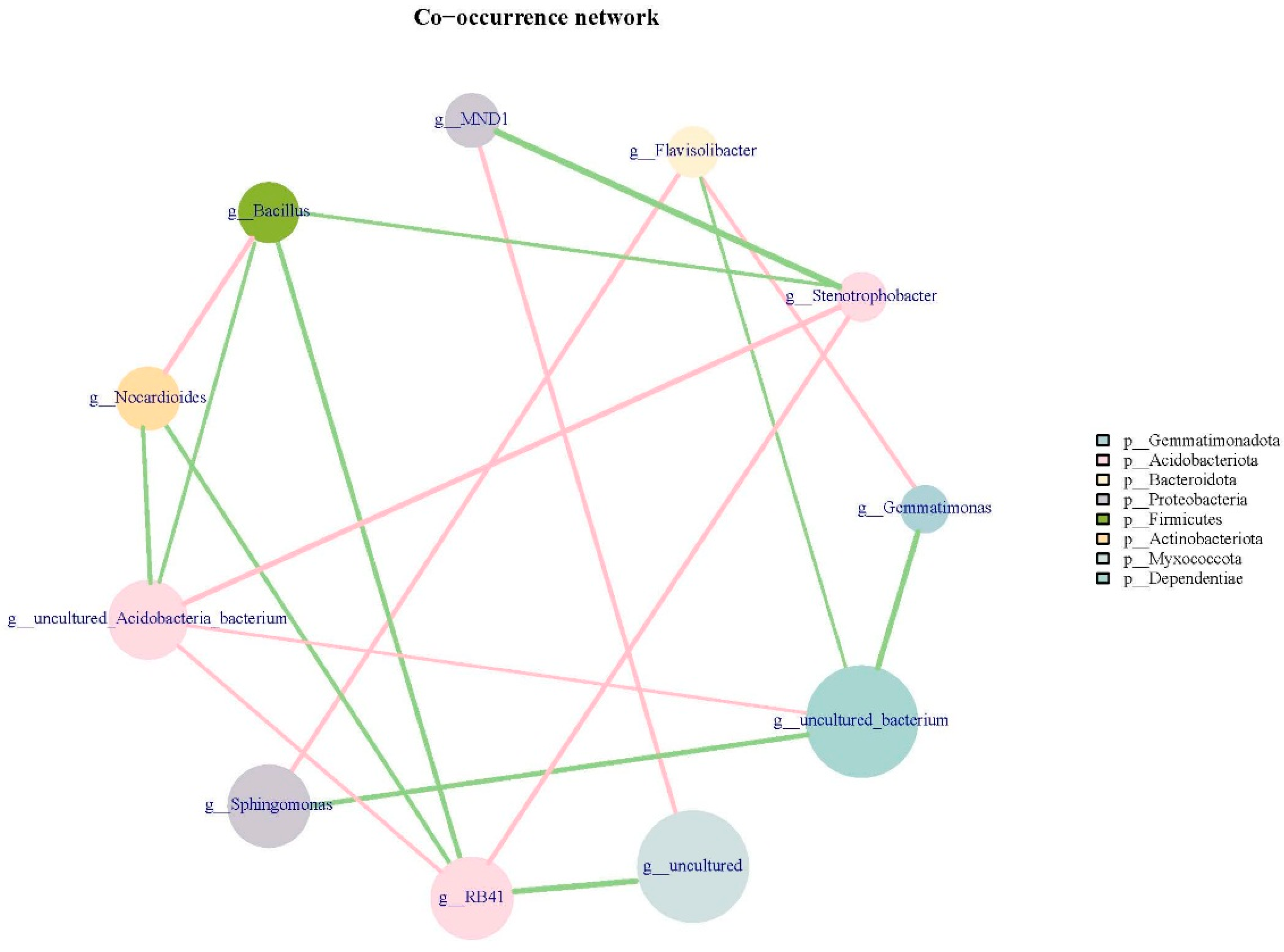
3.3. Correlation Analysis of Bacterial Community Clustering Characteristics and Environmental Factors
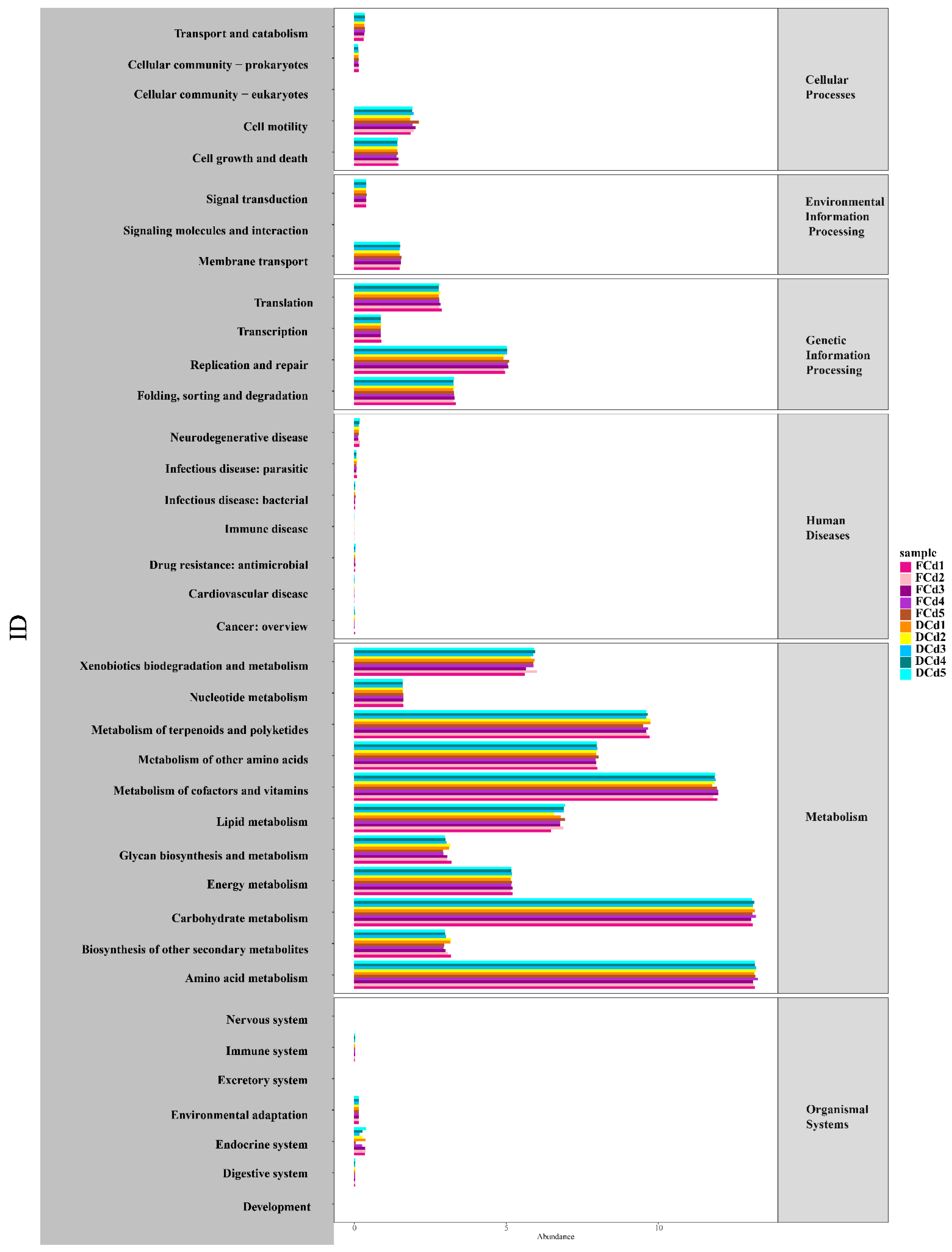
3.4. Functional Prediction of Microbial Communities between Different Treatments
4. Discussion
4.1. Effect of Reclaimed Water Irrigation Amount on the Physicochemical Properties of Rhizosphere Soil with Different Cadmium Concentrations
4.2. Effect of Different Irrigation Levels of Reclaimed Water on the Structural Diversity of Rhizosphere Soil Microbial Communities Contaminated with Different Cadmium Concentrations
4.3. Correlation Analysis of Bacterial Community Clustering Characteristics of Rhizosphere Soil and Environmental Factors with Reclaimed Water Irrigation
4.4. Prediction of Bacterial Community Function in Rhizosphere Soil under Cadmium Concentrations with Reclaimed Water Irrigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World 2021; 978-92-5-134325-8; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; ISBN 978-92-5-134325-8. [Google Scholar]
- Yin, L.; Feng, X.; Fu, B.; Chen, Y.; Wang, X.; Tao, F. Irrigation water consumption of irrigated cropland and its dominant factor in China from 1982 to 2015. Adv. Water Resour. 2020, 143, 103661. [Google Scholar] [CrossRef]
- China, The reuse of urban recycling water—Water quality standard for green space irrigation. In Standardization Administration of the People’s Republic of China; Standards Press of China: Beijing, China, 2010; Volume GB/T 25499-2010.
- Li, K.; Wei, Y.; Wang, J.; Cheng, Y.; Cheng, M.; Li, Y. Water reclamation: Standards comparison and cost analysis. Acta Sci. Circumstantiae 2014, 34, 1635–1653. [Google Scholar]
- Chen, Y.; Geng, H.; Liu, J.; Ouyang, R.; Han, Y. Problems and thinking of reclaimed water utilization planning and management. China Water Resour. 2021, 15, 52–54. [Google Scholar]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Leonel, L.P.; Tonetti, A.L. Wastewater reuse for crop irrigation: Crop yield, soil and human health implications based on giardiasis epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Amori, P.N.; Mierzwa, J.C.; Bartelt-Hunt, S.; Guo, B.; Saroj, D.P. Germination and growth of horticultural crops irrigated with reclaimed water after biological treatment and ozonation. J. Clean. Prod. 2022, 336, 130173. [Google Scholar] [CrossRef]
- Lequette, K.; Ait-Mouheb, N.; Wéry, N. Hydrodynamic effect on biofouling of milli-labyrinth channel and bacterial communities in drip irrigation systems fed with reclaimed wastewater. Sci. Total Environ. 2020, 738, 139778. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Zaccaria, D.; Camposeo, S.; Pasanisi, F.; Salcedo, F.P.; Portoghese, I. Appraising water and nutrient recovery for perennial crops irrigated with reclaimed water in Mediterranean areas through an index-based approach. Sci. Total Environ. 2022, 820, 152890. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Cytryn, E.; Donner, E.; Zhang, T. Challenges related to antimicrobial resistance in the framework of urban wastewater reuse. Water Res. 2020, 170, 115308. [Google Scholar] [CrossRef]
- Shtull-Trauring, E.; Cohen, A.; Ben-Hur, M.; Israeli, M.; Bernstein, N. NPK in treated wastewater irrigation: Regional scale indices to minimize environmental pollution and optimize crop nutritional supply. Sci. Total Environ. 2022, 806, 150387. [Google Scholar] [CrossRef]
- Weber, E.; Grattan, S.R.; Hanson, B.R.; Vivaldi, G.A.; Meyer, R.D.; Prichard, T.L.; Schwankl, L.J. Recycled water is suitable for irrigation of vineyards in Napa County. Calif. Agric. 2014, 68, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Grattan, S.R.; Díaz, F.J.; Pedrero, F.; Vivaldi, G.A. Assessing the suitability of saline wastewaters for irrigation of citrus spp. emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015, 157, 48–58. [Google Scholar] [CrossRef]
- Zaragoza, C.A.; García, I.F.; García, I.M.; Poyato, E.C.; Díaz, J.A.R. Spatio-temporal analysis of nitrogen variations in an irrigation distribution network using reclaimed water for irrigating olive trees. Agric. Water Manag. 2022, 262, 107353. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Ruiz-Navarro, A.; Alarcón, J.J.; García, C.; Nicolás, E. Comparing the impacts of drip irrigation by freshwater and reclaimed wastewater on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar] [CrossRef]
- Cui, B.; Hu, C.; Fan, X.; Cui, E.; Li, Z.; Ma, H.; Gao, F. Changes of endophytic bacterial community and pathogens in pepper (Capsicum annuum L.) as affected by reclaimed water irrigation. Appl. Soil Ecol. 2020, 156, 103627. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, W.; Liu, W.; Guo, X. Effect of reclaimed water effluent on bacterial community structure in the Typha angustifolia L. rhizosphere soil of urbanized riverside wetland, China. J. Environ. Sci. 2017, 55, 58–68. [Google Scholar] [CrossRef]
- Cui, B.; Luo, J.; Jin, D.; Jin, B.; Zhuang, X.; Bai, Z. Investigating the bacterial community and amoebae population in rural domestic wastewater reclamation for irrigation. J. Environ. Sci. 2018, 70, 97–105. [Google Scholar] [CrossRef]
- Guo, W.; Qi, X.; Xiao, Y.; Li, P.; Andersen, M.N.; Zhang, Y.; Zhao, Z. Effects of Reclaimed Water Irrigation on Microbial Diversity and Composition of Soil with Reducing Nitrogen Fertilization. Water 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Mahjoub, O.; Mauffret, A.; Michel, C.; Chmingui, W. Use of groundwater and reclaimed water for agricultural irrigation: Farmers’ practices and attitudes and related environmental and health risks. Chemosphere 2022, 295, 133945. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Li, X.; Wu, Z.; Gao, F. Genetic Diversity Analysis of 13 Dwarf Tomato Germplasm Resources by Phenotypic Traits. Mol. Plant 2022, 20, 1955–1964. [Google Scholar]
- Lu, Q. Tomato Quality Traits Diversity Analysis and Agronomic Characters Identification. Master’s Thesis, Northwest A&F University Xi’an, Xi’an, China, 2021. [Google Scholar]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637–638, 1400–1412. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Tang, C.; Wu, J.; Muhammad, A.; Wang, H. Application of 16S rDNA-PCR amplification and DGGE fingerprinting for detection of shift in microbial community diversity in Cu-, Zn-, and Cd-contaminated paddy soils. Chemosphere 2006, 62, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Ruangdech, T.; Wongphatcharachai, M.; Staley, C.; Sadowsky, M.; Sajjaphan, K. Influence of heavy metals on rhizosphere microbial communities of Siam weed (Chromolaena odorata (L.)) using a 16S rRNA gene amplicon sequencing approach. Agric. Nat. Resour. 2017, 51, 137–141. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil organic matter prevails over heavy metal pollution and vegetation as a factor shaping soil microbial communities at historical ZnePb mining sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Nazaries, L.; Tottey, W.; Robinson, L.; Khachane, A.; Abu Al-Soud, W.; Sørensen, S.; Singh, B.K. Shifts in the microbial community structure explain the response of soil respiration to land-use change but not to climate warming. Soil Biol. Biochem. 2015, 89, 123–134. [Google Scholar] [CrossRef]
- Xiao, W.; Lin, G.; He, X.; Yang, Z.; Wang, L. Interactions among heavy metal bioaccessibility, soil properties and microbial community in phyto-remediated soils nearby an abandoned realgar mine. Chemosphere 2022, 286, 131638. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.; Li, M. Responses of soil and earthworm gut bacterial communities to heavy metal contamination. Environ. Pollut. 2020, 265, 114921. [Google Scholar] [CrossRef]
- Yao, H.; He, Z.; Wilson, M.J.; Campbell, C.D. Microbial Biomass and Community Structure in a Sequence of Soils with Increasing Fertility and Changing Land Use. Microb. Ecol. 2000, 40, 223–237. [Google Scholar] [CrossRef]
- Smalla, K.; Wieland, G.; Buchner, A.; Zock, A.; Parzy, J.; Kaiser, S.; Roskot, N.; Heuer, H.; Berg, G. Bacterial bulk and rhizosphere communities studied by denaturing gradient gel electrophoresis of PCR-ampli®ed fragments of 16S rRNA genes: Plantdependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol. 2001, 67, 4742–4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shidan, B. The Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, A.; Wu, L.; Crowley, D. Assessing the soil quality of long-term reclaimed wastewater-irrigated cropland. Geoderma 2003, 114, 261–278. [Google Scholar] [CrossRef]
- Jiao, Z.; Huang, Z.; Li, Y.; Wang, W.; Yan, B.; Peng, L.; Li, H. The Effect of Reclaimed Water Irrigation on Soil Performance and the Microorganism. J. Agro-Environ. Sci. 2010, 29, 319–323. [Google Scholar]
- Li, Z.; Fan, X.; Qi, X.; Qiao, D.; Li, P.; Zhao, Z. Effect of reclaimed municipal wastewater on ryegrass growth and soil phosphorus conversion. Chin. J. Eco-Agric. 2012, 20, 1072–1076. [Google Scholar] [CrossRef]
- Bandara, T.; Chathurika, J.; Franks, A.; Xu, J.; Tang, C. Interactive effects ofbiochar type and pH on the bioavailability ofAs and Cd and microbial activities in co-contaminated soils. Environ. Technol. Innov. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Yao, B.; Wang, S.; Xie, S.; Li, G.; Sun, G. Optimal soil Eh, pH for simultaneous decrease of bioavailable Cd, As in co-contaminated paddy soil under water management strategies. Sci. Total Environ. 2022, 806, 151342. [Google Scholar] [CrossRef]
- Hu, K.; Yu, H.; Feng, W.; Qin, Y.; Lan, L.; Liao, M.; Wang, C.; Tu, S. Effect of Different Fertilizers of Secondary, Micro-and Beneficial Elements on Soil pH and Cd Availability under Waterlogged Condition. Southwest China J. Agric. Sci. 2010, 23, 1188–1193. [Google Scholar]
- Wang, Y.; Cao, X.; Tan, C.; Huang, D.; Wang, T.; Tan, C.; He, Q.; Liang, Y. Effects of Different Soil pH on Cadmium Fractions and Cadmium Accumulation in Rice. J. Nat. Sci. Hunan Norm. Univ. 2017, 40, 10–16. [Google Scholar]
- Xu, X.; Sun, W.; Wu, W.; Liu, H. Effect of irrigation with reclaimed water on soil salt and ion content in Beijing. Trans. CSAE 2010, 26, 34–39. [Google Scholar] [CrossRef]
- Zalacáin, D.; Martínez-Pérez, S.; Bienes, R.; García-Díaz, A.; Sastre-Merlín, A. Salt accumulation in soils and plants under reclaimed water irrigation in urban parks of Madrid (Spain). Agric. Water Manag. 2019, 213, 468–476. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Mazahreh, N. Changes in Soil Fertility Parameters in Response to Irrigation of Forage Crops with Secondary Treated Wastewater. Commun. Soil Sci. Plant Anal. 2003, 34, 1281–1294. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Pan, N.; Wang, Y.; Wu, L. Impact of reclaimed water irrigation on soil health in urban green areas. Chemosphere 2015, 119, 654–661. [Google Scholar] [CrossRef]
- Singh, B.R.; Myhr, K. Cadmium uptake by barley as affected by Cd sources and pH levels. Geoderma 1998, 84, 185–194. [Google Scholar] [CrossRef]
- Zu, Y.; Li, Y.; Chen, H.; Guhur, M.; Schvartz, C. Research on Factors Influencing Concentrations Pb, Cd, Cu and Zn in Vegetables. J. Agro -Environ. Sci. 2003, 22, 289–292. [Google Scholar]
- Hongwei, J.; Li, N.; Su, Y.; Dou, Y.; Mamat, A. Effects of long-term reclaimed water irrigation on soil properties and heavy metal content in arid mountainous area. J. Arid. Land Resour. Environ. 2020, 34, 181–186. [Google Scholar]
- Liu, M.; Wang, X.; Ji, H. Research Progress of Heavy Metal Accumulation in Reclaimed Water Agricultural Irrigation. J. Irrig. Drain. 2021, 40 (Suppl. 2), 77–80. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater-a case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Qian, X.; Lü, Q.; He, X.; Wang, Y.; Li, H.; Xiao, Q.; Zheng, X.; Lin, R. Pseudomonas sp. TCd-1 significantly alters the rhizosphere bacterial community of rice in Cd contaminated paddy field. Chemosphere 2022, 290, 133257. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 1–6. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolarik, M.; Štursová, M.; Kopecky, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. Int. Soc. Microb. Ecol. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmat-Jou, M.H.; Safari-Sinegani, A.A.; Mirzaie-Asl, A.; Tahmourespour, A. Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 2018, 27, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Cui, E.; Hu, C.; Fan, X.Y.; Gao, F. Effects of Selected Biochars Application on the Microbial Community Structures and Diversities in the Rhizosphere of Water Spinach (Ipomoea aquatica Forssk) Irrigated with Reclaimed Water. Environ. Sci. 2020, 41, 5636–5647. [Google Scholar]
- Guo, W.; Andersen, M.N.; Zhou, Y.; Qi, X.; Li, P.; Li, Z.; Fan, X. Effects of reclaimed water irrigation and nitrogen fertilization on the chemical properties and microbial community of soil. J. Ofintegrativeagriculture 2017, 16, 679–690. [Google Scholar] [CrossRef]
- Li, S.; Fan, X.; Cui, E.; Gao, F.; Wu, H.; Li, S.; Cui, B.; Hu, C. Effects of Dripping Rate with Reclaimed Water on Typical Microbial Community Structure in the Root Zone Soil of Tomato. J. Irrig. Drain. 2021, 40, 26–35. [Google Scholar] [CrossRef]
- Xian, W.; Zhang, X.; Li, W. Research status and prospect on bacterial phylum Chloroflexi. Microbiol. Technol. Microb. Ecol. Theory 2020, 60, 1801–1820. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yu, Z.; Wang, X.; Jin, J.; Liu, X. Research Progress of Acidobacteria Ecology in Soils. Biotechnol. Bull. 2016, 32, 14–20. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Sun, X.; Häggblom, M.M.; Dong, Y.; Zhang, M.; Yang, Z.; Xiao, E.; Xiao, T.; Gao, P.; Li, B.; et al. Metabolic potentials of members of the class Acidobacteriia in metal-contaminated soils revealed by metagenomic analysis. Environ. Microbiol. 2021, 24, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and distribution patterns of acidobacterial communities in_the black soil zone of northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Shang, Y.; Zhao, Q.; Shi, J. Effects of biochar (BC) on microbial diversity of cadmium (Cd) contaminated soil. China Environ. Sci. 2017, 37, 252–262. [Google Scholar]
- Li, B.; Cao, Y.; Guan, X.; Li, Y.; Hao, Z.; Hu, W.; Chen, L. Microbial assessments of soil with a 40-year history of reclaimed wastewater irrigation. Sci. Total Environ. 2019, 651, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, D.H.; Tan, W.B.; Yu, H.; Xi, B.D.; Jiang, Y.H.; Dang, Q.L. Different Responses of Soil Microbial Community Structure to Irrigation with Treated Wastewater from Domestic and Industrial Sources. Environ. Sci. 2020, 41, 4253–4261. [Google Scholar]
- Frenk, S.; Hadar, Y.; Minz, D. Resilience of soil bacterial community to irrigation with water of different qualities under Mediterranean climate. Environ. Microbiol. 2013, 16, 559–569. [Google Scholar] [CrossRef]
- Sun, H.; Terhonen, E.; Koskinen, K.; Paulin, L.; Kasanen, R.; Asiegbu, F.O. Bacterial diversity and community structure along different peat soils in boreal forest. Appl. Soil Ecol. 2014, 74, 34–45. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Yano, I.; Oyaizu, H.; Hashimoto, Y.; Ezaki, T.; Yamamoto, H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. n ov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and Two Genospecies of the Genus Sphingomonas. Microbiol. Immunol. 1990, 34, 99–119. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, Y.K.; Yoon, J.H.; Takeuchi, M.; Pyun, Y.R.; Park, Y.H. Sphingomonas aquatilis sp. nov., Sphingomonas koreensis sp. nov., and Sphingomonas taejonensis sp. nov., yellow-pigmented bacteria isolated from natural mineral water. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt 4, 1491–1498. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Liang, X.; Duan, R.; Yang, L.; Du, Y.; Wu, L.; Huang, J.; Xiang, G.; Bai, J.; et al. Assessment of rhizosphere bacterial diversity and composition in a metal hyperaccumulator (Boehmeria nivea) and a nonaccumulator (Artemisia annua) in an antimony mine. J. Appl. Microbiol. 2022, 132, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Gao, F.; Hu, C.; Li, Z.Y.; Fan, X.Y.; Cui, E.P. Effect of Different Reclaimed Water Irrigation Methods on Bacterial Community Diversity and Pathogen Abundance in the Soil-Pepper Ecosystem. Environ. Sci. 2019, 40, 5151–5163. [Google Scholar]
- Deng, Y.; Jiang, Y.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyse. BMC Bioinform. 2012, 13, 1471–2105. [Google Scholar] [CrossRef] [Green Version]
- Guimerà, R.; Sales-Pardo, M.; Amaral, L.A.N. Classes of complex networks defined by role-to-role connectivity profiles. Nat. Phys. 2007, 3, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Irrigation Level | Irrigation Level Symbol | Cadmium Pollution Concentration | |
|---|---|---|---|---|
| FCd1 | Full irrigation | F | Cd1 | no pollution |
| FCd2 | F | Cd2 | slight pollution | |
| FCd3 | F | Cd3 | light pollution | |
| FCd4 | F | Cd4 | moderate pollution | |
| FCd5 | F | Cd5 | moderate pollution | |
| DCd1 | Deficit irrigation | D | Cd1 | no pollution |
| DCd2 | D | Cd2 | slight pollution | |
| DCd3 | D | Cd3 | light pollution | |
| DCd4 | D | Cd4 | moderate pollution | |
| DCd5 | D | Cd5 | moderate pollution | |
| Treatment | pH | EC Value/μs·cm−1 | Organic Matter Content/g·kg−1 | Total N Content/mg·g−1 | Total P Content/mg·g−1 | Available P Content/mg·kg−1 | Available K Content/mg·kg−1 | Total Cd Content/mg·kg−1 |
|---|---|---|---|---|---|---|---|---|
| FCd1 | 8.53 ± 0.03 bc | 515.67 ± 7.77 d | 29.34 ± 2.76 abc | 0.73 ± 0.04 a | 0.97 ± 0.03 a | 50.72 ± 1.12 a | 254.73 ± 1.85 b | 0.27 ± 0.02 f |
| FCd2 | 8.22 ± 0.02 f | 758.33 ± 8.33 a | 26.53 ± 0.57 abcd | 0.66 ± 0.02 b | 0.71 ± 0.06 b | 40.76 ± 3.03 c | 220.34 ± 2.22 c | 0.61 ± 0.16 e |
| FCd3 | 8.35 ± 0.02 e | 627.00 ± 9.17 b | 22.7 ± 1.64 cd | 0.52 ± 0.02 c | 0.55 ± 0.01 c | 35.86 ± 0.26 de | 201.4 ± 1.57 de | 1.10 ± 0.09 d |
| FCd4 | 8.34 ± 0.01 e | 598.67 ± 9.50 c | 22.07 ± 2.9 e | 0.51 ± 0.02 c | 0.54 ± 0.02 c | 32.89 ± 1.43 f | 185.74 ± 0.63 g | 1.89 ± 0.15 c |
| FCd5 | 8.47 ± 0.02 d | 472.67 ± 6.51 e | 21.93 ± 0.15 e | 0.48 ± 0.01 c | 0.57 ± 0.03 c | 32.89 ± 1.57 f | 197.31 ± 1.27 ef | 2.51 ± 0.06 b |
| DCd1 | 8.52 ± 0.01 c | 438.33 ± 2.08 g | 29.48 ± 0.64 a | 0.75 ± 0.02 a | 0.96 ± 0.01 a | 46.15 ± 1.26 b | 260.88 ± 7.37 a | 0.27 ± 0.02 f |
| DCd2 | 8.52 ± 0.02 c | 451.67 ± 4.51 f | 29.44 ± 0.28 ab | 0.63 ± 0.02 b | 0.67 ± 0.01 b | 42.58 ± 1.26 c | 218.19 ± 0.46 c | 0.55 ± 0.05 e |
| DCd3 | 8.69 ± 0.01 a | 358.00 ± 6.56 h | 25.45 ± 2.31 abcd | 0.51 ± 0.02 c | 0.50 ± 0.01 c | 34.32 ± 1.67 def | 216.24 ± 1.4 c | 1.09 ± 0.11 d |
| DCd4 | 8.55 ± 0.01 b | 471.67 ± 6.03 e | 24.38 ± 3 abcd | 0.49 ± 0.01 c | 0.53 ± 0.04 c | 36.66 ± 0.48 d | 202.37 ± 2.96 d | 1.96 ± 0.08 c |
| DCd5 | 8.47 ± 0.02 d | 459.00 ± 9.00 f | 23.31 ± 7.72 bcd | 0.52 ± 0.03 c | 0.55 ± 0.08 c | 33.64 ± 0.41 ef | 196.27 ± 1.15 f | 2.68 ± 0.15 a |
| Treatment | Chao1 | Goods Coverage | Observed Species | PD Whole Tree | Shannon | Simpson |
|---|---|---|---|---|---|---|
| FCd1 | 4632.02 ab | 0.96 ab | 3230.37 ab | 258.02 abcd | 9.78 ab | 1 a |
| FCd2 | 4978.66 a | 0.96 b | 3406.77 a | 267.94 ab | 10.02 a | 1 a |
| FCd3 | 4798.4 ab | 0.96 ab | 3304.3 ab | 273.03 a | 9.95 ab | 1 a |
| FCd4 | 4424.68 b | 0.96 a | 3073.63 b | 248.33 cd | 9.65 b | 1 a |
| FCd5 | 4757.18 ab | 0.96 ab | 3299.2 ab | 264.26 abc | 9.98 ab | 1 a |
| DCd1 | 4587.39 ab | 0.96 ab | 3174.1 ab | 244.97 d | 9.78 ab | 1 a |
| DCd2 | 4759.15 ab | 0.96 ab | 3297.1 ab | 258.81 abcd | 9.87 ab | 1 a |
| DCd3 | 4567.93 ab | 0.96 ab | 3117.7 ab | 250.78 bcd | 9.8 ab | 1 a |
| DCd4 | 4644.97 ab | 0.96 ab | 3147.27 ab | 252.89 bcd | 9.84 ab | 1 a |
| DCd5 | 4389.39 b | 0.96 ab | 3080.23 b | 248 cd | 9.78 ab | 1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Li, P.; Qi, X.; Rahman, S.U.; Zhang, Z. Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water. Sustainability 2022, 14, 8891. https://doi.org/10.3390/su14148891
Cui J, Li P, Qi X, Rahman SU, Zhang Z. Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water. Sustainability. 2022; 14(14):8891. https://doi.org/10.3390/su14148891
Chicago/Turabian StyleCui, Jiaxin, Ping Li, Xuebin Qi, Shafeeq Ur Rahman, and Zulin Zhang. 2022. "Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water" Sustainability 14, no. 14: 8891. https://doi.org/10.3390/su14148891
APA StyleCui, J., Li, P., Qi, X., Rahman, S. U., & Zhang, Z. (2022). Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water. Sustainability, 14(14), 8891. https://doi.org/10.3390/su14148891










