Effects of Combined Application of Salicylic Acid and Proline on the Defense Response of Potato Tubers to Newly Emerging Soft Rot Bacteria (Lelliottia amnigena) Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Materials
2.2. Experimental Design
2.3. Effect of SA and Pro on Extracellular Enzyme Production by L. amnigena
2.4. Effect of SA and Pro on Potato Soft Rot
2.5. Disease Assessment
2.6. Sampling
2.7. Malondialdehyde and Hydrogen Peroxide Content in Potato Tuber
2.8. Assay of Some Enzymatic Activities
2.9. Quantitative Real-Time (qRT) PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of SA and Pro on Extracellular Enzyme Production by L. amnigena
3.2. Disease Assessment
3.3. Effects of SA and Pro on MDA and H2O2 Content in Potato Tubers Inoculated with L. amnigena
3.4. Effects of SA and Pro on NOX, SOD, POD, PPO, and CAT Activity in Potato Tubers Inoculated with L. amnigena
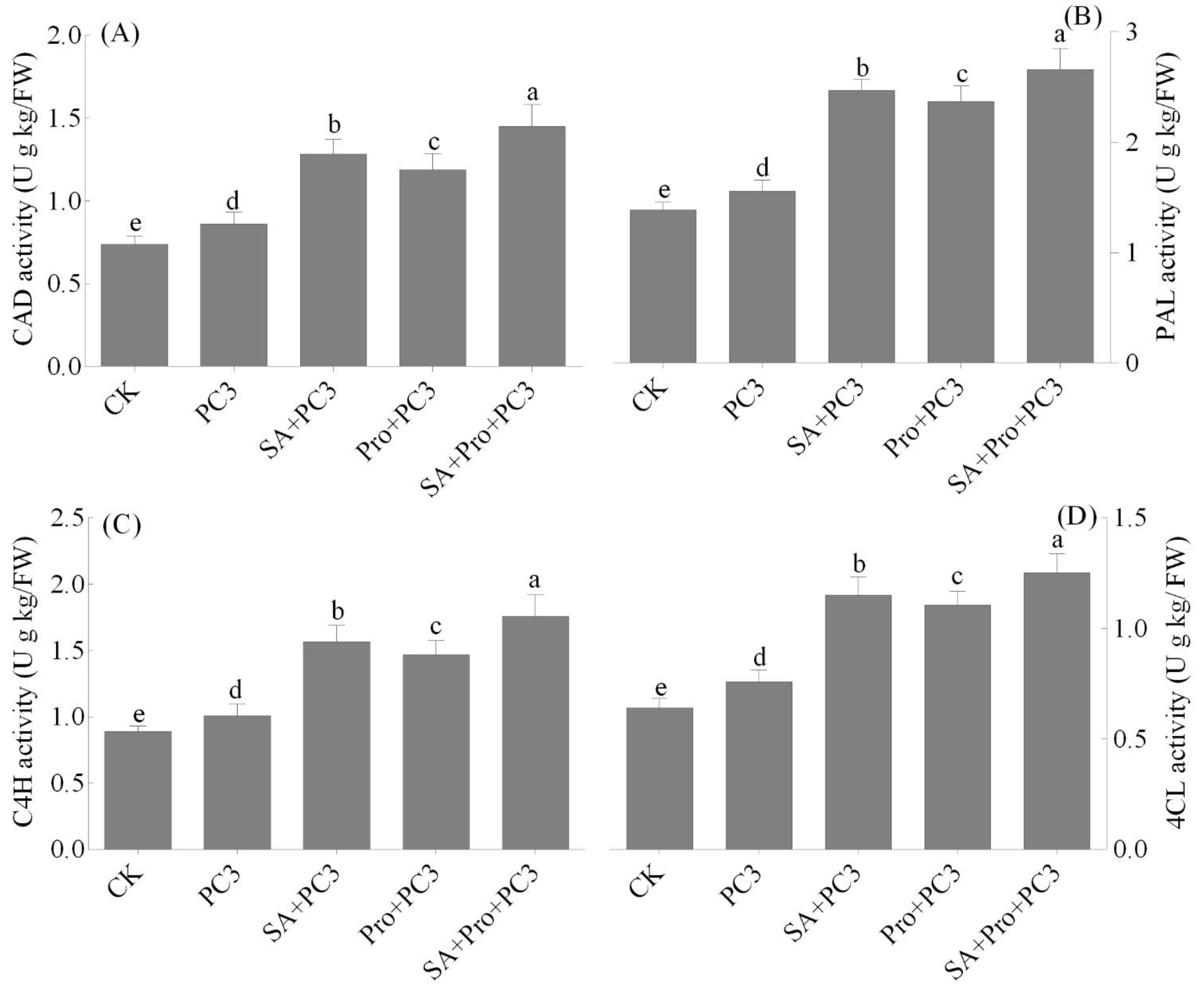
3.5. SA and Pro Treatment Increased PAL, CAD, 4CL, and C4H Activity in Potaato Tubers Inoculated with L. amanigena
3.6. SA and Pro Treatment Up-Regulated NOX, PAL, CAD, 4CL, and C4H Genes in Potato Tubers Inoculated with L. amnigena
3.7. Effects of Combined SA and Pro Treatment on the Expression Levels of Plant Defense-Related Genes in Potato Tubers Inoculated with L. amnigena
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, S.; Chisholm, K.; Coffin, R.H.; Peters, R.D.; Al-Mughrabi, K.I.; Wang-Pruski, G.; Pinto, D.M. Protein profiling in potato (Solanum tuberosum L.) leaf tissues by differential centrifugation. J. Proteome Res. 2012, 11, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Bi, Y.; Liu, J.; Yin, Y. Investigation analysis on potato disease during storage in northwest China. Chin. Potato J. 2009, 23, 364–365. [Google Scholar]
- Bojanowski, A.; Avis, T.J.; Pelletier, S.; Tweddell, R.J. Management of potato dry rot. Postharvest Biol. Technol. 2013, 84, 99–109. [Google Scholar] [CrossRef]
- Czajkowski, R.; Perombelon, M.C.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. J. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Dees, M.W.; Lebecka, R.; Perminow, J.I.S.; Czajkowski, R.; Motyka, A.; Zoledowska, S.; Śliwka, J.; Lojkowska, E.; Brurberg, M.B. Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. Eur. J. Plant Pathol. 2017, 148, 839–851. [Google Scholar] [CrossRef]
- Duarte, V.; De Boer, S.; Ward, L.d.; De Oliveira, A. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. J. Appl. Microbiol. 2004, 96, 535–545. [Google Scholar] [CrossRef]
- Orsini, F.; Pennisi, G.; Mancarella, S.; Al Nayef, M.; Sanoubar, R.; Nicola, S.; Gianquinto, G. Hydroponic lettuce yields are improved under salt stress by utilizing white plastic film and exogenous applications of proline. Sci. Hortic. 2018, 233, 283–293. [Google Scholar] [CrossRef]
- Bateman, D. Plant cell wall hydrolysis by pathogens. In Biochemical Aspects of Plant-Parasite Relationships; Friend, J., Threlfall, D.R., Eds.; Academic Press: London, UK, 2012; pp. 79–103. [Google Scholar]
- Jess, S.; Kildea, S.; Moody, A.; Rennick, G.; Murchie, A.K.; Cooke, L.R. European Union policy on pesticides: Implications for agriculture in Ireland. Pest Manag. Sci. 2014, 70, 1646–1654. [Google Scholar] [CrossRef]
- Torres, R.; Vilanova, L.; Usall, J.; Teixidó, N. Insights into Fruit Defense Mechanisms Against the Main Postharvest Pathogens of Apples and Oranges. In Postharvest Pathology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–40. [Google Scholar] [CrossRef]
- Tian, S.; Torres, R.; Ballester, A.; Li, B.; Vilanova, L.; González-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.; Porat, R. Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Jacobs, J.M.; Ailloud, F.; Fochs, B.; Prior, P.; Allen, C. Degradation of the plant defense signal salicylic acid protects Ralstonia solanacearum from toxicity and enhances virulence on tobacco. MBio 2016, 7, e00656-16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Wang, Y.K.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Lu, F.F.; Jin, S.F.; Zhu, H.M.; Yang, H. Reduced phytotoxicity of propazine on wheat, maize and rapeseed by salicylic acid. Ecotoxicol. Environ. Saf. 2018, 162, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Afroz, A.; Khan, M.R.; Ahsan, N.; Komatsu, S. Comparative proteomic analysis of bacterial wilt susceptible and resistant tomato cultivars. Peptides 2009, 30, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Narasimhamurthy, K.; Soumya, K.; Udayashankar, A.; Srinivas, C.; Niranjana, S. Elicitation of innate immunity in tomato by salicylic acid and Amomum nilgiricum against Ralstonia solanacearum. Biocatal. Agric. Biotechnol. 2019, 22, 101414. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.M.; Galarneau, E.R. Phenolic Compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 2034. [Google Scholar] [CrossRef]
- Poveda, J. Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiol. Mol. Plant Pathol. 2020, 111, 101521. [Google Scholar] [CrossRef]
- Zeng, K.; Cao, J.; Jiang, W. Enhancing disease resistance in harvested mango (Mangifera indica L. cv.‘Matisu’) fruit by salicylic acid. J. Sci. Food Agric. 2006, 86, 694–698. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Cai, Y.; Zheng, Y. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innov. Food Sci. Emerg. Technol. 2011, 12, 310–314. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, L.; Jiang, S.; Dong, Y.; Zheng, X. Biocontrol of gray mold decay in peach fruit by integration of antagonistic yeast with salicylic acid and their effects on postharvest quality parameters. Biol. Control 2008, 47, 60–65. [Google Scholar] [CrossRef]
- Ezzat, A.; Szabó, S.; Szabó, Z.; Hegedűs, A.; Berényi, D.; Holb, I.J. Temporal patterns and inter-correlations among physical and antioxidant attributes and enzyme activities of apricot fruit inoculated with Monilinia laxa under salicylic acid and methyl jasmonate treatments under shelf-life conditions. Fungi 2021, 7, 341. [Google Scholar] [CrossRef]
- Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Blenzar, A.; Barka, E.A. Improving the biocontrol potential of bacterial antagonists with salicylic acid against brown rot disease and impact on nectarine fruits quality. Agronomy 2021, 11, 209. [Google Scholar] [CrossRef]
- Mattioli, R.; Palombi, N.; Funck, D.; Trovato, M. Proline accumulation in pollen grains as potential target for improved yield stability under salt stress. Front. Plant Sci. 2020, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ami, K.; Planchais, S.; Cabassa, C.; Guivarc’h, A.; Véry, A.-A.; Khelifi, M.; Djebbar, R.; Abrous-Belbachir, O.; Carol, P. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol. Plant. 2020, 42, 21. [Google Scholar] [CrossRef]
- Trovato, M.; Forlani, G.; Signorelli, S.; Funck, D. Proline metabolism and its functions in development and stress tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 41–72. [Google Scholar]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Tonhati, R.; Mello, S.C.; Momesso, P.; Pedroso, R.M. L-proline alleviates heat stress of tomato plants grown under protected environment. Sci. Hortic. 2020, 268, 109370. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crops Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.; Desoky, E.-S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J. Plant Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Osei, R.; Yang, C.; Cui, L.; Ma, T.; Li, Z.; Boamah, S. Isolation, identification, and pathogenicity of Lelliottia amnigena causing soft rot of potato tuber in China. Microb. Pathog. 2022, 164, 105441. [Google Scholar] [CrossRef]
- Ben-David, A.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Osei, R.; Yang, C.; Cui, L.; Wei, L.; Jin, M.; Boamah, S. Salicylic acid effect on the mechanism of Lelliottia amnigena causing potato soft rot. Folia Hortic. 2021, 33, 376–389. [Google Scholar] [CrossRef]
- Perveen, S.; Nazir, M. Proline treatment induces salt stress tolerance in maize (Zea Mays L. CV. Safaid Afgoi). Pak. J. Bot. 2018, 50, 1265–1271. [Google Scholar]
- Scherf, J.M.; Milling, A.; Allen, C. Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum race 3, biovar 2, in infected geranium, tomato, and potato plants. Appl. Environ. Microbiol. 2010, 76, 7061–7067. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zong, Y.; Li, Z.; Yang, R.; Li, Z.; Bi, Y.; Prusky, D. Postharvest Pichia guilliermondii treatment promotes wound healing of apple fruits. Postharvest Biol. Technol. 2020, 167, 111228. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bandara, M.B.; Zhu, H.; Sankaridurg, P.R.; Willcox, M.D. Salicylic acid reduces the production of several potential virulence factors of Pseudomonas aeruginosa associated with microbial keratitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4453–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, D.M.; King, B.C.; Hayes, M.L.; Bergstrom, G.C. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 2011, 14, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Ma, B.; Perna, N.T.; Charkowski, A.O. Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Appl. Environ. Microbiol. 2009, 75, 4539–4549. [Google Scholar] [CrossRef] [Green Version]
- Stępień, Ł. Fusarium: Mycotoxins, Taxonomy, Pathogenicity. Microorganisms 2020, 8, 1404. [Google Scholar] [CrossRef] [PubMed]
- López, M.M.; López-López, M.J.; Martí, R.; Zamora, J.; López-Sanchez, J.; Beltra, R. Effect of acetylsalicylic acid on soft rot produced by Erwinia carotovora subsp. carotovora in potato tubers under greenhouse conditions. Potato Res. 2001, 44, 197–206. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.d.; Kasnak, C.; Palamutoglu, R. Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Eshghpour, E.; Shahryari, F.; Ghasemi, A. Evaluation of salicylic acid effect on the reduction of Pectobacterium carotovorum damage in potato tubers. In Proceedings of the 22nd Iranian Plant Protection Congress, Karaj, Iran, 27–30 August 2016. [Google Scholar]
- Bawa, G.; Feng, L.; Yan, L.; Du, Y.; Shang, J.; Sun, X.; Wang, X.; Yu, L.; Liu, C.; Yang, W. Pre-treatment of salicylic acid enhances resistance of soybean seedlings to Fusarium solani. Plant Mol. Biol. 2019, 101, 315–323. [Google Scholar] [CrossRef]
- Li, L.; Zou, Y. Induction of disease resistance by salicylic acid and calcium ion against Botrytis cinerea in tomato (Lycopersicon esculentum). Emir. J. Food Agric. 2017, 29, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Cecchini, N.M.; Monteoliva, M.I.; Alvarez, M.E. Proline dehydrogenase contributes to pathogen defense in Arabidopsis. Plant Physiol. 2011, 155, 1947–1959. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wang, L.; Zhang, S.; Sun, L.; Luo, W.; Posny, D.; Xu, S.; Tang, C.; Ma, M.; Zhang, C. Investigation of proline in superficial scald development during low temperature storage of ‘Dangshansuli’pear fruit. Postharvest Biol. Technol. 2021, 181, 111643. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Yastreb, T. Physiological functions of nonenzymatic antioxidants of plants. Bull. Kharkiv Natl. Agrar. Univ. Ser. Biol. 2015, 2, 6–25. [Google Scholar]
- Mishra, A.K.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, B.L.; Alansi, S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J. Biol. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef]
- Estaji, A. Impact of exogenous applications of salicylic acid on the tolerance to drought stress in pepper (Capsicum Annuum L.) plants. Res. Sq. 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Sayyari, M.; Ghavami, M.; Ghanbari, F.; Kordi, S. Assessment of salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. Int. J. Agric. Crop Sci. 2013, 5, 1951–1957. [Google Scholar]
- Naeem, M.; Basit, A.; Ahmad, I. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Gesunde Pflanz. 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Hayat, K.; Khan, J.; Khan, A.; Ullah, S.; Ali, S.; Fu, Y. Ameliorative effects of exogenous proline on photosynthetic attributes, nutrients uptake, and oxidative stresses under cadmium in Pigeon Pea (Cajanus cajan L.). Plants 2021, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant. 2009, 31, 261–269. [Google Scholar] [CrossRef]
- de Carvalho, K.; de Campos, M.K.F.; Domingues, D.S.; Pereira, L.F.P.; Vieira, L.G.E. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, L.; Yuan, F.; Chen, M. Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress. Plant Signal. Behav. 2019, 14, e1644595. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010, 178, 307–311. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P. Osmoprotectant-mediated abiotic stress tolerance in plants. In Proline Metabolism and Its Functions in Development and Stress Tolerance; Psikol. Çalışmaları; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Burritt, D.J. Proline and the cryopreservation of plant tissues: Functions and practical applications. Curr. Front. Cryopreserv. 2012, 14, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical changes, osmolytes accumulation and distribution in the native plants growing on Pb-contaminated sites. Environ. Geochem. Health 2021, 43, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Singh, I.; Bains, T.; Siddique, K.H.; Bindumadhava, H.; Nair, R.M.; Nayyar, H. Securing reproductive function in mungbean grown under high temperature environment with exogenous application of proline. Plant Physiol. Biochem. 2019, 140, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Banu, M.N.A.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate–glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. plants. J. Hortic. Sci. Biotechnol. 2013, 88, 439–446. [Google Scholar] [CrossRef]
- Tabssum, F.; uz Zaman, Q.; Chen, Y.; Riaz, U.; Ashraf, W.; Aslam, A.; Ehsan, N.; Nawaz, R.; Aziz, H. Exogenous application of proline improved salt tolerance in rice through modulation of antioxidant activities. Pak. J. Agric. Res. 2019, 32, 140. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Abdallah, M.M.; El-Bassiouny, H. Impact of exogenous proline or tyrosine on growth, some biochemical aspects and yield components of quinoa plant grown in sandy soil. Int. J. Pharm. Technol. 2016, 9, 12–23. [Google Scholar]
- Yang, R.; Han, Y.; Han, Z.; Ackah, S.; Li, Z.; Bi, Y.; Yang, Q.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Peng, C.; Shang, X.; Lv, X.; Sun, J.; Li, C.; Wei, L.; Liu, X. Postharvest benzothiazole treatment enhances healing in mechanically damaged sweet potato by activating the phenylpropanoid metabolism. J. Sci. Food Agric. 2020, 100, 3394–3400. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidokoro, S.; Maruyama, K.; Nakashima, K.; Imura, Y.; Narusaka, Y.; Shinwari, Z.K.; Osakabe, Y.; Fujita, Y.; Mizoi, J.; Shinozaki, K. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009, 151, 2046–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavrova, V.; Udalova, Z.V.; Matveeva, E.; Khasanov, F.; Zinovieva, S. Mi-1 gene expression in tomato plants under root-knot nematode invasion and treatment with salicylic acid. Dokl. Biochem. Biophys. 2016, 471, 413–416. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef]
- Mutlu, S.; Atıcı, Ö.; Nalbantoğlu, B.; Mete, E. Exogenous salicylic acid alleviates cold damage by regulating antioxidative system in two barley (Hordeum vulgare L.) cultivars. Front. Life Sci. 2016, 9, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef]
- Mahesh, H.; Murali, M.; Pal, M.A.C.; Melvin, P.; Sharada, M. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef]
- Sekhon, P.; Sangha, M. Influence of different SAR elicitors on induction and expression of PR-proteins in Potato and Muskmelon against Oomycete pathogens. Indian Phytopathol. 2019, 72, 43–51. [Google Scholar] [CrossRef]
- Ghanbari, S.; Fakheri, B.A.; Mahdinezhad, N.; Khedri, R. Systemic acquired resistance. J. New Biol. Rep. 2015, 23, 105–110. [Google Scholar]
- Chen, J.; Zhang, Y.; Wang, C.; Lü, W.; Jin, J.B.; Hua, X. Proline induces calcium-mediated oxidative burst and salicylic acid signaling. Amino Acid 2011, 40, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Aslund, F.; Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 1998, 279, 1718–1721. [Google Scholar] [CrossRef] [PubMed]






| Gene Symbol | Description | Primer Sequence (5′-3′) | Gene ID | Activity |
|---|---|---|---|---|
| Act | Actin | F: ACAATGCTTGCACGTTTCCTC R: TTAGCTGGGACCATTGCCTG | 102605823 | Antioxidant |
| NOXB | NADPH oxidase | F: CATTGCTTCTTCAGGCTCCG R: CCACAAAGCCATCACCCAAA | 111509039 | Antioxidant |
| PAL | Phenylalanine ammonia-lyase | F: GAGGAGTATAGGAAGCCGG R: CTCATCCCTTCCATCACCCA | 102596017 | Antioxidant |
| CAD | Cinnamyl alcohol dehydrogenase | F: GGCCTGATGATGTGCAAGTC R: CCAACAAGCAATCCAACTCCA | 102584791 | Antioxidant |
| 4CL | 4-counmaryl-CoA ligase | F: GCCCTGAATTTGTGTTTGCG R: CCTTCACTTTCCCCGCAAAA | 102596056 | Antioxidant |
| C4H | Cinnamate-4-hdroxylase | F: AGTCTGAGGCTGCTAGTGT R: GAGTCTGAGGCTGCTAGTGT | 817599 | Antioxidant |
| PR 1 | Pathogenesis-related protein | F: GCCAATCCAGGCTGTAGCA R: AGTGGGGAAGAAGAATGTGGAC | 102580826 | Plant defense |
| PR 2 | Tyrosine-protein kinase | F: ACCGCCTTCGAGAACTAGAG R: CCACAAACTTGCCATATCACCA | 111517981 | Plant defense |
| PRH3 | Pathogenesis-related homeodomain protein | F: GCAAAGGGGAAGCTGGGTAA R: TGTTACTTTCAGCTGCATCCTCT | 102596310 | Plant defense |
| CTL1 | Chitinase-like protein 1 | F: ATTACGGTCGTGGTGCCTTG R: ATCTGCAACTGCTTTCCGTG | 102595303 | Plant defense |
| PAL | Phenylalanine ammonia-lyase | F: TGGTGGTGCCCTTCAAAAAG R: CGTAGCTTGTTATGTCATGATGAT | 102596017 | Plant defense |
| SPI1 | Serine protease inhibitor-1 | F: TAGGTGGCCAGAACTGGTTG R: TGTGTTAGCGATTGTCCTTCGA | 823839 | Plant defense |
| Act | Actin | F: ACAATGCTTGCACGTTTCCTC | 102593148 | Plant defense |
| R: TTAGCTGGGACCATTGCCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osei, R.; Yang, C.; Wei, L.; Jin, M.; Boamah, S. Effects of Combined Application of Salicylic Acid and Proline on the Defense Response of Potato Tubers to Newly Emerging Soft Rot Bacteria (Lelliottia amnigena) Infection. Sustainability 2022, 14, 8870. https://doi.org/10.3390/su14148870
Osei R, Yang C, Wei L, Jin M, Boamah S. Effects of Combined Application of Salicylic Acid and Proline on the Defense Response of Potato Tubers to Newly Emerging Soft Rot Bacteria (Lelliottia amnigena) Infection. Sustainability. 2022; 14(14):8870. https://doi.org/10.3390/su14148870
Chicago/Turabian StyleOsei, Richard, Chengde Yang, Lijuan Wei, Mengjun Jin, and Solomon Boamah. 2022. "Effects of Combined Application of Salicylic Acid and Proline on the Defense Response of Potato Tubers to Newly Emerging Soft Rot Bacteria (Lelliottia amnigena) Infection" Sustainability 14, no. 14: 8870. https://doi.org/10.3390/su14148870
APA StyleOsei, R., Yang, C., Wei, L., Jin, M., & Boamah, S. (2022). Effects of Combined Application of Salicylic Acid and Proline on the Defense Response of Potato Tubers to Newly Emerging Soft Rot Bacteria (Lelliottia amnigena) Infection. Sustainability, 14(14), 8870. https://doi.org/10.3390/su14148870





