Combined Treatment of Cr(VI)-Contaminated Soils by Reduction, Adsorption, and Solidification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contaminated Soil
2.2. Specimen Preparation
2.3. Experimental Scheme
2.4. Leaching and UCS Test
3. Results and Discussions
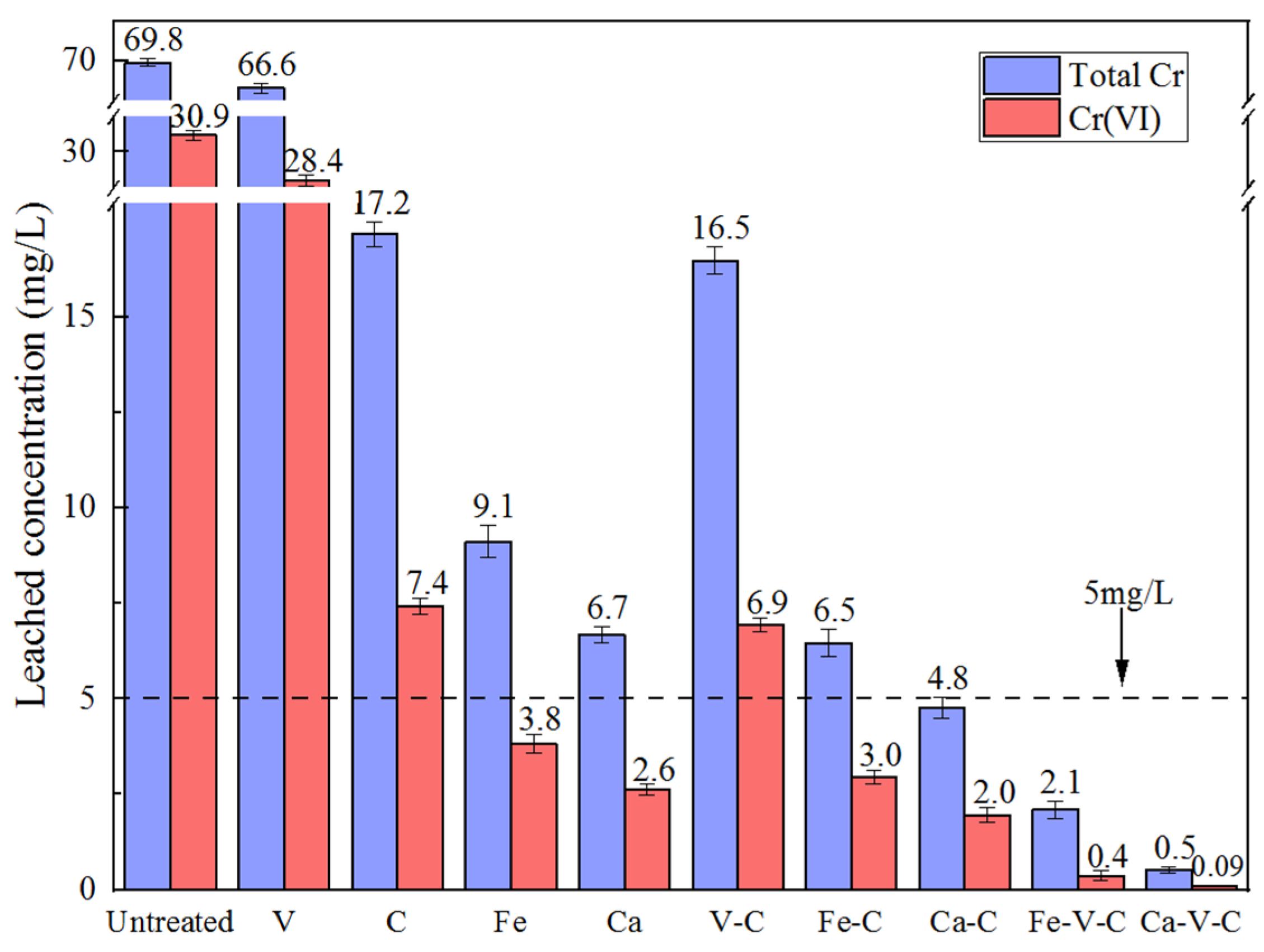
3.1. Leached Concentration from SPLP Test
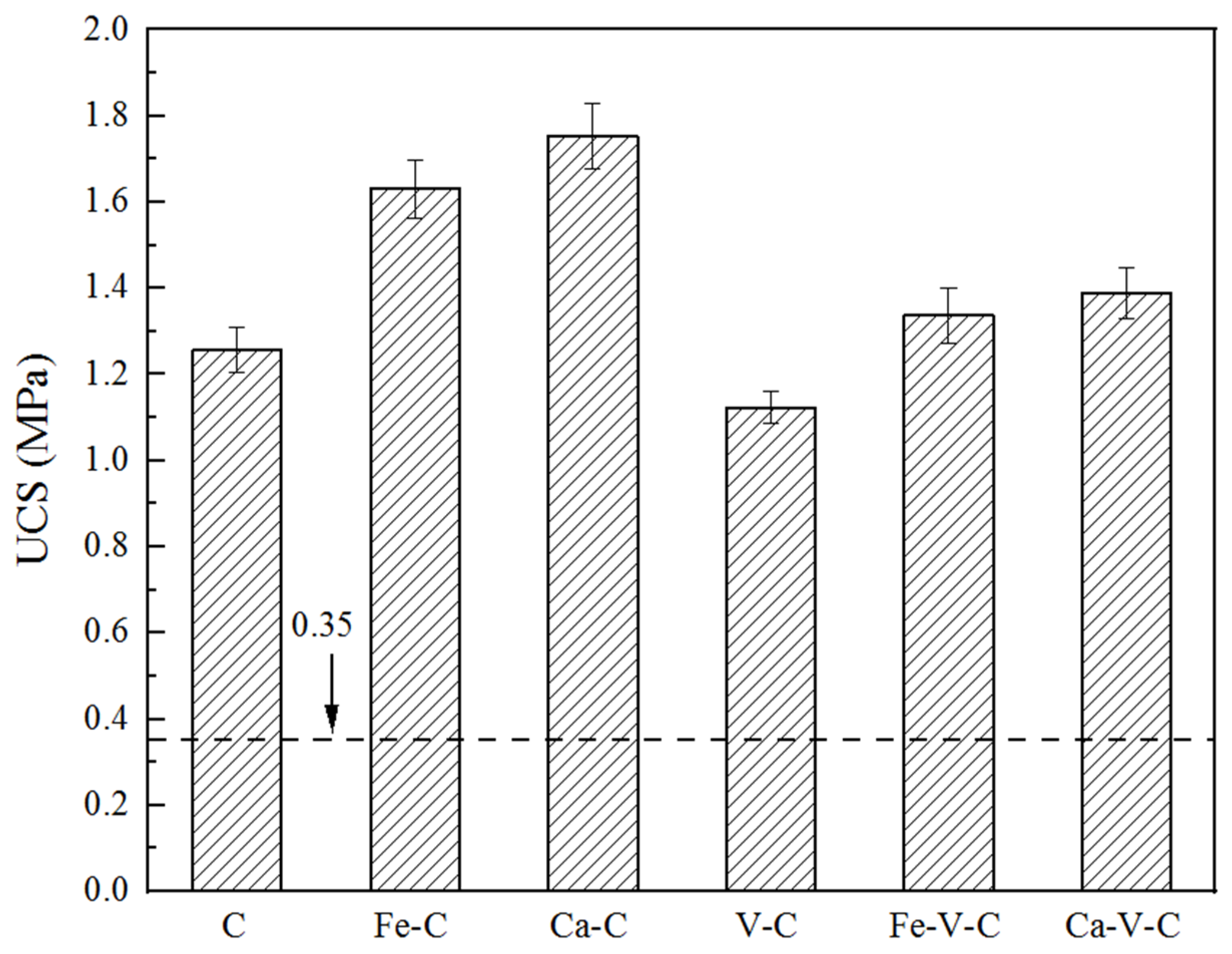
3.2. UCS of Solidified Specimens
3.3. Influence of Different Agent-Adding Procedures
3.4. Results of SEM Analysis
3.5. Results of the Orthogonal Experiments
4. Conclusions
- The Ca-V-C combination was optimum for treatment of Cr(VI)-contaminated soil. For the soil with an initial total Cr content of 1200 mg/kg (Cr(VI) content of 823 ± 20 mg/kg), the leached Cr(VI) concentration could be reduced to 0.09 mg/L by the combined treatment with 2 stoichiometric amounts of CaS5, 15% vermiculite and 20% cement, corresponding to a weight percentage of 75.93% of the treated soil.. Meanwhile, the microstructure of the combined reduction/adsorption/solidification remediation of Cr-contaminated soil was analyzed by SEM in agreement with the results of UCS and toxic leaching. From a microscopic point of view, the engineering properties of composite preparations for repairing Cr-contaminated soils were revealed.
- The leached Cr concentration decreased with the increase of any of the three agents. For soil with a relatively low Cr(VI) content, the influence of CaS5 dosage on the leached concentration was dominant, whereas for soil with a high Cr(VI) content, the impacts of cement and vermiculite were more significant. The UCS increased with the increasing dosages of cement and CaS5, whereas it decreased with an increasing vermiculite dosage.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Lin, M. Influence of redox potential on leaching behavior of a solidified chromium contaminated soil. Sci. Total Environ. 2020, 733, 139410. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, N.; Sarrafi, A.; Ramezanianpour, A. Immobilization of hexavalent chromium in cement mortar: Leaching properties and microstructures. Environ. Sci. Pollut. Res. 2019, 26, 20829–20838. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Z.; Zhao, L.; Ma, J.; Li, X.; He, F.; Hou, H. Toxicity of exogenous hexavalent chromium to soil-dwelling springtail Folsomia candida in relation to soil properties and aging time. Chemosphere 2019, 224, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Zhu, F.; Kang, B.; Xu, L.; Deng, Y.; Yang, C.; Chu, C. Experimental investigation of cement/soda residue for solidification/stabilization of Cr-contaminated soils. Adv. Civ. Eng. 2020, 2020, 8890149. [Google Scholar] [CrossRef]
- Park, J.; Kang, W.; Hwang, I. Hexavalent chromium uptake and release in cement pastes. Environ. Eng. Sci. 2006, 23, 133–140. [Google Scholar] [CrossRef]
- Zha, F.; Liu, J.; Xu, L.; Cui, K. Effect of cyclic drying and wetting on engineering properties of heavy metal contaminated soils solidified/stabilized with fly ash. J. Cent. South Univ. 2013, 20, 1947–1952. [Google Scholar] [CrossRef]
- Montañés, M.; Sánchez-Tovar, R.; Roux, M. The effectiveness of the stabilization/solidification process on the leachability and toxicity of the tannery sludge chromium. Environ. Manag. 2014, 143, 71–79. [Google Scholar] [CrossRef]
- Singh, T.; Pant, K. Solidification/stabilization of arsenic containing solid wastes using portland cement, fly ash and polymeric materials. J. Hazard. Mater. 2006, 131, 29–36. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Cui, J.; Poon, C.; Beiyuan, J.; Tsang, D.; Li, X. Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: Spectroscopic investigation and leaching tests. Sci. Total Environ. 2018, 631–632, 1486–1494. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.; Li, J.; Yeung, T.; Ding, S.; Poon, C. Green remediation of contaminated sediment by stabilization/solidification with industrial by-products and CO2 utilization. Sci. Total Environ. 2018, 631–632, 1321–1327. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; McCabe, B.; Chen, Y.; Morrison, L. Dredged marine sediments stabilized/solidified with cement and GGBS: Factors affecting mechanical behaviour and leachability. Sci. Total Environ. 2020, 733, 138551. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Du, Y.; Zhou, A.; Zhang, M.; Li, J.; Zhou, S.; Xia, W. Geoenvironmental properties of industrially contaminated site soil solidified/stabilized with a sustainable by-product-based binder. Sci. Total Environ. 2021, 765, 142778. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.; Hug, S. Influence of mineral surfaces on chromium(VI) reduction by iron(II). Environ. Sci. Technol. 1999, 33, 4285–4291. [Google Scholar] [CrossRef]
- Wazne, M.; Jagupilla, S.; Moon, D.; Jagupilla, S.; Christodoulatos, C.; Kim, M. Assessment of calcium polysulfide for the remediation of hexavalent chromium in chromite ore processing residue (COPR). J. Hazard. Mater. 2007, 143, 620–628. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Y.; Qiao, J.; Pan, B.; Zhang, W.; Guan, X. Enhanced chromium(VI) removal by zero-valent iron in the presence of anions and a weak magnetic field: Batch and column tests. Chem. Eng. J. 2018, 354, 445–453. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Liang, J.; Chen, X.; Ye, J.; Liu, Y.; Liu, Y.; Wei, Y. Remediation of hexavalent chromium in contaminated soil using amorphous iron pyrite: Effect on leachability, bioaccessibility, phytotoxicity and long-term stability. Environ. Pollut. 2020, 264, 114804. [Google Scholar] [CrossRef]
- Bulut, U.; Ozverdi, A.; Erdem, M. Leaching behavior of pollutants in ferrochrome arc furnace dust and its stabilization/solidification using ferrous sulphate and Portland cement. J. Hazard. Mater. 2009, 162, 893–898. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Chen, Z.; Li, T.; Shen, C.; Xuan, L.; Xu, L. Remediation of contaminated soil and groundwater using chemical reduction and solidification/ stabilization method: A case study. Environ. Sci. Pollut. R. 2021, 28, 12766–12779. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhou, S.; Lei, X. Reduction/immobilization processes of hexavalent chromium using metakaolin-based geopolymer. J. Environ. Eng. 2017, 5, 373–380. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef]
- Badawy, N.; El-Bayaa, A.; Alkhalik, E. Vermiculite as an exchanger for copper(II) and Cr(III) ions, kinetic studies. Ionics 2010, 16, 733–739. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef]
- Abbaslou, H.; Poorangha, P.; Bakhtiari, S.; Ghanizadeh, A. Anionic contamination and its stabilization on the strength and plastic features of clay soils (vermiculite and sepiolite). Soil Sediment Contam. 2020, 29, 557–568. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, Z.; Fan, X.; Jing, R.; Yang, J.; Shao, Y.; Liu, X.; Wu, M.; Zhang, Q.; Liu, A. Removal of heavy metals from soil by vermiculite supported layered double hydroxides with three-dimensional hierarchical structure. Chem. Eng. J. 2020, 390, 124554. [Google Scholar] [CrossRef]
- Wang, S.; Vipulanandan, C. Solidification/stabilization of Cr(VI) with cement: Leachability and XRD analyses. Cem. Concr. Res. 2000, 30, 385–389. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Li, C.; Jin, Y.; Nie, Y.; Li, J. Comparison of the fixation effects of heavy metals by cement rotary kiln co-processing and cement based solidification/stabilization. J. Hazard. Mater. 2009, 165, 1179–1185. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Ferreira, D.; Johnston, C. Calcium polysulfide treatment of Cr(VI)-contaminated soil. J. Hazard. Mater. 2010, 179, 650–657. [Google Scholar] [CrossRef]
- Kameswari, S.; Narasimman, L.; Pedaballe, V.; Kalyanaraman, C. Diffusion and leachability index studies on stabilization of chromium contaminated soil using fly ash. J. Hazard. Mater. 2015, 297, 52–58. [Google Scholar] [CrossRef]
- US EPA. Method 1312: Synthetic Precipitation Leaching Procedure; US EPA: Washington, DC, USA, 1994. [Google Scholar]
- Dahlawi, S.; Siddiqui, S. Calcium polysulphide, its applications and emerging risk of environmental pollution-a review article. Environ. Sci. Pollut. R. 2017, 24, 92–102. [Google Scholar] [CrossRef]
- Graham, M.; Farmer, J.; Anderson, P.; Paterson, E.; Hillier, S.; Lumsdon, D.; Bewley, R. Calcium polysulfide remediation of hexavalent chromiumcontamination from chromite ore processing residue. Sci. Total Environ. 2006, 364, 32–44. [Google Scholar] [CrossRef]
- Koksal, F.; Sahin, Y.; Gencel, O. Influence of expanded vermiculite powder and silica fume on properties of foam concretes. Constr. Build. Mater. 2020, 257, 119547. [Google Scholar] [CrossRef]
- US EPA. Prohibition on the Disposal of Bulk Liquid Hazardous Waste in Landfills-Statutory Interpretive Guidance; US EPA: Washington, DC, USA, 1996. [Google Scholar]
- Yin, C.; Shaaban, M.; Mahmud, H. Chemical stabilization of scrap metal yard contaminated soil using ordinary Portland cement: Strength and leachability aspects. Build. Environ. 2007, 42, 794–802. [Google Scholar] [CrossRef]




| Metal Ion | Al | Mg | Fe | Mn | K | Ca | Zn | Cr | Pb | Cd |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/g) | 11.07 | 7.36 | 18.05 | 0.47 | 21.20 | 5.43 | 0.06 | 0.08 | 0.04 | 0.03 |
| Specimen No. | Agent Combination | Reducing Agent | Adsorbent | Solidified Agent |
|---|---|---|---|---|
| 1 | / | / | / | / |
| 2 | C | / | / | Cement |
| 3 | Fe | FeSO4 | / | / |
| 4 | Ca | CaS5 | / | / |
| 5 | V | / | Vermiculite | / |
| 6 | V-C | / | Vermiculite | Cement |
| 7 | Fe-C | FeSO4 | / | Cement |
| 8 | Ca-C | CaS5 | / | Cement |
| 9 | Fe-V-C | FeSO4 | Vermiculite | Cement |
| 10 | Ca-V-C | CaS5 | Vermiculite | Cement |
| 11 | FeV-C | FeSO4 + Vermiculite | Cement | |
| 12 | CaV-C | CaS5 + Vermiculite | Cement | |
| 13 | Fe-VC | FeSO4 | Vermiculite + Cement | |
| 14 | Ca-VC | CaS5 | Vermiculite + Cement | |
| Level | CaS5 (Times) | Vermiculite (%) | Cement (%) |
|---|---|---|---|
| 1 | 1 | 5 | 10 |
| 2 | 1.5 | 10 | 15 |
| 3 | 2 | 15 | 20 |
| Test No. | Agent Dosage Level | SPLP (mg/L) | UCS (MPa) | ||||
|---|---|---|---|---|---|---|---|
| CaS5 (Molar Times) | Vermiculite (%) | Cement (%) | Soil No.1 | Soil No.2 | Soil No.1 | Soil No.2 | |
| 1 | 1 (1) | 1 (5) | 1 (10) | 6.31 | 10.46 | 0.73 | 0.67 |
| 2 | 1 (1) | 2 (10) | 2 (15) | 3.43 | 6.58 | 0.88 | 0.80 |
| 3 | 1 (1) | 3 (15) | 3 (20) | 2.31 | 2.78 | 1.19 | 1.08 |
| 4 | 2 (1.5) | 1 (5) | 2 (15) | 2.02 | 5.55 | 1.05 | 0.96 |
| 5 | 2 (1.5) | 2 (10) | 3 (20) | 1.48 | 2.17 | 1.32 | 1.21 |
| 6 | 2 (1.5) | 3 (15) | 1 (10) | 1.95 | 4.79 | 0.60 | 0.56 |
| 7 | 3 (2) | 1 (5) | 3 (20) | 1.12 | 3.64 | 1.43 | 1.27 |
| 8 | 3 (2) | 2 (10) | 1 (10) | 1.41 | 4.46 | 0.73 | 0.71 |
| 9 | 3 (2) | 3 (15) | 2 (15) | 0.62 | 1.88 | 0.90 | 0.80 |
| Parameter | Soil No.1 | Soil No.2 | ||||
|---|---|---|---|---|---|---|
| CaS5 | Vermiculite | Cement | CaS5 | Vermiculite | Cement | |
| K1 | 4.02 | 3.15 | 3.22 | 6.38 | 6.55 | 6.57 |
| K2 | 1.82 | 2.11 | 2.02 | 4.17 | 4.40 | 4.67 |
| K3 | 1.05 | 1.63 | 1.64 | 3.33 | 2.92 | 2.63 |
| R | 2.97 | 1.52 | 1.59 | 3.05 | 3.63 | 3.94 |
| Parameter | Soil No.1 | Soil No.2 | ||||
|---|---|---|---|---|---|---|
| CaS5 | Vermiculite | Cement | CaS5 | Vermiculite | Cement | |
| K1 | 0.93 | 1.07 | 0.69 | 0.85 | 0.97 | 0.65 |
| K2 | 0.99 | 0.98 | 0.94 | 0.91 | 0.91 | 0.85 |
| K3 | 1.02 | 0.90 | 1.31 | 0.93 | 0.81 | 1.19 |
| R | 0.09 | 0.17 | 0.62 | 0.08 | 0.16 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhang, W.; Xiao, Y.; Jiang, H.; Ye, L. Combined Treatment of Cr(VI)-Contaminated Soils by Reduction, Adsorption, and Solidification. Sustainability 2022, 14, 8827. https://doi.org/10.3390/su14148827
Ji Y, Zhang W, Xiao Y, Jiang H, Ye L. Combined Treatment of Cr(VI)-Contaminated Soils by Reduction, Adsorption, and Solidification. Sustainability. 2022; 14(14):8827. https://doi.org/10.3390/su14148827
Chicago/Turabian StyleJi, Yongxin, Wenjie Zhang, Yu Xiao, Hong Jiang, and Liaoyu Ye. 2022. "Combined Treatment of Cr(VI)-Contaminated Soils by Reduction, Adsorption, and Solidification" Sustainability 14, no. 14: 8827. https://doi.org/10.3390/su14148827
APA StyleJi, Y., Zhang, W., Xiao, Y., Jiang, H., & Ye, L. (2022). Combined Treatment of Cr(VI)-Contaminated Soils by Reduction, Adsorption, and Solidification. Sustainability, 14(14), 8827. https://doi.org/10.3390/su14148827





