First Report on Reproductive Features of Shadow Driftfish Cubiceps whiteleggii (Perciformes: Nomeidae): An Effort toward Sustainable Management
Abstract
:1. Introduction
2. Materials and Methods
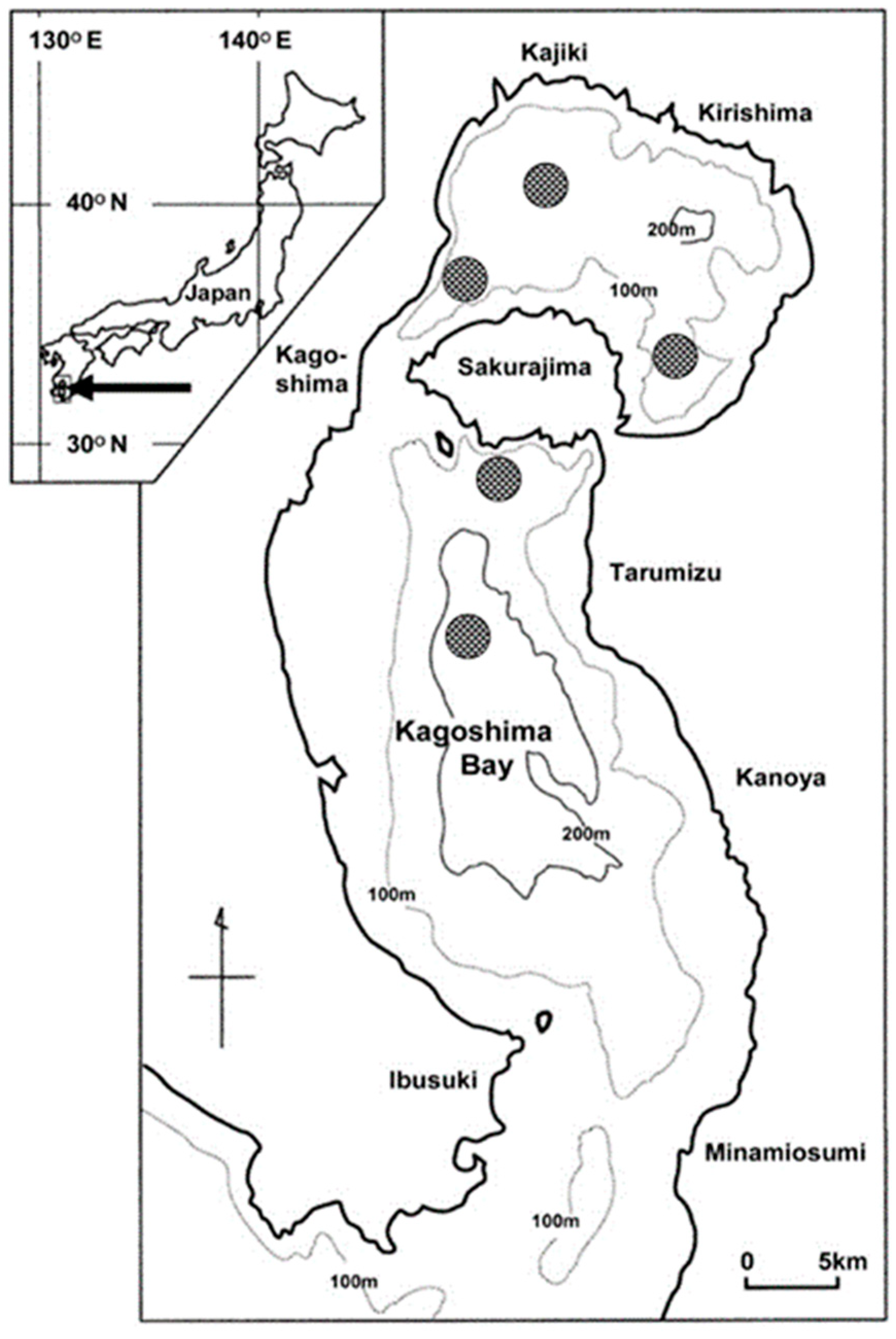
2.1. Study Site
2.2. Sampling
2.3. Measurements and Macroscopic Observation
2.4. Histological Analysis and Measurement of Oocytes
2.5. Size at Sexual Maturity
2.6. Annual Reproductive Cycle
3. Results
3.1. Macroscopic Stages of Ovaries
3.2. Classification of Oocyte Developmental Stages
3.3. Classification of Ovarian Maturity Stages
3.4. Size at Sexual Maturity
3.5. Annual Reproductive Cycle
3.6. Bottom Water Temperature
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potier, M.; Romanov, E.; Cherel, Y.; Sabatié, R.; Zamorov, V.; Ménard, F. Spatial distribution of Cubiceps pauciradiatus (Perciformes: Nomeidae) in the tropical Indian Ocean and its importance in the diet of large pelagic fishes. Aquat. Living Resour. 2008, 21, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Abe, T. Notes on the adult of Cubiceps gracilis from the western Pacific. J. Oceanogr. Sci. 1955, 11, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Romanov, E.V. Bycatch in the tuna purse-seine fisheries of the western Indian Ocean. Fish Bull. 2002, 100, 90–105. [Google Scholar]
- Escalle, L.; Gaertner, D.; Chavance, P.; Murua, H.; Simier, M.; Pascual-Alayón, P.J.; Ménard, F.; Ruiz, J.; Abascal, F.; Mérigot, B. Catch and bycatch captured by tropical tuna purse-seine fishery in whale and whale shark associated sets: Comparison with free school and FAD sets. Biodivers. Conserv. 2018, 28, 467–499. [Google Scholar] [CrossRef]
- Alverson, F.G. The food of yellowfin and skipjack tunas in the eastern tropical Pacific Ocean. Inter-Am. Trop. Tuna Comm. Bull. 1963, 7, 293–396. [Google Scholar]
- Beauplet, G.; Dubroca, L.; Guinet, C.; Cherel, Y.; Dabin, W.; Gagne, C.; Hindell, M. Foraging ecology of subantarctic fur seals Arctocephalus tropicalis breeding on Amsterdam Island: Seasonal changes in relation to maternal characteristics and pup growth. Mar. Ecol. Prog. Ser. 2004, 273, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Ménard, F.; Stéquert, B.; Rubin, A.; Herrera, M.; Marchal, E. Food consumption of tuna in the equatorial Atlantic Ocean: FAD-associated versus unassociated schools. Aquat. Living Resour. 2000, 13, 233–240. [Google Scholar] [CrossRef]
- Pinaud, D.; Cherel, Y.; Weimerskirch, H. Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in yellow-nosed albatrosses. Mar. Ecol. Prog. Ser. 2005, 298, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Potier, M.; Marsac, F.; Lucas, V.; Sabatié, R.; Hallier, J.-P.; Ménard, F. Feeding Partitioning among Tuna Taken in Surface and Mid-water Layers: The Case of Yellowfin (Thunnus albacares) and Bigeye (T. obesus) in the Western Tropical Indian Ocean. Western Indian Ocean J. Mar. Sci. 2004, 3, 51–62. [Google Scholar]
- Potier, M.; Marsac, F.; Cherel, Y.; Lucas, V.; Sabatier, R.; Maury, O.; Ménard, F. Forage fauna in the diet of three large pelagic fishes (lancetfish, swordfish and yellowfin tuna) in the western equatorial Indian Ocean. Fish. Res. 2007, 83, 60–72. [Google Scholar] [CrossRef]
- Young, J.; Lansdell, M.; Riddoch, S.; Revill, A. Feeding ecology of broadbill swordfish, Xiphias gladius, off eastern Australia in relation to physical and environmental variables. Bull. Mar. Sci. 2006, 79, 793–809. [Google Scholar]
- Young, J.W.; Lansdell, M.J.; Campbell, R.A.; Cooper, S.P.; Juanes, F.; Guest, M.A. Feeding ecology and niche segregation in oceanic top predators off eastern Australia. Mar. Biol. 2010, 157, 2347–2368. [Google Scholar] [CrossRef]
- Perrin, W.F.; Warner, R.R.; Fiscus, C.H.; Holts, D.B. Stomach contents of porpoise, Stenella spp., and yellowfin tuna, Thunnus albacares, in mixed-species aggregations. Fish. Bull. 1973, 71, 1077–1092. [Google Scholar]
- Dollar, M.L.; Walker, W.A.; Kooyman, G.L.; Perrin, W.F. Comparative feeding ecology of spinner dolphins (Stenella longirostris) and Fraser’s dolphins (Lagenodelphis hosei) in the Sulu Sea. Mar. Mamm. Sci. 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Ponnampalam, L.S.; Collins, T.J.Q.; Minton, G.; Schulz, I.; Gray, H.; Ormond, R.F.G.; Baldwin, R.M. Stomach contents of small cetaceans stranded along the Sea of Oman and Arabian Sea coasts of the Sultanate of Oman. J. Mar. Biolog. Assoc. U. K. 2012, 92, 1699–1710. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J. Integrating reproductive biology into scientific advice for fisheries management. J. Northwest Atl. Fish. Sci. 2008, 41, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Nakabo, T.; Doiuchi, R. Nomeidae. In Fishes of Japan with Pictorial Keys to the Species, 3rd ed.; Nakabo, T., Ed.; Tokai University Press: Hadano, Japan, 2013; pp. 1081–1083, 2041–2042. (In Japanese) [Google Scholar]
- Last, P.R. Nomeidae. Drift fishes (Cigarfishes). In FAO Species Identification Guide for Fishery Purposes; Capenter, K.E., Niem, V.H., Eds.; The Living Marine Resources of the Western Central Pacific: Vol 6 Bony Fishes Part 4 (Labridae to Latimeriidae), Estuarine Crocodiles, Sea Turtles, Sea Snakes and Marines Mammals; FAO: Rome, Italy, 2001; pp. 3771–3779. [Google Scholar]
- Zhao, M.; Ma, H.; Ma, C.; Zhang, H.; Zhang, X.; Meng, Y.; Wei, H.; Chen, F.; Ma, L. The complete mitochondrial genome and gene organization of Cubiceps squamiceps (Perciformes: Nomeidae) with phylogenetic consideration. Mitochondrial DNA Part A 2015, 27, 4296–4297. [Google Scholar] [CrossRef]
- Haedrich, R.L. Nomeidae. In Smith’s Sea Fishes; Smith, M.M., Ed.; Macmillan South Africa: Johannesburg, South Africa, 1986; pp. 846–850. [Google Scholar]
- Cabebe, R.A.; Motomura, H. Nomeid fishes (Perciformes) from Kagoshima Prefecture, southern Kyushu, Japan. Nat. Kagoshima 2019, 46, 117–124. [Google Scholar]
- Matsui, S. Notes on the Nomeid fish Cubiceps sqamiceps (Lloyd) from the sea near Kagoshima, Japan. Sci. Bull. Fac. Agr., Kyushu Univ. 1977, 32, 1–7. [Google Scholar] [CrossRef]
- Butler, J.L. The nomeid genus Cubiceps (PISCES) with a description of a new species. Bull. Mar. Sci. 1979, 29, 226–241. [Google Scholar]
- Mizuno, K.; White, W.B. Annual and interannual variability in the Kuroshio Current system. J. Phys. Oceanogr. 1983, 13, 1847–1867. [Google Scholar] [CrossRef]
- Kobari, T.; Akamatsu, H.; Minowa, M.; Ichikawa, T.; Iseki, K.; Fukuda, R.; Higashi, M. Effects of the copepod community structure on fecal pellet flux in Kagoshima Bay, a deep, semi-enclosed embayment. J. Oceanogr. 2010, 66, 673–684. [Google Scholar] [CrossRef]
- Ohtomi, J. Fisheries resources for small-scale bottom trawlers in Kagoshima Bay, Japan. Bull. Mar. Resour. Environ. Kagoshima Univ. 2001, 9, 19–23. (In Japanese) [Google Scholar]
- Ohtomi, J.; Fujieda, S.; Higashi, M.; Habano, A. Preliminary trawl survey for estimating distributions of benthic animals and marine debris in Kagoshima Bay. Bull. Jpn. Soc. Fish. Oceanogr. 2004, 68, 158–164, (In Japanese with English abstract). [Google Scholar]
- Fulanda, B.; Ohtomi, J. Determination of effective tow duration for estimation of fish and shellfish abundance in deeper-water bottom trawl surveys. Fish. Sci. 2011, 77, 487–495. [Google Scholar] [CrossRef]
- Yamamoto, K. Studies on the formation of fish eggs. Annual cycle in the development of ovarian eggs in the flounder Liopsetta obscura. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1956, 12, 362–374. [Google Scholar]
- Granada, P.V.; Masuda, Y.; Matsuoka, T. Annual reproductive cycle and spawning frequency of the female yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay, southern Japan. Suisanzoshoku 2004, 52, 329–340. [Google Scholar] [CrossRef]
- Havimana, L.; Ohtomi, J.; Masuda, Y.; Vazquez Archdale, M. The reproductive biology of female crimson sea bream Evynnis tumifrons off the southwestern coast of Kyushu, Japan. Fish. Sci. 2020, 86, 65–75. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J. Measurement of spawning frequency in multiple spawning fishes. In An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy, Engraulis mordax; Lasker, R., Ed.; NOAA Technical Report NMFS 36; US Department of Commerce: Washington, DC, USA, 1985; pp. 79–94. [Google Scholar]
- Alejo-Plata, C.; Díaz-Jaimes, P.; Salgado-Ugarte, I.H. Sex ratios, size at sexual maturity and spawning seasonality of dolphinfish Coryphaena hippurus captured in the gulf of Tehuantepec, Mexico. Fish. Res. 2011, 110, 207–216. [Google Scholar] [CrossRef]
- Sun, C.; Chang, H.; Liu, T.; Yeh, S.; Chang, Y. Reproductive biology of the black marlin Istiompax indica off southwestern and eastern Taiwan. Fish. Res. 2015, 166, 12–20. [Google Scholar] [CrossRef]
- Okochi, Y.; Abe, O.; Tanaka, S.; Ishihara, Y.; Shimizu, A. Reproductive biology of female Pacific bluefin tuna Thunnus orientalis in the Sea of Japan. Fish. Res. 2016, 174, 30–39. [Google Scholar] [CrossRef]
- West, G. Methods of assessing ovarian development in fishes: A review. Aust. J. Mar. Freshw. Res. 1990, 41, 199–222. [Google Scholar] [CrossRef]
- Farrell, E.D.; Hussy, K.; Coad, J.O.; Clausen, L.W.; Clarke, M.W. Oocyte development and maturity classification of boarfish (Capros aper) in the northeast Atlantic. ICES J. Mar. Sci. 2012, 69, 498–507. [Google Scholar] [CrossRef] [Green Version]
- King, M. Fisheries Biology, Assessment and Management; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. XIV–382. [Google Scholar]
- Rahman, M.M.; Ohtomi, J. Reproductive biology of the deep-water velvet shrimp Metapenaeopsis sibogae (De Man, 1907) (Decapoda: Penaeidae). J. Crustac. Biol. 2017, 37, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ohtomi, J. Ovarian maturation, size at sexual maturity and spawning season of Metapenaeopsis provocatoria owstoni Shinomiya & Sakai, 2000 (Decapoda: Penaeidae). Crustacean Res. 2020, 49, 109–120. [Google Scholar] [CrossRef]
- Vitale, F.; Svedäng, H.; Cardinale, M. Histological analysis invalidates macroscopically determined maturity ogives of the Kattegat cod (Gadus morhua) and suggests new proxies for estimating maturity status of individual fish. ICES J. Mar. Sci. 2006, 63, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ohtomi, J. Relative growth and morphological sexual maturity of the deep-water velvet shrimp Metapenaeopsis sibogae (Crustacea, Decapoda, Penaeidae). Invertebr. Reprod. Dev. 2018, 62, 125–132. [Google Scholar] [CrossRef]
- Kume, G.; Yamaguchi, A.; Aoki, I.; Taniuchi, T. Reproductive biology of the cardinalfish Apogon lineatus in Tokyo Bay, Japan. Fish. Sci. 2000, 66, 947–954. [Google Scholar] [CrossRef]
- Iqbal, K.M.; Ohtomi, J.; Suzuki, H. Reproductive biology of the Japanese silver-biddy, Gerres equulus, in western Kyushu, Japan. Fish. Res. 2007, 83, 145–150. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J.; Lo, N.C.; Kimbrell, C.A. Fecundity, spawning and maturity of female dover sole Microstomus pacifcus with an evaluation of assumptions and precision. Fish. Bull. 1992, 90, 101–128. [Google Scholar]
- Kurita, Y.; Fujinami, Y.; Amano, M. The effect of temperature on the duration of spawning markers-migratory-nucleus and hydrated oocytes and postovulatory follicles-in the multiple-batch spawner Japanese founder (Paralichthys olivaceus). Fish. Bull. 2011, 109, 79–89. [Google Scholar]
- Matsuyama, M.; Adachi, S.; Nagahama, Y.; Matsuura, S. Diurnal rhythm of oocyte development and plasma steroid hormone levels in the female red sea bream Pagrus major during the spawning season. Aquaculture 1988, 73, 357–372. [Google Scholar] [CrossRef]
- Yoda, M.; Yoneda, M. Assessment of reproductive potential in multiple-spawning fish with indeterminate fecundity: A case study of Yellow Sea bream Dentex hypselosomus in the East China Sea. J. Fish. Biol. 2009, 74, 2338–2354. [Google Scholar] [CrossRef]
- Hayashi, I. On the ovarian maturation of the Japanese sea bass Lateolabrax japonicus. Jpn. J. Ichthyol. 1972, 19, 243–254. [Google Scholar] [CrossRef]
- Kunishima, T.; Higuchi, S.; Kawabata, Y.; Furumitsu, K.; Nakamura, I.; Yamaguchi, A.; Tachihara, K.; Tokeshi, M.; Arakaki, S. Age, growth, and reproductive biology of the blackfin seabass Lateolabrax latus, a major predator in rocky coastal ecosystems of southwestern Japan. Reg. Stud. Mar. Sci. 2021, 41, 101597. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Lowerre, J.M.; Barbieri, L.R. Multiple spawning and the dynamics of fish populations: Inferences from an individual-based simulation model. Can. J. Fish. Aquat. Sci. 1998, 55, 2244–2254. [Google Scholar] [CrossRef]
- Luo, J.; Musick, J.A. Reproductive biology of the bay anchovy in Chesapeake Bay. Trans. Am. Fish. Soc. 1991, 120, 701–710. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rodhouse, P.G.; Nolan, C.P. Modeling the selective effects of fishing on reproductive potential and population structure of squid. ICES J. Mar. Sci. 1994, 51, 299–313. [Google Scholar] [CrossRef]
- Zhu, G.P.; Dai, X.J.; Song, L.M.; Xu, L.X. Size at sexual maturity of bigeye tuna Thunnus obesus (Perciformes: Scombridae) in the tropical waters: A comparative analysis. Turk. J. Fish. Aquat. Sci. 2011, 11, 149–156. [Google Scholar] [CrossRef]
- Anzawa, W.; Tomioka, N.; Yamabuki, K. Report on the stock enhancement of sea bream Evynnis japonica; Fisheries Experimental Station of Niigata Prefecture: Niigata, Japan, 1987; pp. 1–31. (In Japanese) [Google Scholar]
- DeMartini, E.E.; Uchiyama, J.H.; Williams, H.A. Sexual maturity, sex ratio, and size composition of swordfish Xiphias gladius caught by the Hawaii-based pelagic longline fishery. Fish. Bull. 2000, 98, 489–506. [Google Scholar]
- Yoneda, M.; Tokimura, M.; Fujita, H.; Takeshita, N.; Takeshita, K.; Matsuyama, M.; Matsuura, S. Ovarian structure and batch fecundity in Lophiomus setigerus. J. Fish. Biol. 1998, 52, 94–106. [Google Scholar] [CrossRef]
- Migaud, H.; Fontaine, P.; Kestemont, P.; Wang, N.; Brun-Bellut, J. Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture 2004, 241, 561–574. [Google Scholar] [CrossRef]
- Kobari, T.; Habano, A.; Ichikawa, T. Seasonal variations in phyto- and zooplankton biomass in Kagoshima Bay. Mem. Fac. Fish. Kagoshima Univ. 2002, 51, 19–25. [Google Scholar]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Norberg, B.; Brown, C.L.; Halldorsson, O.; Stensland, K.; Björnsson, B.T. Photoperiod regulates the timing of sexual maturation, spawning, sex steroid and thyroid hormone profiles in the Atlantic cod (Gadus morhua). Aquaculture 2004, 229, 451–467. [Google Scholar] [CrossRef]
- Bromage, N.R.; Elliott, J.A.; Springate, J.R.; Whitehead, C. The effects of constant photoperiods on the timing of spawning in the rainbow trout. Aquaculture 1984, 43, 213–223. [Google Scholar] [CrossRef]
- Campos-Mendoza, A.; Bromage, N.; McAndrew, B.J. The effect of photoperiod on the reproductive performance of the Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2003, 28, 503–504. [Google Scholar] [CrossRef]
- Giannecchini, L.G.; Haluko, M.; João Batista, K.F. Effects of photoperiod on reproduction of Siamese fighting fish Betta splendens. Rev. Bras. De Zootec. 2012, 41, 821–826. [Google Scholar] [CrossRef] [Green Version]
- Shahjahan, M.; Al-Emran, M.; Islam, S.M.; Baten, S.A.; Rashid, H.; Haque, M.M. Prolonged photoperiod inhibits growth and reproductive functions of rohu Labeo rohita. Aquac. Rep. 2020, 16, 100272. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. Available online: https://www.esrl.noaa.gov/gmd/grad/solcalc/ (accessed on 16 January 2022).
- Russell, M.W.; Luckhurst, B.E.; Lindeman, K.C. Management of spawning aggregations. In Reef Fish Spawning Aggregations: Biology, Research and Management; de Mitcheson, Y.S., Colin, P.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 371–404. [Google Scholar]
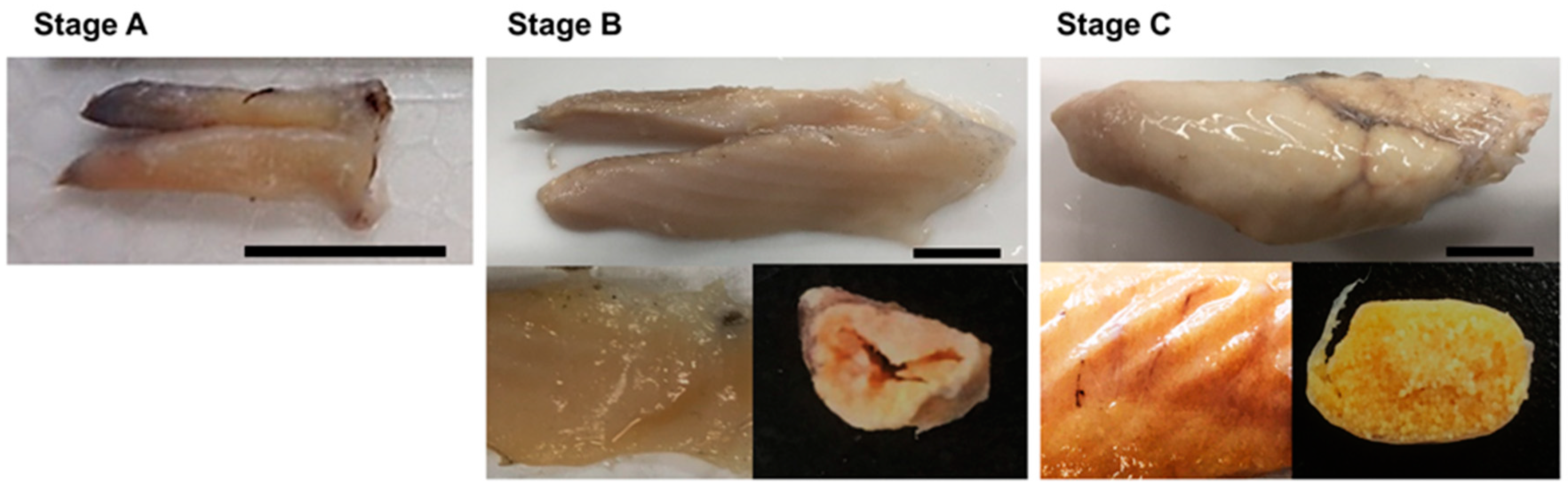









| Ovarian Maturity Stage | Developmental Stage of Oocytes |
|---|---|
| Immature | Only unyolked oocytes, such as PN and PV, were present. Oocytes ranged from 30 to 120 μm in diameter. |
| Maturing | The most advanced oocyte was in EYG or LYG. Oocytes were 110 to 280 μm in diameter |
| Mature | The most advanced oocyte was in MN or H. Oocytes were 260 to 410 μm in diameter |
| Spawned | Yolked oocytes and POF were present |
| Spent | More than 50% of yollked oocytes were in EA. POF was absent |
| Resting | Unyolked oocytes and LA oocytes were present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtomi, J.; Hirowatari, K.; Rahman, M.M.; Havimana, L.; Masuda, Y. First Report on Reproductive Features of Shadow Driftfish Cubiceps whiteleggii (Perciformes: Nomeidae): An Effort toward Sustainable Management. Sustainability 2022, 14, 8813. https://doi.org/10.3390/su14148813
Ohtomi J, Hirowatari K, Rahman MM, Havimana L, Masuda Y. First Report on Reproductive Features of Shadow Driftfish Cubiceps whiteleggii (Perciformes: Nomeidae): An Effort toward Sustainable Management. Sustainability. 2022; 14(14):8813. https://doi.org/10.3390/su14148813
Chicago/Turabian StyleOhtomi, Jun, Kaito Hirowatari, Md Mosaddequr Rahman, Lindon Havimana, and Yasuji Masuda. 2022. "First Report on Reproductive Features of Shadow Driftfish Cubiceps whiteleggii (Perciformes: Nomeidae): An Effort toward Sustainable Management" Sustainability 14, no. 14: 8813. https://doi.org/10.3390/su14148813
APA StyleOhtomi, J., Hirowatari, K., Rahman, M. M., Havimana, L., & Masuda, Y. (2022). First Report on Reproductive Features of Shadow Driftfish Cubiceps whiteleggii (Perciformes: Nomeidae): An Effort toward Sustainable Management. Sustainability, 14(14), 8813. https://doi.org/10.3390/su14148813







