Flood Resilience, Viability, and Growth Response to Seawater Immersion of Bermudagrass (Cynodon dactylon (L.) Pers.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flood Resilience Experiment
2.2. Post-Submergence Growth and Biomass Allocation Experiment
2.3. Statistical Analysis
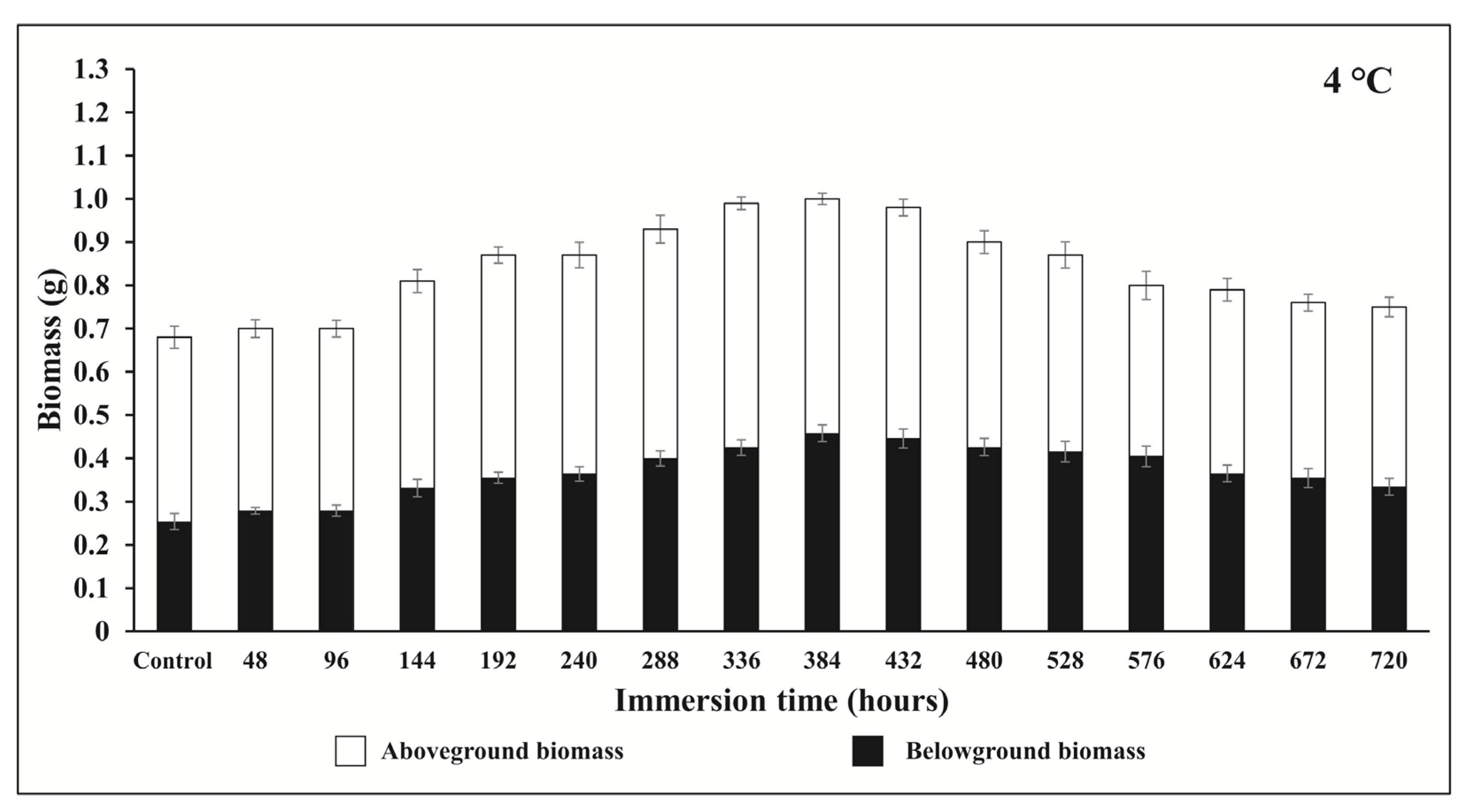
3. Results
3.1. Flood and Salt Resilience
3.2. Viability
3.3. Post-Submergence Changes
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholls, R.J.; Cazenave, A. Sea-level rise and its impact on coastal zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Hoggart, S.; Hanley, M.; Parker, D.; Simmonds, D.; Bilton, D.; Filipova-Marinova, M.; Franklin, E.; Kotsev, I.; Penning-Rowsell, E.; Rundle, S.; et al. The consequences of doing nothing: The effects of seawater flooding on coastal zones. Coast. Eng. 2014, 87, 169–182. [Google Scholar] [CrossRef]
- Weisse, R.; Bellafiore, D.; Menéndez, M.; Méndez, F.; Nicholls, R.J.; Umgiesser, G.; Willems, P. Changing extreme sea levels along European coasts. Coast. Eng. 2014, 87, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.; Nicholls, R.J.; Goodwin, P.; Haigh, I.D.; Lincke, D.; Vafeidis, A.T.; Hinkel, J. Quantifying Land and People Exposed to Sea-Level Rise with No Mitigation and 1.5 °C and 2.0 °C Rise in Global Temperatures to Year 2300. Earth’s Future 2018, 6, 583–600. [Google Scholar] [CrossRef]
- Hallegatte, S.; Green, C.; Nicholls, R.J.; Corfee-Morlot, J. Future flood losses in major coastal cities. Nat. Clim. Chang. 2013, 3, 802–806. [Google Scholar] [CrossRef]
- Recanatesi, F.; Petroselli, A.; Ripa, M.N.; Leone, A. Assessment of stormwater runoff management practices and BMPs under soil sealing: A study case in a peri-urban watershed of the metropolitan area of Rome (Italy). J. Environ. Manag. 2017, 201, 6–18. [Google Scholar] [CrossRef]
- Durant, D.; Kernéïs, E.; Meynard, J.M.; Choisis, J.P.; Chataigner, C.; Hillaireau, J.M.; Rossignol, C. Impact of storm Xynthia in 2010 on coastal agricultural areas: The Saint Laurent de la Prée research farm’s experience. J. Coast. Conserv. 2018, 22, 1177–1190. [Google Scholar] [CrossRef]
- Narayan, S.; Nicholls, R.; Trifonova, E.; Filipova-Marinova, M.; Kotsev, I.; Vergiev, S.; Hanson, S.; Clarke, D. Coastal habitats within flood risk assessments: Role of the 2D SPR approach. Coast. Eng. Proc. 2012, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dávila, O.; Stithou, M.; Pescaroli, G.; Pietrantoni, L.; Koundouri, P.; Díaz-Simal, P.; Rulleau, B.; Touili, N.; Hissel, F.; Penning-Rowsell, E. Promoting resilient economies by exploring insurance potential for facing coastal flooding and erosion: Evidence from Italy, Spain, France and United Kingdom. Coast. Eng. 2014, 87, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Trifonova, E.; Valchev, N.; Keremedchiev, S.; Kotsev, I.; Eftimova, P.; Todorova, V.; Konsulova, T.; Doncheva, V.; Filipova-Marinova, M.; Vergiev, S.; et al. Case studies world-wide: Mitigating flood and erosion risk using sediment management for a tourist City: Varna, Bulgaria. In Coastal Risk Management in a Changing Climate, 1st ed.; Zanuttigh, B., Nicholls, R., Vanderlinden, J., Burcharth, H., Thompson, R., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 358–383. [Google Scholar] [CrossRef]
- Teng, J.; Jakeman, A.; Vaze, J.; Croke, B.F.; Dutta, D.; Kim, S. Flood inundation modelling: A review of methods, recent advances and uncertainty analysis. Environ. Model. Softw. 2017, 90, 201–216. [Google Scholar] [CrossRef]
- Rubinato, M.; Heyworth, J.; Hart, J. Protecting Coastlines from Flooding in a Changing Climate: A Preliminary Experimental Study to Investigate a Sustainable Approach. Water 2020, 12, 2471. [Google Scholar] [CrossRef]
- Vergiev, S. Tall Wheatgrass (Thinopyrum ponticum): Flood Resilience, Growth Response to Sea Water Immersion, and Its Capacity for Erosion and Flooding Control of Coastal Areas. Environments 2019, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Maun, M.A. The Biology of Coastal Sand Dunes, 1st ed.; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Schoutens, K.; Heuner, M.; Minden, V.; Schulte Ostermann, T.; Silinski, A.; Belliard, J.; Temmerman, S. How effective are tidal marshes as nature-based shoreline protection throughout seasons? Limnol. Oceanogr. 2019, 64, 1750–1762. [Google Scholar] [CrossRef]
- Clark, J.R. Coastal Zone Management Handbook, 1st ed.; CRC Press/Lewis Publishers: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Vergiev, S. Comparative study of the capacity of three plant species from the Poaceae family for erosion and flooding control of coastal areas. In Sustainable Development and Innovations in Marine Technologies, Proceedings of the 18th International Congress of the International Maritime Association of the Mediterranean, Varna, Bulgaria, 9–11 September 2019; CRC Press: London, UK; pp. 597–602.
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Borsjea, B.W.; van Wesenbeeck, B.K.; Dekker, F.; Paalvast, P.; Bouma, T.J.; van Katwijk, M.M.; de Vries, M.B. How ecological engineering can serve in coastal protection. Ecol. Eng. 2011, 37, 113–122. [Google Scholar] [CrossRef]
- Hart, A.T.; Hilton, M.J.; Wakes, S.J.; Dickinson, K.J.M. The impact of Ammophila arenaria foredune development on downwind aerodynamics and parabolic dune development. J. Coast. Res. 2012, 28, 112–122. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Sources of Variation in Cynodon dactylon (L). Pers. Crop. Sci. 1969, 9, 774–778. [Google Scholar] [CrossRef]
- Vergiev, S. The impact of sea water immersion on the viability of psammophilous species Carex colchica and its capacity as dune stabilizer. C. R. Acad. Bulg. Sci. 2018, 71, 648–654. [Google Scholar] [CrossRef]
- Vergiev, S. Comparative study of the response of four native to the Bulgarian Black Sea Coast psammophytes to simulated flooding experiments. Annu. Res. Rev. Biol. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, W.H.J.M.; Lenssen, J.P.M.; van de Steeg, H.M.; Blom, C.W.P.M.; de Kroon, H. Seasonal Dependent Effects of Flooding on Plant Species Survival and Zonation: A Comparative Study of 10 Terrestrial Grassland Species. Hydrobiologia 2006, 565, 59–69. [Google Scholar] [CrossRef]
- Ye, T.; Shi, H.; Wang, Y.; Chan, Z. Contrasting Changes Caused by Drought and Submergence Stresses in Bermudagrass (Cynodon dactylon). Front. Plant Sci. 2015, 6, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachel, A.; Marcel, R. The effect of sea-water submergence on rhizome bud viability of the introduced Ammophila arenaria and the native Leymus mollis in California. J. Coast. Conserv. 2000, 6, 107–111. [Google Scholar] [CrossRef]
- Konlechner, T.M.; Hilton, M.J. The potential for marine dispersal of Ammophila arenaria (marram grass) rhizome. J. Coast. Res. 2009, 56, 434–437. [Google Scholar]
- Vergiev, S.; Filipova-Marinova, M.; Trifonova, E.; Kotsev, I.; Pavlov, D. The impact of sea water immersion on the viability of psammophilous species Leymus racemosus subsp. sabulosus and Ammophila arenaria. C. R. Acad. Bulg. Sci. 2013, 66, 211–216. [Google Scholar] [CrossRef]
- Konlechner, T.M.; Orlovich, D.A.; Hilton, M.J. Restrictions in the sprouting ability of an invasive coastal plant, Ammophila arenaria, from fragmented rhizomes. Plant Ecol. 2016, 217, 521–532. [Google Scholar] [CrossRef]
- Hanley, M.E.; Yip, P.Y.S.; Hoggart, S.; Bilton, D.T.; Rundle, S.D.; Thompson, R.C. Riding the storm: The response of Plantago lanceolata to simulated tidal flooding. J. Coast. Conserv. 2013, 17, 799–803. [Google Scholar] [CrossRef]
- White, A.C.; Colmer, T.D.; Cawthray, G.R.; Hanley, M.E. Variable response of three Trifolium repens ecotypes to soil flooding by seawater. Ann. Bot. 2014, 114, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Hanley, M.E.; Gove, T.L.; Cawthray, G.R.; Colmer, T.D. Differential responses of three coastal grassland species to seawater flooding. J. Plant Ecol. 2017, 10, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Hanley, M.E.; Hartley, F.C.; Hayes, L.; Franco, M. Simulated seawater flooding reduces oilseed rape growth, yield and progeny performance. Ann. Bot. 2020, 125, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Vergiev, S. Sea water flood resilience of five plant species with conservation status over the Bulgarian Black Sea Coast. GSC Biol. Pharm. Sci. 2021, 16, 19–23. [Google Scholar] [CrossRef]
- Liew, J.; Andersson, L.; Boström, U.; Forkman, J.; Hakman, I.; Magnuski, E. Regeneration capacity from buds on roots and rhizomes in five herbaceous perennials as affected by time of fragmentation. Plant. Ecol. 2013, 214, 1199–1209. [Google Scholar] [CrossRef]
- Hilton, M.; Harvey, N.; Hart, A.; James, K.; Arbuckle, C. The impact of exotic dune grass species on foredune development in Australia and New Zealand: A case study of Ammophila arenaria and Thinopyrum junceiforme. Aust. Geog. 2006, 37, 313–334. [Google Scholar] [CrossRef]
- Upreti, K.K.; Murti, G.S.R. Response of grape rootstocks to salinity: Changes in root growth, polyamines and abscisic acid. Biol. Plant. 2010, 54, 730–734. [Google Scholar] [CrossRef]
- Harris, D.; Davy, A.J. Regenerative potential of Elymus farctus from rhizome fragments and seed. J. Ecol. 1986, 74, 1057–1067. [Google Scholar] [CrossRef]
- Van Tran, T.; Fukai, S.; Giles, H.; Lambrides, C. Salinity tolerance among a large range of bermudagrasses (Cynodon spp.) relative to other halophytic and non-halophytic perennial C4 grasses. Environ. Exp. Bot. 2018, 145, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Hameed, M.; Ashraf, M. Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the Salt Range (Pakistan) to salinity stress. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 683–694. [Google Scholar] [CrossRef]
- Fan, J.; Xu, J.; Zhang, W.; Amee, M.; Liu, D.; Chen, L. Salt-Induced Damage is Alleviated by Short-Term Pre-Cold Treatment in Bermudagrass (Cynodon dactylon). Plants 2019, 8, 347. [Google Scholar] [CrossRef] [Green Version]


| Parameter (h) | 4 °C | 13 °C | 23 °C |
|---|---|---|---|
| Decomposition onset of leaves | 144 | 144 | 144 |
| Decomposition onset of stems | 264 | 264 | 264 |
| Decomposition onset of roots | 360 | 360 | 360 |
| Full putrefaction of leaves | 408 | 408 | 402 |
| Full putrefaction of stems | n/a | n/a | n/a |
| Full putrefaction of roots | n/a | n/a | n/a |
| Growth of stems | 120 | 120 | 120 |
| Growth of root sprouts | 144 | 144 | 144 |
| Putrefaction onset of newly grown stems | 460 | 460 | 450 |
| Putrefaction onset of newly grown roots | n/a | n/a | n/a |
| Full putrefaction of newly grown stems | n/a | n/a | n/a |
| Full putrefaction of newly grown root | n/a | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergiev, S. Flood Resilience, Viability, and Growth Response to Seawater Immersion of Bermudagrass (Cynodon dactylon (L.) Pers.). Sustainability 2022, 14, 8733. https://doi.org/10.3390/su14148733
Vergiev S. Flood Resilience, Viability, and Growth Response to Seawater Immersion of Bermudagrass (Cynodon dactylon (L.) Pers.). Sustainability. 2022; 14(14):8733. https://doi.org/10.3390/su14148733
Chicago/Turabian StyleVergiev, Stoyan. 2022. "Flood Resilience, Viability, and Growth Response to Seawater Immersion of Bermudagrass (Cynodon dactylon (L.) Pers.)" Sustainability 14, no. 14: 8733. https://doi.org/10.3390/su14148733
APA StyleVergiev, S. (2022). Flood Resilience, Viability, and Growth Response to Seawater Immersion of Bermudagrass (Cynodon dactylon (L.) Pers.). Sustainability, 14(14), 8733. https://doi.org/10.3390/su14148733






