1. Introduction
Fluorine, the thirteenth most prevalent element, is often found in the earth’s crust at a concentration of roughly 0.3 g/kg [
1,
2,
3,
4]. Fluorine is obtained geologically from fluorine-bearing minerals such as fluorite (CaF
2) (48.9% fluorine content), cryolite (Na
3AlF
6), topaz [Al
2SiO
4(F,OH
2)], apatite [Ca
5(PO
4)
3(Cl,F,OH)], micas [AB2–3(X, Si)
4O
10(O,F,OH)
2] and sellaite (MgF
2), fluorapatite (Ca
5FO
12P
3), and biotite K(Mg,Fe)
3(AlSi
3O
10)(F,OH)
2 [
5,
6,
7,
8]. Fluoride is present in the environment in a number of countries, including China, India, Iran, Mexico, Turkey, Sri Lanka, Pakistan, Kenya, Ethiopia, and Thailand [
9,
10,
11,
12].
Because of its location in the Chiang Mai Basin, which comprises the provinces of Chiang Mai, Lamphun, and Mae Hong Son, the Ma Khuea Chae subdistrict in Thailand, which is located between 18.573821° latitude and 99.134547° longitude, has a population that is exposed to fluoride [
13,
14,
15,
16]. For many years, this population has suffered from fluorosis caused by drinking fluoride-contaminated groundwater. Athikhomrungsarit (2002) revealed that fluoride was detected in drinking water drawn from groundwater in San Pa Tieng communities, with the maximum concentration being 17 ppm. Dean’s community fluorosis index revealed fluoride concentrations ranging from 0.2 to 18.9 mg/L, 0.1 to 2.3 mg/L, and 0.25 mg/L, respectively [
17].
These communities are exposed to fluoride by natural and human-caused processes [
15,
18,
19,
20]. Several natural elements, including rock type, weathering, leaching, soil, tec-tonics, hydrogeology, geothermal, and topographical climate, contribute significantly to increased fluoride levels in drinking water [
21,
22,
23]. Fluoride concentrations vary according to geography. Fawell’s prior research indicated that fluoride is found in soil at concentrations ranging from 10 to 1000 ppm, and in water at concentrations ranging from 0.5 to 2000 ppm [
22].
Fluoride pollution might arise as a result of human, agricultural, and industrial activity. Typically, a place is exposed to fluoride as a result of its vicinity to paddy fields or an industrial zone (as
Figure 1 and
Figure 2). The findings indicated that fluoride-affected regions contaminate the surrounding area. Inappropriate phosphorus fertilizer application contributes to fluoride pollution of paddy fields. Fluoride levels in the soil are reported to be 0.34 mg/L due to the usage of phosphate fertilizers in rice fields [
24]. Gray (2018) found that total fluoride concentrations increased from 251 mg/kg to 349 and 430 mg/kg in topsoil (0–7.5 cm) that received 188 kg/ha and 376 kg/ha fertilizer, respectively [
23]. Further, the long-term application was to establish a relationship between higher fluoride concentrations in soil and subsequent fluoride transfer to vegetables and livestock. Momdal and Gupta (2015) showed that irrigated water had been contaminated with fluoride accumulated in soil, crops, and vegetables [
20].
Additionally, industrial sources emit fluoride. Aluminum smelting, glass processing, phosphate fertilizer production, commercial brick production, industrial pesticide pro-duction, fluorine-containing product (PTFE) mining and manufacturing, and coal-fired power plants all add fluoride to the environment [
2,
25]. Numerous parts of the world, particularly those adjacent to industrial zones, are commonly contaminated with fluoride (35). Fluoride enters the food chain after it is released into the environment, via soil to plants and animals, as well as from plants and animals to humans [
26,
27]. Populations living in contaminated areas have been affected and are at risk of developing fluorosis. Populations living in fluoride-affected areas have consumed fluoride-infected food, vegetables, and fruits, as well as contaminated drinking water [
28]. The high-fluoride waters in the Chiang Mai basin originate from a nearby geothermal field. Furthermore, 35% of deep wells had fluoride at a concentration of at least 1.5 mg/L, compared to only 7% of shallow wells [
16]. Numerous investigations indicate that the distribution of fluoride in food roots, such as
Triticum aestivum,
Oryza sativa,
Cajanus cajan, and
Capsicum annuum, that grow in fluoride-contaminated areas corresponds to the area’s fluoride distribution [
26,
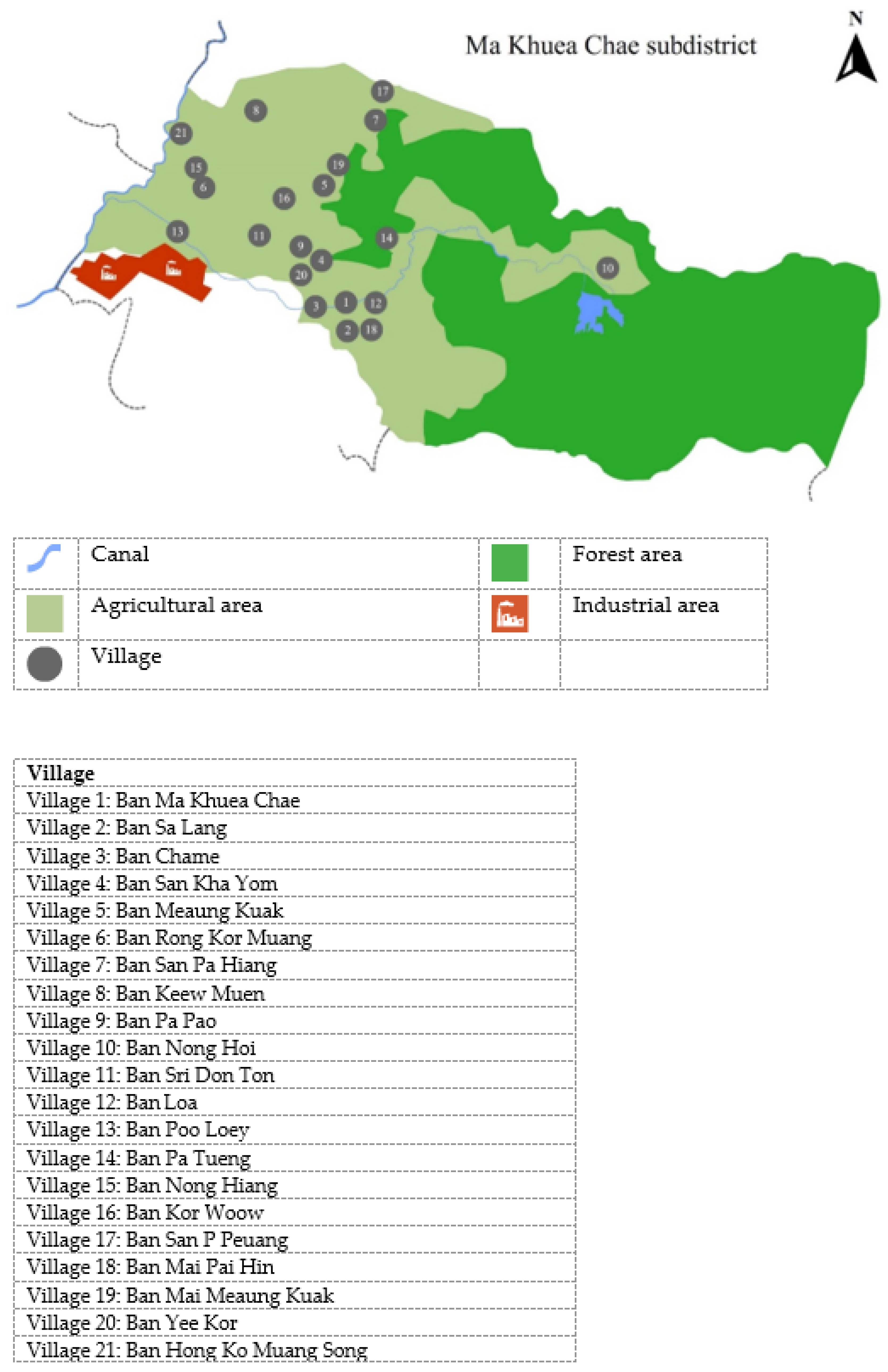
27]. Our previous research also examined the urinary fluoride levels of village health volunteers (VHVs), who represent the public health ministry, with a particular emphasis on residents of 21 villages in the Ma Khuea Chae subdistricts (
Figure 3). There, 51.3% of urine samples had fluoride concentrations greater than the threshold (0.2–3.2 ppm). These values were greater than the industry guideline (3.20 mg/L) [
24]. Chaiwong et al. (2020) demonstrated biological monitoring in these locations through plant, animal, and commercial bottled water production. According to the study, the average fluoride concentration of fruit plants, young plants, whole plants, and poultry was 560.00, 510.00, 610.00, and 336.67 mg/kg dry weight, respectively [
24]. The values are much greater than the EPA and WHO’s maximum contaminant levels for food (4.00 mg/kg) and a dose capable of causing sickness (0.30 mg/kg). As a result, this study focused on analyzing the health risk assessment (HRA) of participants ingesting locally grown vegetables and fruits, as well as poultry and meat, in locations and groundwater contaminated with fluoride. Fluoride is a “double-edged sword”: insufficient consumption results in mottling teeth, softening of bones and skeletons, neurological damage, dental and skeletal fluorosis, soft tissues, and reduced thyroid function [
18,
22,
29].
3. Results
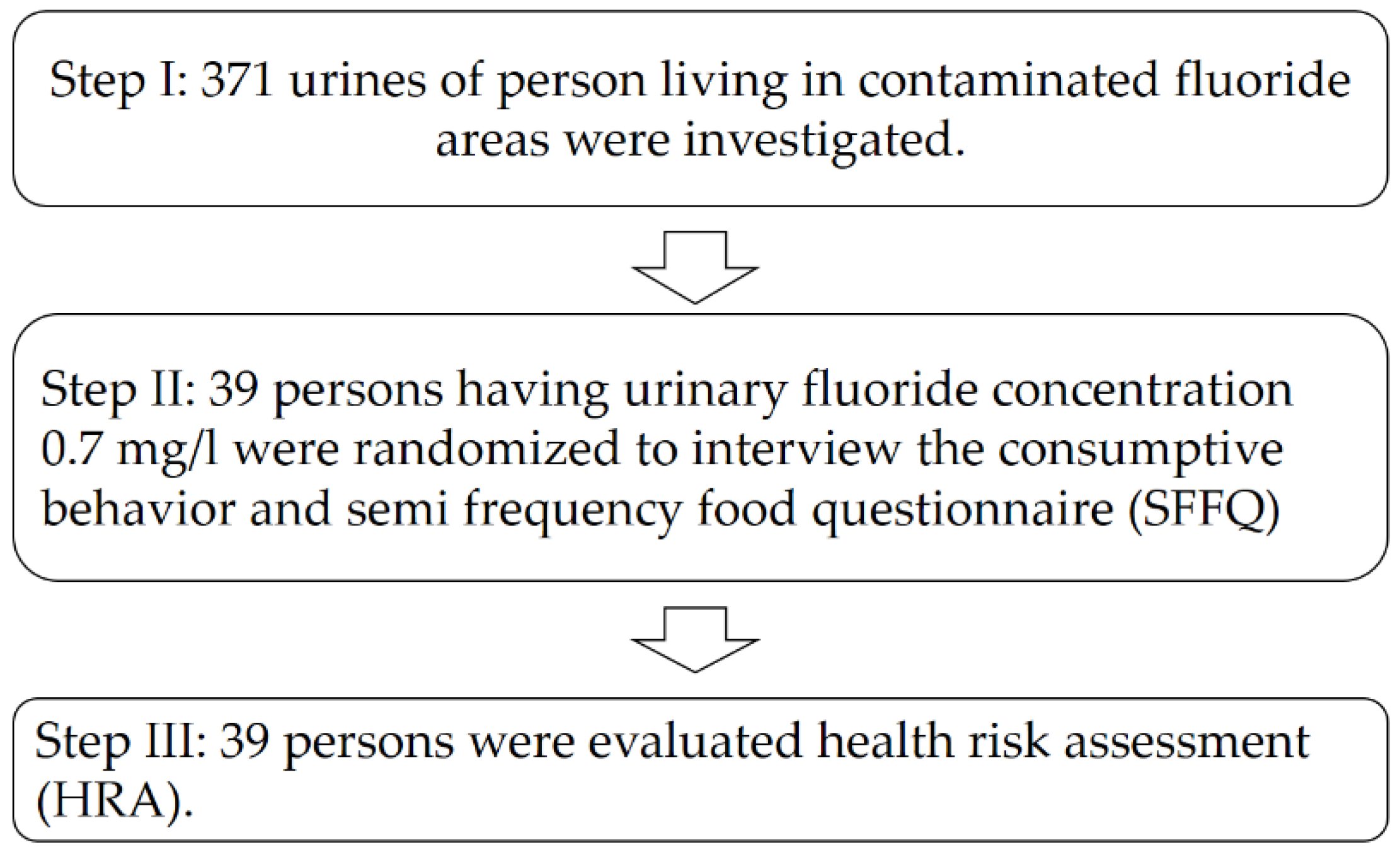
The data are presented in the form of personal dates of village health volunteers in
Table 3. The information was sorted into groups. The volunteers’ ages varied from 51 to 60 years old, with 48.25% between those ages. The majority were female, accounting for 60.38%, with married status accounting for 79.51%. About 44.20% of people weigh between 51 and 60 kg, while 47.71% of people are between 151 and 160 cm tall. Primary school education was around 67.65%, employment was around 59.03%, and income was around 34.77% in the range of 151–300 EUR. Housing ownership was around 87.33%, while living in the same house since birth was around 78.44%, and residing in the same house for more than five years was around 68.75%. Furthermore, commercially bottled water was the primary source of drinking water for 65.14% of the time and drinking water quality was enhanced by 87.06% by filtering 60.37% of the time. About 65.14% of the cooked water came from commercially bottled water, and 61.32% of the cooked water was enhanced by filtration. The never-tested group had a 100% history of fluoride investigation in urine, while the never-tested group had a 100% history of fluoride in water. Apparent
Camellia sinensis chewing was reported at 88.44% in the group of no chewing and 40.90% in the group of chewing during one to five years. Tea drinking behavior was identified in 90.84% of those who did not drink, and in 64.72% of those who drank during one to five years. In fluoride-contaminated areas, the consumption of vegetables, fruits, poultry, and animal meats was studied.
Brassica chinensis Jusl var.
parachinensis (Bailey) and Tsen and Lee ranked first among the vegetables consumed by VHHs, with 29.43% purchased from the village farm and 44.20% purchased from the market. Furthermore, the top ranking of poultry and animal foods was
Sus scrofa domesticus, which accounted for 38.02% of the total purchased from the village’s farm, which accounted for 73.09%.
In
Table 4, it is reported that fluoride increased the incidence of village health volunteers (VHVs). The findings were divided into three categories once the level was evaluated. The results show a 0.00% score from 11 to 14, which was interpreted very broadly. Furthermore, 34.77% of the 7–10 score range and 65.23% of the 1–6 score range were interpreted at a medium and low level, respectively.
The fluoride concentration and hazard quotient (HQ) of commercial ground water bottles consumed in different areas (N = 8) are shown in
Table 5. The highest fluoride concentration was detected in Village 20: Ban Yee Kor in the type of water supply brand F at a concentration of 15.20 mg/L with HQ 1.10, while the lowest fluoride concentration was detected in Village 1: Ban Ma Khuea Chae in the type of bottle water brand A at a concentration of 0.29 mg/L with HQ 0.84. The average fluoride concentration and HQ were found to be 3.51 mg/L.
The fluoride level and hazard quotient (HQ) of the various varieties of fruits grown in the Ma Khuea Chae subdistrict (N = 7) were also determined and are reported in
Table 6. The maximum concentration of fluoride was identified in the fruit of
Mangidera indica at 0.46 mg/g with HQ 1.78, while the lowest concentration was found in
Musa acuminata Colla at 0.01 mg/g with HQ 0.03. The concentrations of fluoride in
Psidium guajava and
Carica papaya L. were found to be similar at 0.38 and 0.35 mg/g, respectively. Additionally, an average fluoride content of 0.26 mg/g was determined.
The fluoride concentration and hazard quotient (HQ) of several types of young plants in Ma Khuea Chae subdistrict (N = 9) were determined, and the results are presented in
Table 7. Fluoride concentrations ranged between 0.07 and 0.91 mg/g.
Momordica charantia L. had the highest fluoride concentration of 0.91 mg/g with HQ 3.53, and
Cucurbita moschata Decne had the lowest fluoride concentration of 0.07 mg/g with HQ 0.25. Fluoride concentrations in young plants were found to be on average 0.44 mg/g.
The fluoride concentration and hazard quotient (HQ) of various mature plants grown in the Ma Khuea Chae subdistrict (N = 5) are shown in
Table 8. Fluoride levels in these mature plants ranged between 0.43 and 3.07 mg/g.
Brassica juncea (L.)
Czern. and Coss. had the highest fluoride concentration at 3.07 mg/g with HQ 11.92, while
Ocimum sanctum had the lowest at 0.43 mg/g with HQ 1.67. Fluoride concentrations in mature
Ocimum sanctum,
Brassica pekinensis,
Brassica chinensis L. var.
parachinesis Tsen and
Lee,
and Piper sarmentosum plants were 0.43, 0.46, 0.44, and 0.49 mg/g, respectively. Fluoride concentrations averaged 0.98 mg/g.
The fluoride concentration and hazard quotient (HQ) of various types of whole plants were determined in the Ma Khuea Chae subdistrict (N = 3), and the results are summarized in
Table 9. Fluoride concentrations in
Ipomoea aquatica,
Gymnema inodorum, and
Neptunia oleracea were 0.76, 0.58, and 0.77 mg/g, respectively. The results indicate that
Neptunia oleracea contains the most fluoride at HQ 2.99 and
Gymnema inodorum contains the least fluoride at HQ 2.25. The average fluoride concentration in whole plants was 0.70 mg/g.
The fluoride concentrations and hazard quotient (HQ) of several food crops grown in the Ma Khuea Chae subdistrict (N = 7) are shown in
Table 10. Fluoride concentrations ranged between 0.12 and 0.62 mg/g. The highest concentration of fluoride was found in the
Lablab purpureus plant at 0.62 mg/g with HQ 2.40, while the lowest concentration was found in the
Solanum melongena plant at 0.12 mg/g. Fluoride concentrations in food crops were on average 0.29 mg/g.
The fluoride concentration and hazard quotient (HQ) of various types of cooked plants grown in the Ma Khuea Chae subdistrict (N = 5) were determined, and the results are shown in
Table 11. Fluoride concentrations in these cooked plants ranged from 0.11 to 1.44 mg/g. The highest fluoride concentration, 1.44 mg/g, was found in
Citrus hystrix with HQ 5.59, while the lowest fluoride concentration, 0.11 mg/g with HQ 0.42, was found in
Capsicum annuum ‘Bird’s Eye’. Fluoride concentrations in cooked plants were on average 0.49 mg/g.
The fluoride concentration and hazard quotient (HQ) in various poultry and meat farmed in the Ma Khuea Chae subdistrict (N = 3) were determined, and the results are illustrated in
Table 12. The fluoride concentration range found in between 0.10 and 0.81 mg/g. The highest fluoride concentration at 0.81 mg/g was found in
Bos Taurus with HQ 3.14, and the fluoride concentration found in
Gallus gallus and
Oreochromis niloticus were at the same levels at 0.10 mg/g with HQ 0.38. Additionally, the result shows the average fluoride concentration at 0.34 mg/g.
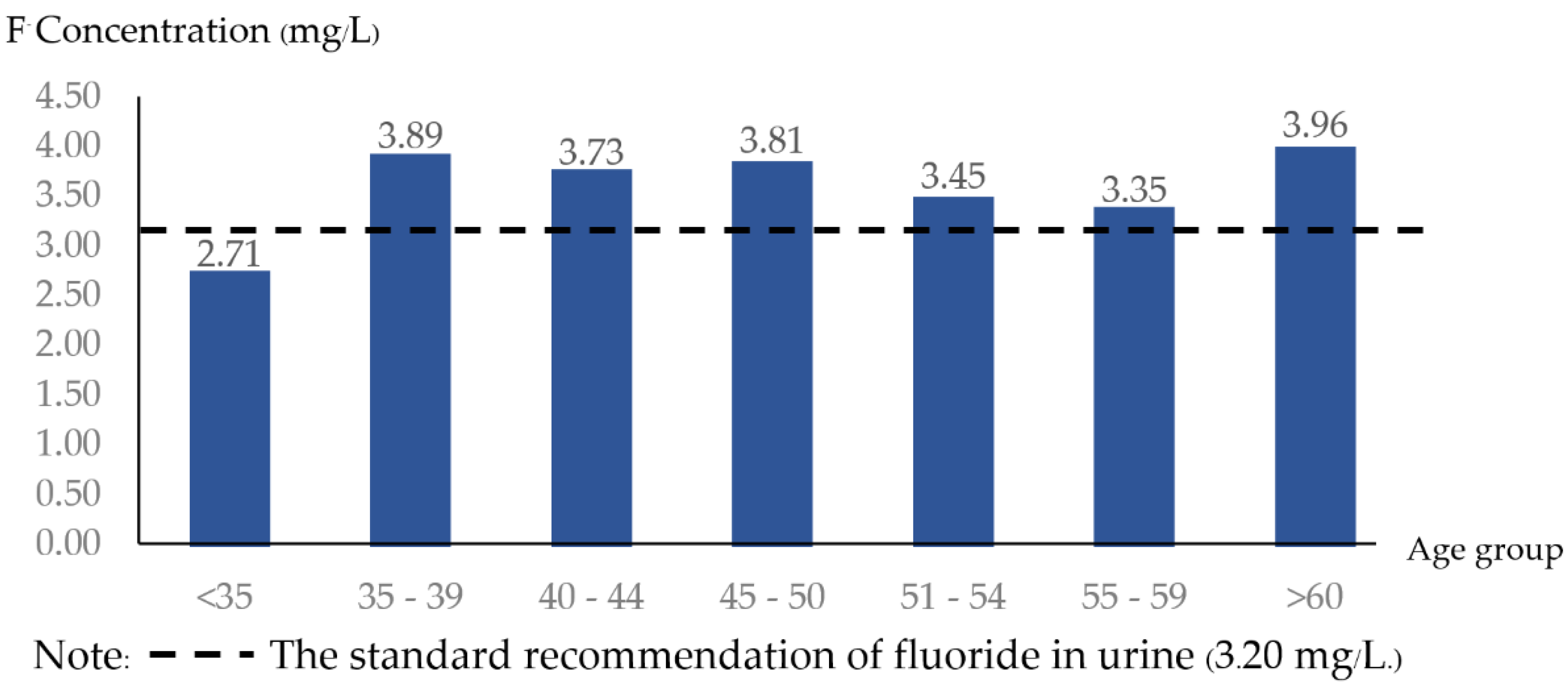
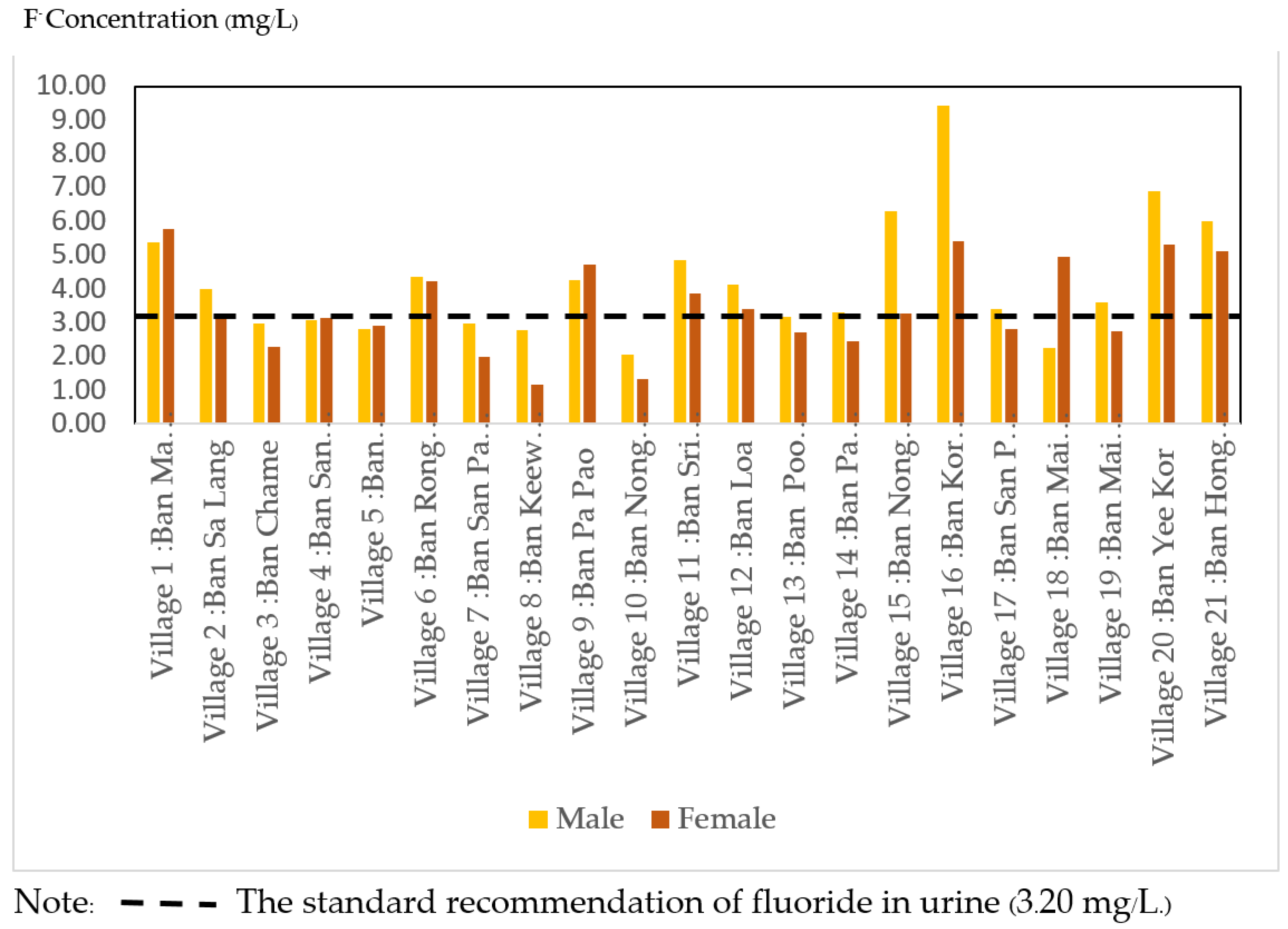
The amount of fluoride detected in the urine of village health volunteers (VHVs) in the 21 villages of the Ma Khuea Chae subdistrict is shown in
Figure 4. The over-60 age group (mean = 3.96 ± 2.82 mg/L), the 35–39 age group (mean = 3.89 ± 2.71 mg/L), and the 45–50 age group (mean = 3.81 ± 2.32 mg/L) have the highest urinary fluoride concentrations. Fluoride levels in the urine were 3.54 ± 2.16 mg/L on average. Except for the 35-year-old age group, all age groups have urinary fluoride levels greater than the standard recommendation (3.20 mg/L).
The fluoride assays performed on the urine of village health volunteers (VHVs) in the Ma Khuea Chae subdistrict’s 21 villages are shown in
Figure 5. Ban Kor Woow (N = 7) village (mean = 6.57 ± 2.13 mg/L), Ban Nong Hiang (N = 22) village (mean = 5.79 ± 2.20 mg/L), and Ban Yee Kor village (mean = 5.64 ± 3.24 mg/L). Urinary fluoride concentrations were 3.55 ± 2.16 mg/L on average (N = 401). The amount of fluoride detected in the urine of village health volunteers (VHVs) in the 21 villages of the Ma Khuea Chae subdistrict is shown in
Figure 4. Males had a mean fluoride level of 3.66 mg/L (SD. = 2.71, min–max = 1.13–13.60 mg/L) and females had a mean fluoride level of 3.47 mg/L (SD = 2.18, min–max = 0.71–12.50 mg/L). Male and female concentrations were not significantly different (
p > 0.05). Notably, nine villages had males with a higher average than females. According to
Figure 3 and
Figure 4, 11 villages (52.38%) had a fluoride level in urine greater than the standard recommendation (3.20 mg/L).
4. Discussion
The conclusion indicated that most village health volunteers had a limited understanding of fluoride and were unable to assess the health hazards associated with food consumption by sampling vegetables, fruits, poultry, and animal meats consumed by village residents. Commercially bottled water received 87.59% of the total consumption of popular and commercially bottled water sourced from groundwater, followed by several fruits at 51.14%, young plants at 88.89%, mature plants at 100.00%, whole plants at 100.00%, food crops at 42.86%, cooked plants at 60%, and meat from poultry and animals at 33.33%. The Ma khuea Chae subdistrict has been found to contain fluoride. Fluoride levels were determined in shallow unconfined groundwater used for drinking across the plain. The results indicated that concentrations in the plain’s center ranged from less than 0.5 to 10 ppm. Numerous minerals, including apatite, fluorite, biotite, and hornblende, contain fluoride. Fluoride concentrations in groundwater are increased due to weathering and infiltration of rainfall through these rocks. The central section of the basin is composed of Holocene alluvial deposits, whereas the flood plain is composed of well-sorted sand and gravel covered by a few meters of clay. The formation is composed of thick beds of fine sediments, including kaolinite, interspersed with lenses of sand and gravel [
16,
37]. These thin layers of fine material act as aquitards, preventing water from flowing between aquifers and mixing. The majority of groundwaters east and north of Lamphun are Na-HCO
3, Ca-Na-HCO
3, or Ca-HCO
3 in composition [
38]. It has been established that waters containing sodium hydroxide conform [
37]. The region’s redominance of sodium is due to cation exchange. Additionally, geothermal water is the primary source of high fluoride concentrations in the water, as demonstrated by a test of 70 hot springs known to contain high fluoride levels [
16,
39]. Kim et al. (2005) identified two environmental processes in Korea that contribute to the occurrence of high fluoride levels: weathering of fluoride-bearing rocks in faults and upward flow of deep fluoride-enriched groundwater along fault zones [
40]. This process appears to occur at the Lamphun site, which has an intrusion of fluoride-bearing biotite and granite in Palaeozoic rock. Fluoride concentrations in water are high because the calcium (Ca
2+) ion is removed from groundwater and replaced by the sodium ion (Na
+) found in clay minerals. This material has a high capacity for cation exchange, preventing the precipitation of highly insoluble calcium fluoride (CaF
2), resulting in fluoride accumulation in groundwater [
16,
37]. The HQ values in
Table 2 represent the effects of oral ingestion (of fluoride-related adverse effects at HQ > 1). One brand in village 6, Ban Rong Kor Muang, had more than ten headquarters. The greater the value, the more intolerable the risk of non-carcinogenic adverse health effects. The Ma khuea Chae subdistrict is located in Lamphun [
31,
41,
42]. It is a zone with elevated fluoride levels near the prominent Mae Tha fault and is most likely associated with groundwater resources. The fluoride concentration of the urine and consumption patterns of each participant was used to generate the calculated HQ values. The amount of food ingested during a specified period of time (l/day), the frequency of exposure (days/year), the duration of exposure, your body weight (BW), and the average time (AT) that it will be in the area––these considerations play a significant role in determining whether or not the area’s residents will inevitably acquire fluoride via the food chain. In India, Pakistan, West Africa, Thailand, China, Sri Lanka, and Southern Africa, elevated groundwater fluoride concentrations have been associated with igneous and metamorphic rocks such as granites and gneisses. Fluoride levels in drinking water exceeded 10 mg/L in the Ma Khuea Chae subdistrict, in Thailand’s north region. Around 1% of the area’s natural water sources are estimated to contain fluoride concentrations greater than 2 mg/L. [
43]. Fluoride levels may be elevated in northern Thailand due to geothermal water sources [
44]. Fluoride concentrations in groundwater sources reached a maximum of 0.92 mg/L. [
45]. For the majority of people, the primary sources of fluoride in their diet are drinking water, food, and beverages [
46]. The presence of fluoride in drinking water is widely regarded as one of the most significant health risks [
30,
47].
Ma Khuea Chae is located on the Chiang Mai–Lampang highway, near the northern region industrial estate. The modern production and use of chemicals such as hydrogen fluoride (HF), calcium fluoride (CaF
2), sodium fluoride (NaF), fluorosilicic corrosive (H
2SiF
6), sodium hexafluorosilicate (Na
2SiF
6), sulphur hexafluoride (SF
6), and phosphate manures are all anthropogenic sources of fluoride in the earth. Composts made of phosphoric acid are a significant source of fluoride contamination in agricultural soils [
48,
49]. The proximity of the smelter increased the mean fluoride concentration in vegetables grown in the area, which ranged from 0.36–0.69 to 0.71–0.90 ppm. The location closest to the smelter had the highest mean concentration (0.00 km). These values exceed the 1.00 mg/kg
3 cutoff. Mezghani et al. (2005) confirmed that vegetation in close proximity to the factory accumulates significant amounts of fluoride with variable specific symptoms of toxicity, and that, as expected, fluoride concentrations decreased as the distance from the pollution source increased [
42]. According to Brougham et al. (2013), fluoride concentrations in vegetation and soils near an aluminum smelter decrease after 36 weeks of the smelter’s shutdown. The current study indicates that fluoride pollution of soil is inversely proportional to its distance from the source of pollution [
50]. In terms of soil layers, it was observed that the mean fluoride concentration was significantly higher in areas closer to the smelter in the upper and deeper soil layers. Additionally, at all distances, the upper soil layer had a significantly higher mean fluoride concentration than the deeper soil layers. Okibe et al. (2010) and Yadhav et al. (2012) demonstrate that irrigating water affects the fluoride content of vegetables and soil. They discovered that applying fertilizer can also have an effect on the fluoride concentration in the soil and, consequently, on the vegetables. Water and fertilizer samples were collected from various distances and fluoride concentrations [
8,
51]. Fluoride concentrations in fertilizer and water samples collected at various distances were between 1.4–1.5 ppm and 1.8–1.9 ppm, respectively. Notably, fluoride concentrations were found to be higher in areas adjacent to the zinc smelter and lower in areas distant from the zinc smelter. Fluorosis is associated with fluoride-rich alkaline groundwaters (pH range 7.0 to 8.5 in India and Sri Lanka [
25,
47,
52]). Fluoride is released into the environment through natural sources as well as the aluminum and coal industries, fertilizer use, and manufacturing processes [
47]. Bartram and Balance (1996); Haidouti (1995) demonstrated that total soil fluoride collected at depths of 0–5, 5–15, and 15–30 cm near an alumina production plant decreased with distance from the emission source and reached background levels at approximately 20 km
2. Additionally, the total soil fluoride content decreased with depth in high-impact areas and increased with depth in low-impact areas [
53,
54]. This high fluoride content in soil may leach into groundwater via the unsaturated zone during precipitation, increasing fluoride levels. Agricultural fertilizers and coal combustion are anthropogenic sources of fluoride, whereas phosphate fertilizers contribute to fluoride enrichment in irrigation lands [
55].
Fluoride bioaccumulation in various plant parts varies according to the mechanisms by which it is transported from soil solution to roots and then translocated from root to shoot. Fluoride is more soluble in acid soils and primarily accumulates in the leaf [
56,
57]. According to a related study conducted by Edmunds and Smedley (2013), the fluoride content of the leafy part of vegetables grown in this area is significantly higher than the fluoride content of the fruits and tubers [
58]. Seeds accumulate very little fluoride in comparison to other parts of the plant. To minimize the risk of human exposure to fluoride, it is critical to minimize the use of fluoride-contaminated irrigation water, which is especially detrimental for crops that accumulate fluoride. The majority of fluoride occurs naturally in the environment as a result of various sources, including rock (100–1300 mg/kg) and soil (20–50 mg/kg). Fluoride is more soluble in acid soils, and its uptake by plants is enhanced in these soils. It is primarily found in the leaf. Fluoride accumulation in excess in vegetables results in visible leaf injury and fruit damage [
8,
56]. Fluoride enters plants via soil and water and then through passive diffusion to the plant roots. Fluoride is then transported into the shoot via the xylem via the apoplastic and symplastic pathways in a unidirectional distal movement. Fluoride’s bioavailability to plants is primarily determined by the pH of the solution and the presence of other metal ions such as calcium, aluminum, and phosphorous, as well as the soil type. Additionally, abiotic factors such as light, humidity, other pollutants, mineral nutrition, and temperature influence the uptake or movement of fluoride in plants and may influence the plant’s response to fluoride.
The residents of the Ma Kheau Chae subdistrict have been exposed via three routes: (1) ingested via drinking water (>1.5 mg F-/L) and fruits, vegetables, edible poultry, and animals produced in exposed areas, (2) inhalation routes, and (3) dermal routes. The food chain is the primary source of fluoride in the human body. Around 90% of fluoride consumed in water is absorbed in the gastrointestinal tract, compared to only 30–60% of fluoride consumed in food [
22,
47]. Fluoride is converted to hydrogen fluoride (HF) in an acidic stomach environment, and up to 40% of ingested fluoride is absorbed from the stomach as HF. By decreasing the quantity of HF absorbed by the stomach, a high stomach pH decreases gastric absorption. Fluoride that does not pass through the stomach is absorbed through the intestine, where it is unaffected by pH. Concentrations of cations (e.g., calcium, magnesium, and aluminum) that form insoluble complexes with fluoride can significantly reduce gastrointestinal fluoride absorption [
59]. Fluoride is easily absorbed by the body, with roughly 99% remaining in calcium-rich regions such as bone and teeth (dentine and enamel), where it is incorporated into the crystal lattice. In infants, 80 to 90% of absorbed fluoride is maintained, but this percentage drops to 60% in adults, damaging the following generation via placental transfer. Fluoride crosses and is present in modest concentrations in mother’s milk, similar to those observed in the blood [
60,
61,
62]. Dental fluorosis is a condition marked by hypomineralization of tooth enamel produced by excessive fluoride intake during enamel creation. It is caused by excessive fluoride intake during tooth formation. Because enamel and primary dentin fluorosis can occur only during tooth formation, fluoride exposure occurs throughout childhood (83). The prediction of dental fluorosis in schoolchildren revealed a favorable and statistically significant association between fluoride in well water and the level of dental fluorosis (r = 0.61;
p <0.01). Mandinic et al. (2010) established an association between the health risk of children exposed to high fluoride levels in endemic areas and dental fluorosis using an odds ratio (OR). The danger increases as fluoride content in drinking water increases [
63].
Fluoride concentrations were determined in the plasma of 50 pregnant women, 44 amniotic fluid samples, and 29 fetal cord blood samples from normal pregnancies. The results indicated that the fluoride concentrations in maternal and fetal plasma did not differ significantly. Opydo-Szymaczek (2007) evaluated placental fluoride transfer in 30 pregnant women in Poznan, Poland, at the time of delivery, where the fluoride concentration in the drinking water ranges between 0.4 and 0.8 mg/L. The mean fluoride concentration in maternal plasma was significantly greater than that in venous cord plasma (3.54 mol/L vs. 2.89 mol/L, respectively), and both values were comparable to those previously reported in pregnant women receiving prenatal fluoride supplements [
64]. The findings confirm that fluoride easily crosses the placenta, and that prenatal fluoride supplementation is not recommended in this population [
65]. In comparison, Gurumurthy et al., (2011a) collected drinking water, ground water, maternal blood, cord blood, and placenta and divided them into three sections––maternal, fetal, and peripheral. The average fluoride concentration in drinking water was 1.64–0.49, 10.94–2.09, 1.62–0.78, 2.50–41.54, and 1.41–0.78 ppm, respectively. The difference in fluoride levels between maternal and cord blood was significant. The association between maternal fluoride accumulation and adverse fetal outcomes is discussed [
65]. Gurumurthy et al. (2011b) demonstrated that 1 ppm of fluoride in maternal serum could result in fetal health risks such as low birth weight, preterm delivery, and a low APGAR score [
66]. Trivedi et al. (2007) established that fluoride can cross the placenta and reach the fetus, and that continued exposure to fluoride during childhood may have negative effects on the developing brain [
67]. Seraj et al. (2012) demonstrated that children who live in areas with higher than normal water fluoride levels have impaired intelligence development. Children’s intelligence may be impacted by high water fluoride levels, as mean IQ scores decreased from 97.77–18.91 in the low fluoride group to 89.03–12.99 in the medium fluoride group to 88.58–16.01 in the high fluoride group (
p = 0.001) [
68].
In my study, the age group > 60 years old had the highest fluoride urine content in the population compared to the age group of 35 years old. None were statistically significant when
p > 0.05 cutoff was used. The higher fluoride levels associated with aging support this idea. Fluoridated water and food can convert the hydroxyl group in hydroxyapatite crystals (Ca
5(PO
4)
3(OH), the primary component of dental enamel and bone minerals, to fluorapatite (Ca
10(PO
4)
6(F
2), which affects the strength of such fluoride-containing bone. Thus, fluoride is added to drinking water and medical products such as toothpaste to prevent tooth decay, as fluoride is incorporated into the formation of apatite crystals [
69]. Fluoride accumulation in agricultural plants and its subsequent entry into the food chain pose a potential threat to human health. Dental fluorosis, mottling of teeth, skeletal fluorosis, and bone deformation are significant health problems associated with excessive fluoride in all age groups [
56,
70].
Regarding gender, males are, on average, larger than females, although the difference is not statistically significant (
p > 0.05). Fluoride is more concentrated in the skeleton than in the teeth, where it combines with calcium. Fluoride accumulation in the male body has an effect on body size. Fluoride’s effect on the structure and shape of apatite. Calcium and magnesium are the primary minerals in the bone that mix and precipitate as fluorapatite. Additionally, males have a quicker rate of skeletal growth than females, which may be combined and stored in bone alongside fluoride. Carbonate and fluoride have opposite effects on crystallinity and solubility in bioapatite: carbonate decreases crystallinity and enhances solubility, whereas fluoride does the opposite [
71,
72]. Finally, more fluoride stores in the bone can be absorbed by the body and excreted in the urine. Levy (2009) investigated the connection and comparison of longitudinal fluoride intake in drinking water at 0.68 mg/day from birth to 11 years of age using dual-energy X-ray absorption (DXA) content bone mineral content (BMC) and bone mineral density (BMD) results indicated a negative correlation between female bone outcome and fluoride intake. Still, male bone outcomes were all good and did not differ by gender [
73].
To mitigate health hazards in the areas covered by the Health Ministry and other relevant authorities, it is essential to develop guidelines for operation in the following categories:
Capacity training to enhance personal competencies of public health volunteers and residents of areas at risk of fluoride contamination in Thailand’s upper north, including raising awareness of fluorides through a comprehensive health belief program comprised of four processes: perceived susceptibility, perceived severity, perceived benefit, and perceived barrier, which encompasses more than fluoride contamination [
74]. The government should have a strategy establishing an integrated body of knowledge on unique pollutant contamination, such as fluoride contamination, in eight provinces in Thailand’s upper north [
24]; arsenic in the Nakhon Si Thammarat province [
75], lead mining is located in the Kanchanaburi Province [
76], and zinc mining material was sent to village health volunteers in the Tak Province to communicate information to those living in the affected areas [
77]. Volunteers who represent the Ministry of Public Health by assisting with the care of people’s health in various settings are referred to as “Village Health Volunteers”. As a result, to assist the Ministry of Public Health in creating a body of fluoride knowledge among the general people, it is required and desirable for the general public to participate in the process, particularly village health volunteers, to take complete care of the neighborhood’s residents. The study by Xiang et al. (2020) revealed that individuals with higher perceived susceptibility, indicated stronger perceived susceptibility, greater severity of oral diseases, less performing of oral health behaviors, and a higher score of decayed missing filled teeth (DMFT) were directly related to increased dental anxiety levels. Other HBM variables, such as perceived susceptibility, self-efficacy beliefs, cues to action, and perceived barriers, may all have an effect on dental anxiety via oral health behaviors and caries status [
78].
It is feasible to limit the risk of long-term exposure to fluoride in the environment by analyzing urine. According to the study’s findings, 100% of the VHV had never had their urine tested for fluoride levels. The presence of fluoride in urine is a biological indicator of fluoride exposure that has an influence on the kidneys’ ability to function properly. Researchers revealed that fluoride concentrations in the urine could be used to forecast a person’s risk of developing kidney illness, and residents will be screened for dental and skeletal fluorosis [
79,
80], and chronic kidney disease (CKDu) [
81,
82].
A health information system is a huge computer database management system that manages a large amount of information. It will include people living in fluoride-contaminated areas who will be given fluoride-related information, the development of an information center will aid in the collection, storage, and management of data. Medical and public health professionals can use this system to access patient information in planning applications. The assessment of patient care according to the clinical information system discovered that there are no sources or information systems that can provide knowledge to people who have been exposed to fluoride and other pollutants in the contaminated areas [
83,
84].
Humans are exposed to environmental fluoride through birth (78.44%) and through drinking groundwater from specific contaminated locations and commercially bottled water. This is because the Lamphun area is located inside the Chiang Mai-Basin basin, well-known for its mineral fluoride concentrations, and commercially bottled water consumption remains a concern in Thailand, although the legal demands that it be supplied at a total concentration of no more than 0.7 mg/L [
85]. We discovered that commercially available bottled drinking water in Bangkok, Thailand, contained varying levels of fluoride, with some having extremely high levels. When prescribing fluoride supplements, health professionals must be aware of the varying fluoride content of bottled drinking water and educate parents of infants and small children. It should be considered to include the fluoride content on the label of bottled water, particularly those with a fluoride content greater than 0.3 mg F/l (4). As a result of the findings (
Table 5), it was determined that the HQ value required to influence health must be more than 1 (HQ > 1). Fluoride concentrations in commercially bottled water were connected with 0.45 mg/L (taking into intake rate (l/day), exposure frequency (days/year), duration of exposure, body weight (BW), and average time (AT). The fluoride concentration in bottled water should be reduced from 0.70 to 0.34 mg/L (HQ = 0.99 mg/L), according to the Food and Drug Administration Ministry of Health Thailand, to decrease the hazard associated with the consumption of bottled water.
Health literacy is defined by the U.S. Department of Health and Human Services (HHS) as “the degree to which persons have the capacity to receive, process, and understand fundamental health information and services required to make informed health decisions” (HHS, 2010). People who reside in more affluent areas should be proficient in self-defense, including fluoride awareness. The ideal fluoride dosage has been found to improve dental health in small doses. Conversely, excessive chronic use may have unfavorable effects, such as the onset of dental fluorosis. Fluoride awareness, toothpaste, and a proper intake (0.05 mg/day/kg body weight) are all important factors in preventing dental caries [
86,
87]. Health literacy and educational attainment both correlated with support for community water fluoridation (CWF), demonstrating the value of both broad and specialized knowledge in voters’ decision-making. Although educational level and other demographic factors are more indicative of participation, health literacy helps to explain fluoride support in a CWF referendum more clearly. Additional sociodemographic factors helped to explain turnout, but these same traits also explain voting behavior more generally [
88].
According to the study’s findings, 51.14% of fruits, 88.89% of young plants, 100.00% of mature plants, 100.00% of whole plants, 42.86% of food crops, 60.00% of cooked plants, and 33.33% of poultry and animal meat had HQ values greater than 1, implying that those who consume them suffer health risks. The primary source of contamination in soil, vegetables, and meat is the food chain (soil fluoride: plants: animal: humans). Fluoride contamination of the surrounding ecology is a product of various constraints, including spatial factors. This is because the Ma Khuea Chae subdistrict is located within the Lamphun Industrial Estate, which contains numerous industrial plants that produce fluoride and emit it into the atmosphere, including a portion of the agricultural region. Chemical fertilizers are also known to contain fluoride, which contributes to the increase in soil fluoride caused by agriculture and when chemical fertilizers are applied over an extended period. As a result, the soil becomes acidic, allowing fluoride to be dissolved into the surrounding environment as ions enter the roots of plants, where they accumulate in various areas of the plant. The precipitate-flotation of fluoride-containing effluent from a semiconductor factory was investigated. The procedure begins with the formation of calcium chloride to form a precipitate, followed by the removal of calcium fluoride (CaF
2). Then fluoride is accumulated at the soil surface, predominantly contaminated in the 0–40 cm layer, which can be addressed by topsoiling [
89,
90].