Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria fendleri Oil for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of a Physaria Fendleri Polyurethane Dispersion
2.3. Preparation of Polyurethane and Acrylic Latex Adhesive
2.4. Preparation of Laminated Poly(Ethylene Terephthalate) Films
2.5. Characterization
2.5.1. Gel Permeation Chromatography
2.5.2. Differential Scanning Calorimetry
2.5.3. Infrared Spectroscopy
2.5.4. Peel Strength
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonymous. Advancing Sustainable Materials Management: 2018 Fact Sheet. 2020. Available online: https://www.epa.gov/sites/default/files/2020-11/documents/2018_ff_fact_sheet.pdf (accessed on 6 April 2021).
- The Pew Charitable Trusts and SYSTEMIQ. Breaking the Plastic Wave: A Comprehensive Assessment of Pathways Towards Stopping Ocean Plastic Pollution; Pew Cheritable Trusts: Washington, DC, USA, 2020. [Google Scholar]
- The Future of Global Packaging to 2024. 2021. Available online: https://www.smithers.com/en-gb/services/market-reports/packaging/future-of-global-packaging-to-2024 (accessed on 14 June 2021).
- Food Packaging Market Size, Share & Trends Analysis Report By Type (Rigid, Flexible), By Material (Paper, Plastic), By Application (Bakery and Confectionery), By Region, And Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/food-packaging-market?utm_source=referral&utm_medium=processing&utm_campaign=guestpost_17august18_food-packaging-market (accessed on 22 April 2021).
- The Global Bioplastics & Biopolymers Market Size Is Expected to Grow from USD 10.5 Billion in 2020 and USD 27.9 Billion by 2025, at a CAGR of 21.7%. 2020. Available online: https://www.globenewswire.com/news-release/2020/04/16/2016985/0/en/The-global-bioplastics-biopolymers-market-size-is-expected-to-grow-from-USD-10-5-billion-in-2020-and-USD-27-9-billion-by-2025-at-a-CAGR-of-21-7.html (accessed on 15 June 2021).
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Sánchez-Adsuar, M.; Pastor-Blas, M.; Torregrosa-Maciá, R.; Martín-Martínez, J. Relevance of polyurethane configuration on adhesion properties. Int. J. Adhes. Adhes. 1994, 14, 193–200. [Google Scholar] [CrossRef]
- Somani, K.P.; Kansara, S.S.; Patel, N.K.; Rakshit, A.K. Castor oil based polyurethane adhesives for wood-to-wood bonding. Int. J. Adhes. Adhes. 2003, 23, 269–275. [Google Scholar] [CrossRef]
- Arán-Aís, F.; Torró-Palau, A.M.; Orgilés-Barceló, A.C.; Martín-Martínez, J.M. Synthesis and characterization of new thermoplastic polyurethane adhesives containing rosin resin as an internal tackifier. J. Adhes. Sci. Technol. 2000, 14, 1557–1573. [Google Scholar] [CrossRef]
- Kutlug, Ö.; Reck, S.; Hartwig, A. Pressure sensitive adhesives with post-crosslinking ability based on acrylic dispersions obtained from solvent-borne copolymers. Int. J. Adhes. Adhes. 2019, 91, 36–42. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhang, L.; Guo, Y.; Song, J.; Lou, J.; Guan, Q.; He, C.; You, Z. Strong, detachable, and self-healing dynamic crosslinked hot melt polyurethane adhesive. Mater. Chem. Front. 2019, 3, 1833–1839. [Google Scholar] [CrossRef]
- Maciel, V.G.; Bockorny, G.; Domingues, N.; Scherer, M.B.; Zortea, R.B.; Seferin, M. Comparative Life Cycle Assessment among Three Polyurethane Adhesive Technologies for the Footwear Industry. ACS Sustain. Chem. Eng. 2017, 5, 8464–8472. [Google Scholar] [CrossRef]
- Ivey, A.; Talbert, J.; Evangelista, R.; Vorst, K.; Curtzwiler, G. Influence of a hydrocarbon side chain on the performance of Physaria fendleri-Castor oil polyurethane packaging adhesives. Clean. Eng. Technol. 2021, 4, 100216. [Google Scholar] [CrossRef]
- Bao, L.; Fan, H.; Chen, Y.; Yan, J.; Yanga, T.; Guo, Y. Effect of surface free energy and wettability on the adhesion property of waterborne polyurethane adhesive. RSC Adv. 2016, 6, 99346–99352. [Google Scholar] [CrossRef]
- Czech, Z.; Pełech, R. Thermal decomposition of polyurethane pressure-sensitive adhesives dispersions. Prog. Org. Coat. 2010, 67, 72–75. [Google Scholar] [CrossRef]
- CakićaIvan, S.M.; Ristić, S.; Marinović-Cincović, M.; Špírková, M. The effects of the structure and molecular weight of the macrodiol on the properties polyurethane anionic adhesives. Int. J. Adhes. Adhes. 2013, 41, 132–139. [Google Scholar]
- Rahman, M.M.; Kim, H.-D.; Lee, W.-K. Properties of Waterborne Polyurethane Adhesives: Effect of Chain Extender and Polyol Content. J. Adhes. Sci. Technol. 2012, 23, 177–193. [Google Scholar] [CrossRef]
- Pérez-Limiñana, M.A.; Arán-Aís, F.; Torró-Palau, A.M.; Orgilés-Barceló, A.C.; Martín-Martínez, J.M. Characterization of waterborne polyurethane adhesives containing different amounts of ionic groups. Int. J. Adhes. Adhes. 2005, 25, 507–517. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Li, Q.; Wang, J.; Kang, M.; Wang, X.; Xiang, H. Synthesis and characterization of waterborne polyurethane adhesive from MDI and HDI. Appl. Polym. Sci. 2008, 110, 1396–1402. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, W.-K. Properties of isocyanate-reactive waterborne polyurethane adhesives: Effect of cure reaction with various polyol and chain extender content. Appl. Polym. Sci. 2009, 114, 3767–3773. [Google Scholar] [CrossRef]
- Lopez, A.; Degrandi-Contraires, E.; Canetta, E.; Creton, C.; Keddie, J.L.; Asua, J.M. Waterborne Polyurethane−Acrylic Hybrid Nanoparticles by Miniemulsion Polymerization: Applications in Pressure-Sensitive Adhesives. Langmuir 2011, 27, 3878–3888. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Hasneen, A.; Kim, H.-D.; Lee, W.-K. Preparation and properties of polydimethylsiloxane (PDMS)/polytetramethyleneadipate glycol (PTAd)-based waterborne polyurethane adhesives: Effect of PDMS molecular weight and content. Appl. Polym. Sci. 2011, 125, 88–96. [Google Scholar] [CrossRef]
- Fuensanta, M.; Jofre-Reche, J.A.; Rodríguez-Llansola, F.; Costa, V.; Iglesias, J.I.; Martín-Martínez, J.M. Structural characterization of polyurethane ureas and waterborne polyurethane urea dispersions made with mixtures of polyester polyol and polycarbonate diol. Prog. Org. Coat. 2017, 112, 141–152. [Google Scholar] [CrossRef]
- Pradhan, S.; Mohanty, S.; Nayak, S.K. Waterborne epoxy adhesive derived from epoxidized soybean oil and dextrin: Synthesis and characterization. Int. J. Polym. Anal. Char. 2017, 22, 318–329. [Google Scholar] [CrossRef]
- Badía, A.; Movellan, J.; Barandiaran, M.J.; Leiza, J.R. High Biobased Content Latexes for Development of Sustainable Pressure Sensitive Adhesives. Ind. Eng. Chem. Res. 2018, 57, 14509–14516. [Google Scholar] [CrossRef]
- Gaddam, S.K.; Kutcherlapati, S.N.R.; Palanisamy, A. Self-Cross-Linkable Anionic Waterborne Polyurethane–Silanol Dispersions from Cottonseed-Oil-Based Phosphorylated Polyol as Ionic Soft Segment. ACS Sustain. Chem. Eng. 2017, 5, 6447–6455. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Biobased Biodegradable Waterborne Hyperbranched Polyurethane as an Ecofriendly Sustainable Material. ACS Sustain. Chem. Eng. 2014, 2, 2730–2738. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Sun, X.S. Soy-oil-based waterborne polyurethane improved wet strength of soy protein adhesives on wood. Int. J. Adhes. Adhes. 2017, 73, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: I. Elastomers. J. Am. Oil Chem. Soc. 2007, 84, 55–63. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Guo, A.; Zhang, W. Structure and properties of polyurethanes based on halogenated and nonhalogenated soy-polyols. Polym. Chem. 2000, 38, 4062–4069. [Google Scholar] [CrossRef]
- Hu, Y.H.; Gao, Y.; Wang, D.N.; Hu, C.P.; Zu, S.; Vanoverloop, L.; Randall, D. Rigid polyurethane foam prepared from a rape seed oil based polyol. Appl. Polym. Sci. 2002, 84, 591–597. [Google Scholar] [CrossRef]
- Dutta, S.; Karak, N. Effect of the NCO/OH ratio on the properties of Mesua Ferrea L. seed oil-modified polyurethane resins. Polym. Int. 2005, 55, 49–56. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. Synthesis and application of new bio-polyols based on mustard oil for the production of selected polyurethane materials. Ind. Crops Prod. 2020, 155, 112831. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Bogusław, M.B.; Tomaszewska, C.E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.R.F.; A. Tasdelen, M. Synthesis and properties of soybean oil-based biodegradable polyurethane films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- He, W.; Kang, P.; Fang, Z.; Hao, J.; Wu, H.; Zhu, Y.; Guo, K. Flow Reactor Synthesis of Bio-Based Polyol from Soybean Oil for the Production of Rigid Polyurethane Foam. Ind. Eng. Chem. Res. 2020, 59, 17513–17519. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Jang, J.H.; Patil, C.; Jirimali, H.; Gite, V.; Hong, S.C. Functional soybean oil-based polyols as sustainable feedstocks for polyurethane coatings. Ind. Crops Prod. 2018, 113, 249–258. [Google Scholar] [CrossRef]
- Xiaa, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- K. Patil, C.; Rajput, S.D.; Marathe, R.J.; Kulkarni, R.D.; Phadnis, H.; Sohn, D.; Mahulikar, P.P.; Gite, V.V. Synthesis of bio-based polyurethane coatings from vegetable oil and dicarboxylic acids. Prog. Org. Coat. 2017, 106, 87–95. [Google Scholar] [CrossRef]
- Knight, S.C.; Schaller, C.P.; Tolman, W.B.; Hillmyer, M.A. Renewable carvone-based polyols for use in polyurethane thermosets. RSC Adv. 2013, 3, 20399–20404. [Google Scholar] [CrossRef]
- Alagi, P.; Jin, Y.; Sung, C.; Hong, C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, H.; Liang, D.; Lin, Z.; Chen, Q.; Feng, P.; Wang, Q. Renewable Castor-Oil-based Waterborne Polyurethane Networks: Simultaneously Showing High Strength, Self-Healing, Processability and Tunable Multishape Memory. Angew. Chem. Int. Ed. 2020, 60, 4289–4299. [Google Scholar] [CrossRef]
- De, B.; Gupta, K.; Mandal, M.; Karak, N. Biodegradable Hyperbranched Epoxy from Castor Oil-Based Hyperbranched Polyester Polyol. ACS Sustain. Chem. Eng. 2014, 2, 445–453. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Cincović, M.M.; Stojiljković, D.T.; János, C.J.; Miroslav, C.J.; Stamenković, J.V. Glycolyzed poly(ethylene terephthalate) waste and castor oil-based polyols for waterborne polyurethane adhesives containing hexamethoxymethyl melamine. Prog. Org. Coat. 2015, 78, 357–368. [Google Scholar]
- Zhang, Y.; Zhang, W.; Wang, X.; Dong, Q.; Zeng, X.; Quirino, R.L.; Lu, Q.; Wang, Q.; Zhang, C. Waterborne polyurethanes from castor oil-based polyols for next generation of environmentally-friendly hair-styling agents. Prog. Org. Coat. 2020, 142, 105588. [Google Scholar] [CrossRef]
- Gurunathan, T.; Arukula, R. High performance polyurethane dispersion synthesized from plant oil renewable resources: A challenge in the green materials. Polym. Degrad. Stab. 2018, 150, 122–132. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, H.; Xia, L.; Shen, L.; Zhang, C.; Lu, Q.; Sun, S. Semi-interpenetrating polymer networks prepared from castor oil-based waterborne polyurethanes and carboxymethyl chitosan. Carbohydr. Polym. 2021, 256, 117507. [Google Scholar] [CrossRef] [PubMed]
- Cakic, S.M.; Valcic, M.D.; Ristic, I.S.; Radusin, T.; Cvetinov, M.J.; Budinski-Simendic, J. Waterborne polyurethanes-silica nanocomposite adhesives based on castor oil-recycled polyols: Effects of (3-aminopropyl)triethoxysilane (APTES) content properties. Int. J. Adhes. Adhes. 2019, 90, 22–31. [Google Scholar] [CrossRef]
- Contreras, J.; Valdés, O.; Mirabal-Gallardo, Y.; de la Torre, A.F.; Navarrete, J.; Lisperguer, J.; Durán-Lara, E.F.; Santos, L.S.; Nachtigall, F.M.; Cabrera-Barjas, G.; et al. Development of eco-friendly polyurethane foams based on Lesquerella fendleri (A. Grey) oil-based polyol. Eur. Polym. J. 2020, 128, 109606. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: II. Foams. J. Am. Oil Chem. Soc. 2007, 84, 65–72. [Google Scholar] [CrossRef]
- Thames, S.F.; Yu, H.; Schuman, T.P.; Wang, M.D. Acrylated lesquerella oil in ultraviolet cured coatings. Prog. Org. Coat. 1996, 28, 299–305. [Google Scholar] [CrossRef]
- Thames, S.F.; Yu, H.; Wang, D. Air-dry primer coatings from dehydrated lesquerella oil. Ind. Crops Prod. 1997, 6, 169–175. [Google Scholar] [CrossRef]
- Evangelista, R.L. Oil extraction from lesquerella seeds by dry extrusion and expelling. Ind. Crops Prod. 2009, 29, 189–196. [Google Scholar] [CrossRef]
- ASTM D1876-08; Standard Test Method for Peel Resistance of Adhesives. ASTM International: West Conshohocken, PA, USA, 2015.
- Sultan, M.; Zia, K.M.; Bhatti, H.N.; Jamil, T.; Hussain, R.; Zuber, M. Modification of cellulosic fiber with polyurethane acrylate copolymers. Part I: Physicochemical properties. Carbohydr. Polym. 2012, 87, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sato, M. The rate of the reaction of isocyanates with alcohols. II. J. Org. Chem. 1962, 27, 819–825. [Google Scholar] [CrossRef]
- Wood, L.A. Glass transition temperature of copolymers. J. Polym. Sci. 1958, 28, 319–330. [Google Scholar] [CrossRef]
- Cassidy, P.E.; Johnson, J.M.; Locke, C.E. The relationship of glass transition temperature to adhesive strength. J. Adhes. 1972, 4, 183–191. [Google Scholar] [CrossRef]
- Karami, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Ghasemi Rad, N. Bio-based thermoset alloys from epoxy acrylate, sesame oil- and castor oil-derived resins. Polym. Renew. Resour. 2019, 10, 27–44. [Google Scholar]
- Chen, H.; Jiang, B.; Cai, Z.-Q. Preparation and properties of paper-plastic laminating adhesive used for medical packaging materials. Polym. Adv. Technol. 2015, 26, 1065–1069. [Google Scholar] [CrossRef]

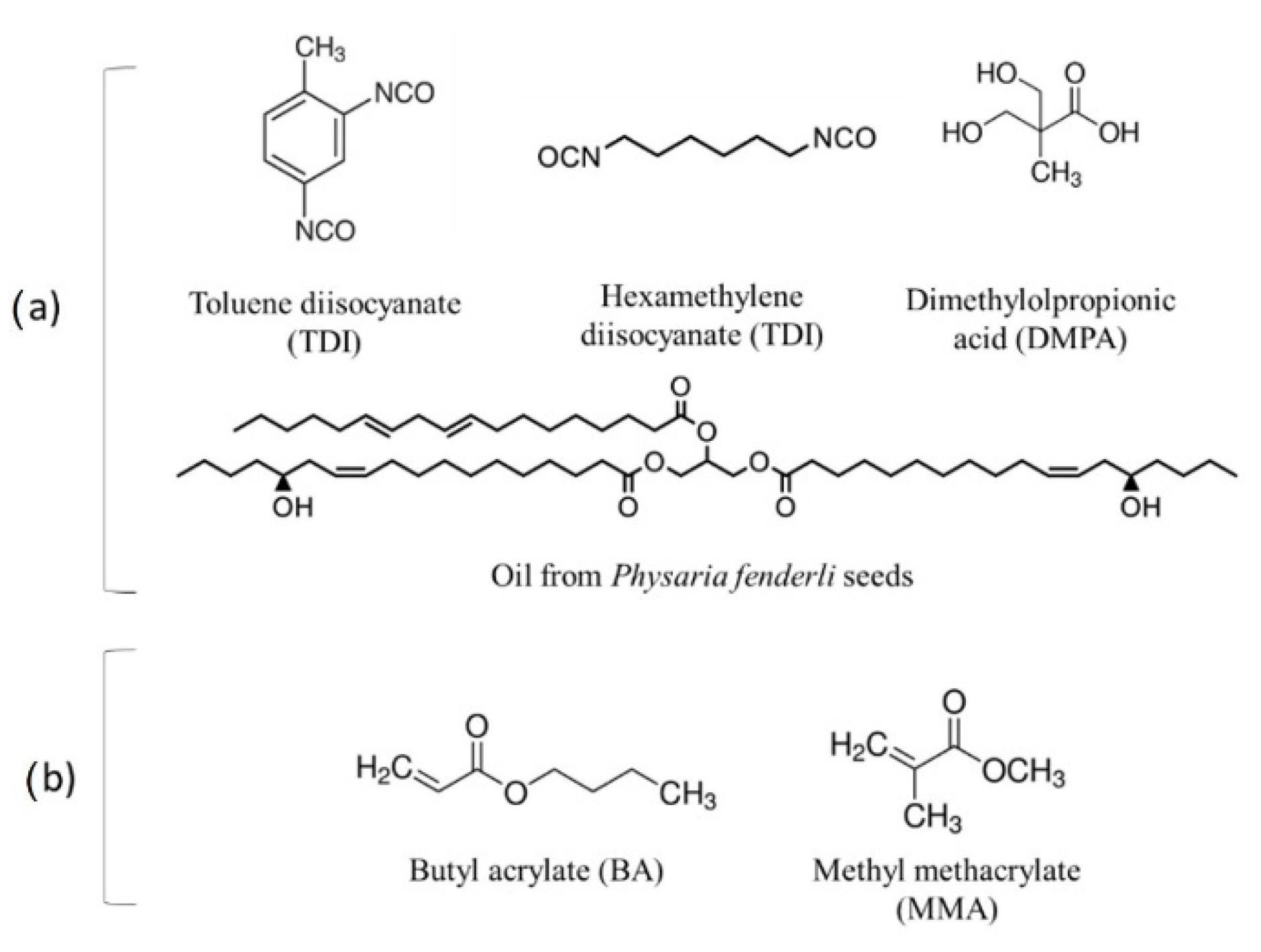
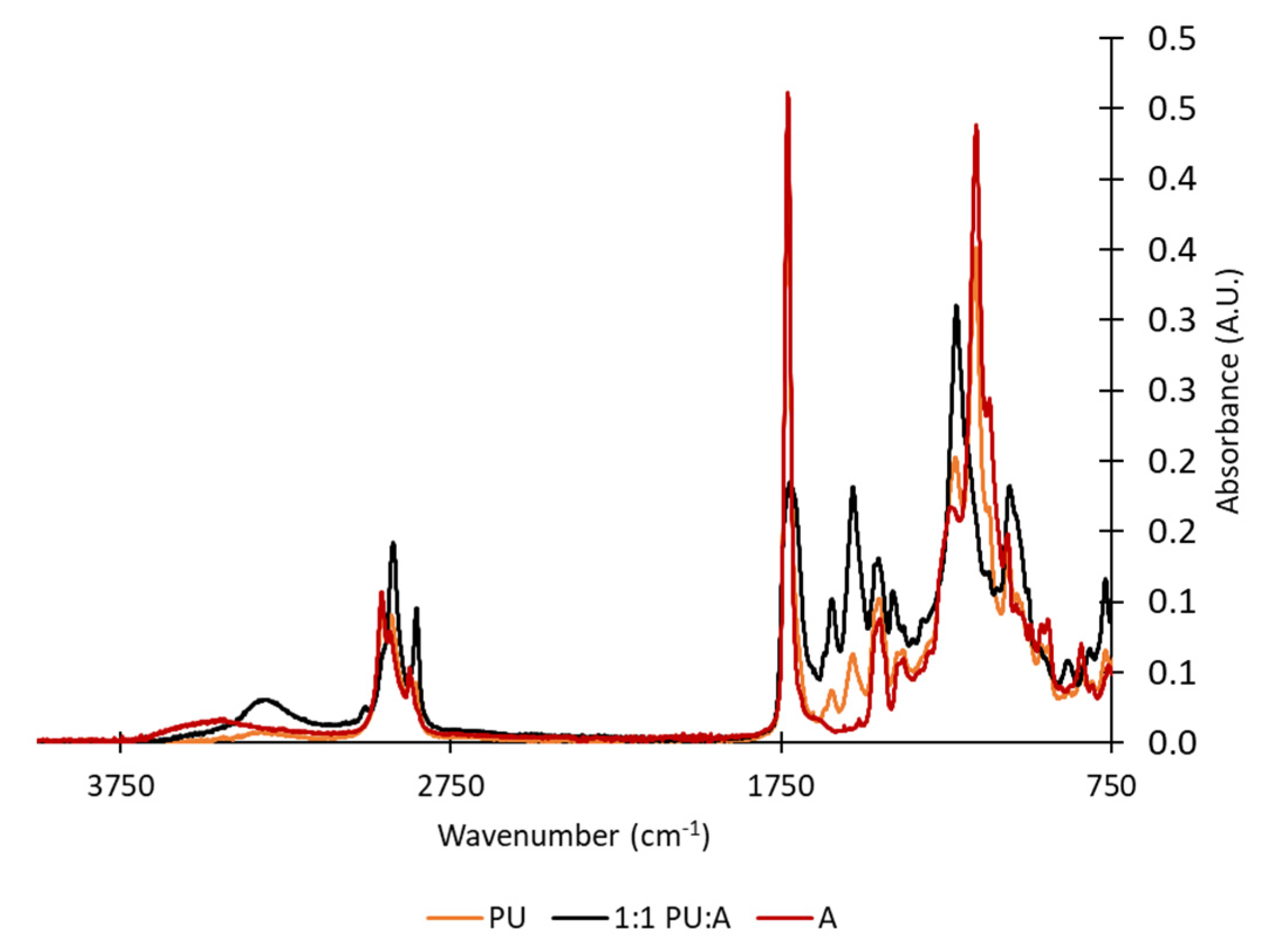


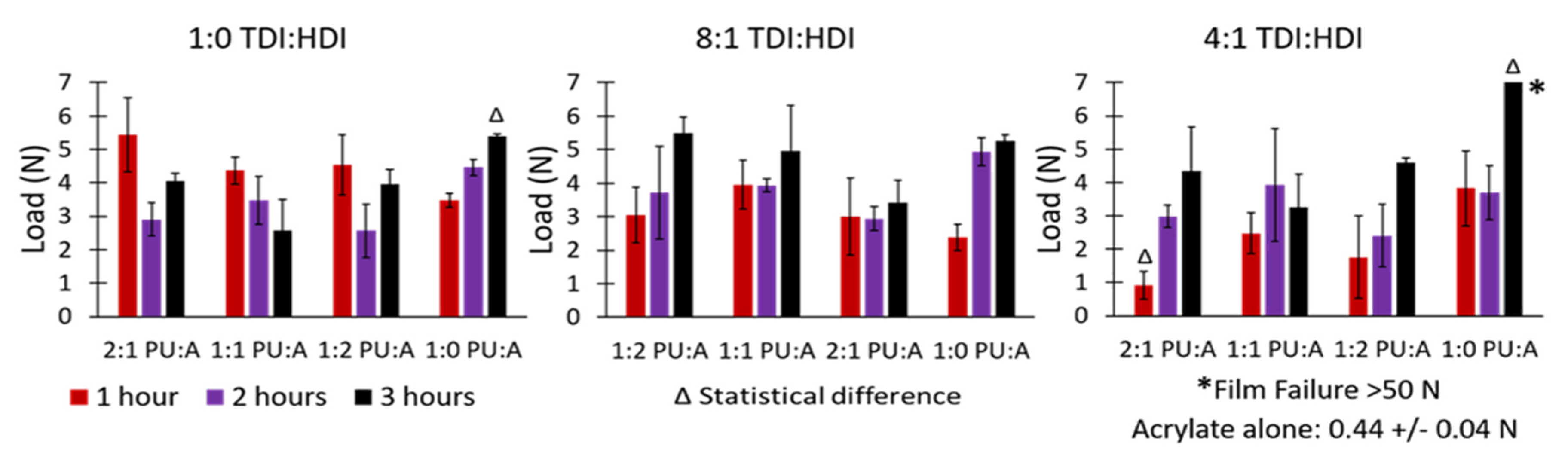
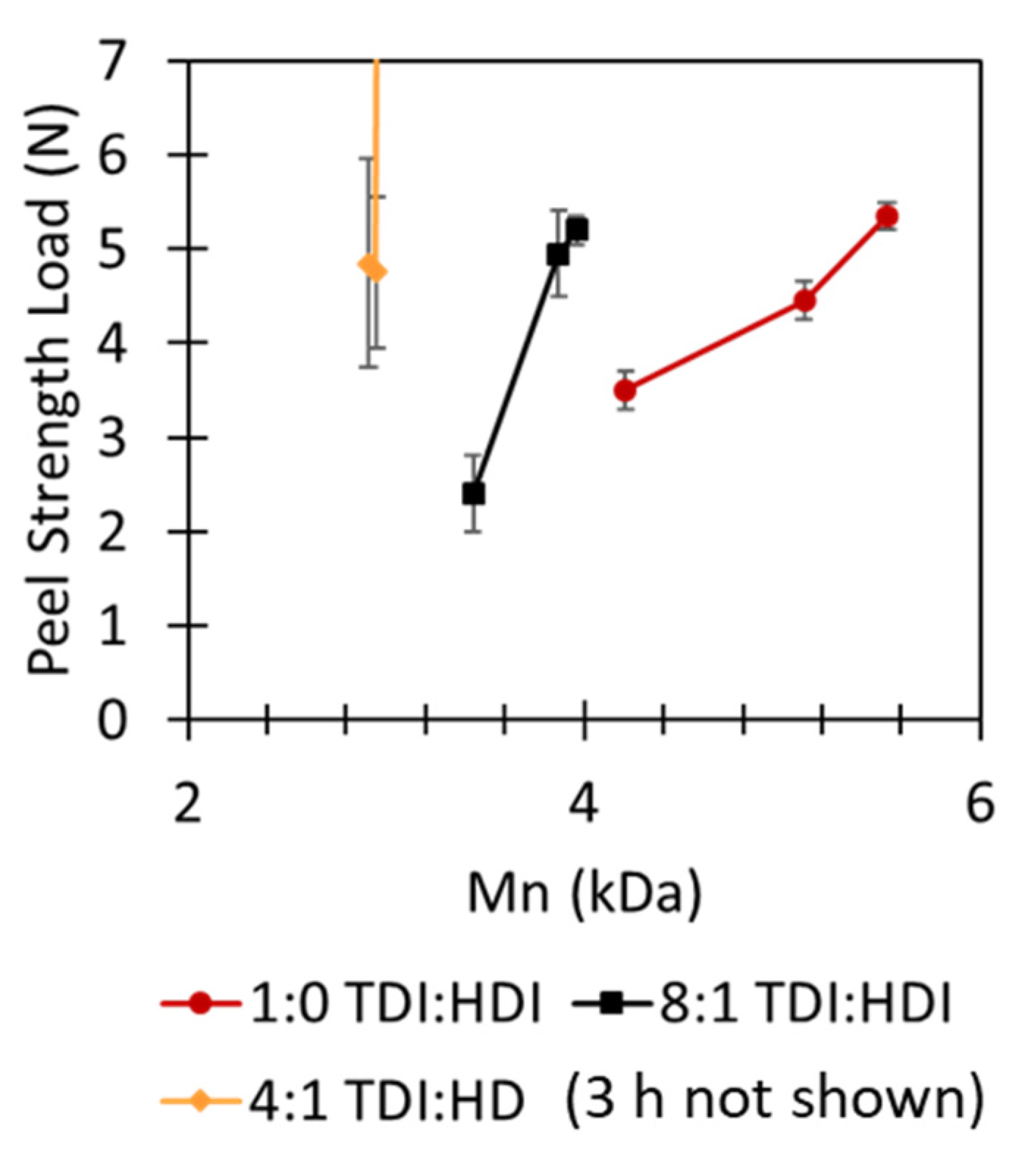
| Formulation # | PU Reaction TDI:HDI Ratio | Acrylate Emulsion BA:MMA Ratio | Final Dispersion PU:Acrylate Ratio |
|---|---|---|---|
| 1 | 1:0 | 8:2 | 1:0 |
| 2 | 1:0 | 8:2 | 2:1 |
| 3 | 1:0 | 8:2 | 1:1 |
| 4 | 1:0 | 8:2 | 1:2 |
| 5 | 8:1 | 8:2 | 1:0 |
| 6 | 8:1 | 8:2 | 2:1 |
| 7 | 8:1 | 8:2 | 1:1 |
| 8 | 8:1 | 8:2 | 1:2 |
| 9 | 4:1 | 8:2 | 1:0 |
| 10 | 4:1 | 8:2 | 2:1 |
| 11 | 4:1 | 8:2 | 1:1 |
| 12 | 4:1 | 8:2 | 1:2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mort, R.; Olson, E.; Thurber, H.; Jiang, S.; Vorst, K.; Curtzwiler, G. Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria fendleri Oil for Food Packaging Applications. Sustainability 2022, 14, 8657. https://doi.org/10.3390/su14148657
Mort R, Olson E, Thurber H, Jiang S, Vorst K, Curtzwiler G. Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria fendleri Oil for Food Packaging Applications. Sustainability. 2022; 14(14):8657. https://doi.org/10.3390/su14148657
Chicago/Turabian StyleMort, Rebecca, Emily Olson, Henry Thurber, Shan Jiang, Keith Vorst, and Greg Curtzwiler. 2022. "Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria fendleri Oil for Food Packaging Applications" Sustainability 14, no. 14: 8657. https://doi.org/10.3390/su14148657
APA StyleMort, R., Olson, E., Thurber, H., Jiang, S., Vorst, K., & Curtzwiler, G. (2022). Waterborne Polyurethane/Acrylic Adhesive Blends from Physaria fendleri Oil for Food Packaging Applications. Sustainability, 14(14), 8657. https://doi.org/10.3390/su14148657







