Hydrodynamic Limitations to Mangrove Seedling Retention in Subtropical Estuaries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Lateral Pull Test
2.2.1. Field Data Collection
2.2.2. Laboratory Data Collection
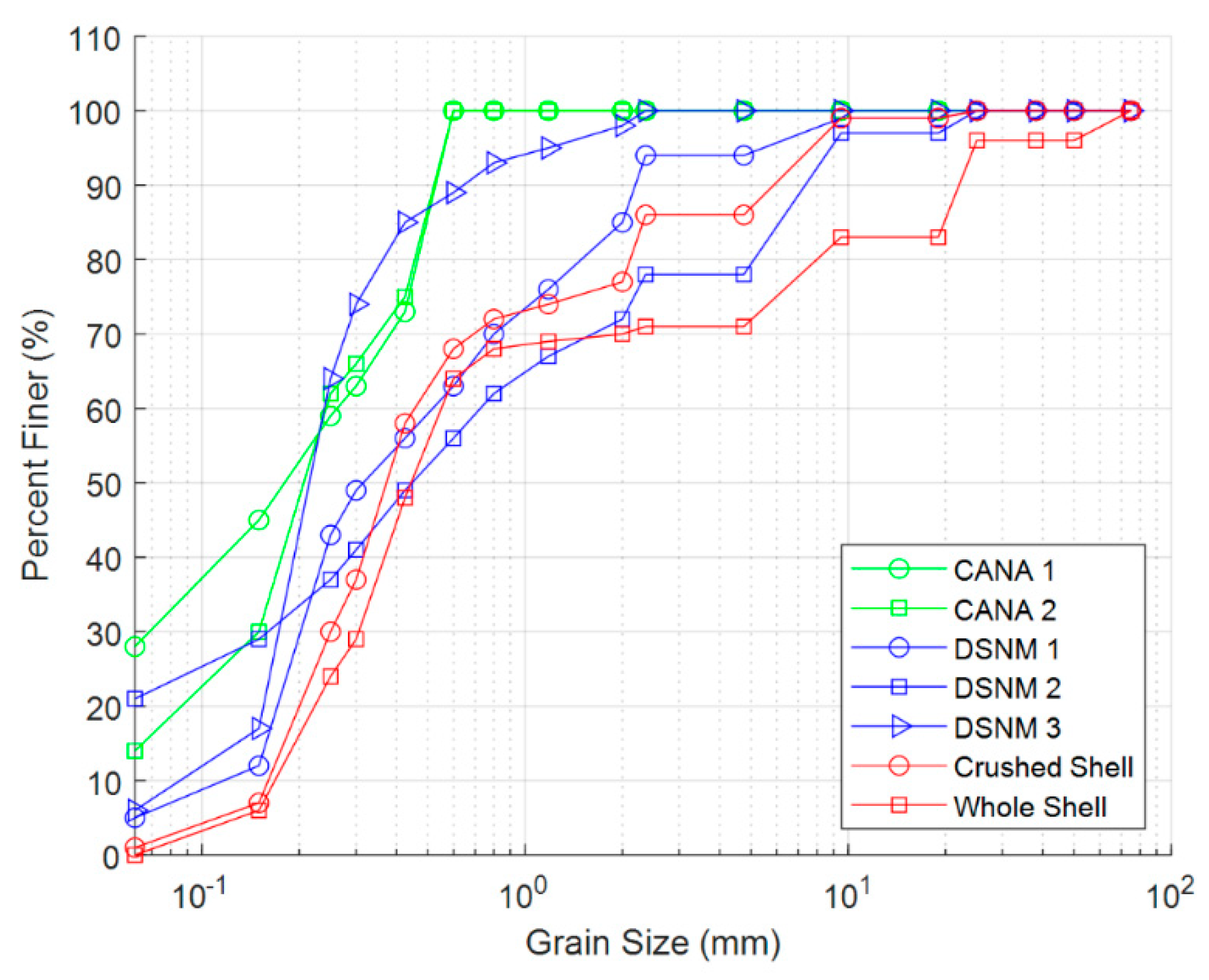
2.3. Seedling and Sediment Characterization
2.4. Data Analysis
3. Results
3.1. Field Pull Tests: Anchoring Force in Mangrove Forest
3.2. Laboratory Pull Tests: Anchoring Force across Seedling Age
3.3. Minimum Flows Required for Type I Dislodgement of Mangrove Seedlings
4. Discussion
4.1. Seedling Size and Age Influence Anchoring Force and Mechanism of Removal
4.2. Influence of Sediment and Surrounding Vegetation to Seedling Anchoring
4.3. Mangrove Seedling Susceptibility to Instantaneous Hydrodynamic Removal
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| # | Model | AICc | Δ AICc |
|---|---|---|---|
| 1 | log(Horiz..Force..N.) ~ BG.Biomass × Sp. + sed × Age | 126.1 | 0 |
| 2 | log(Horiz..Force..N.) ~ BG.Biomass × Sp. + sed + Age | 129.6 | 3.5 |
| 3 | log(Horiz..Force..N.) ~ BG.Biomass × Sp. + Age | 132.5 | 6.4 |
| 4 | log(Horiz..Force..N.) ~ BG.Biomass + Sp. + sed + Age | 140.5 | 14.4 |
| # | Model | AICc | Δ AICc |
|---|---|---|---|
| 1 | log(Horiz..Force..N.) ~ AG.Biomass..g. × Sp. + Per.Veg | 239.5 | 0 |
| 2 | log(Horiz..Force..N.) ~ AG.Biomass..g. × Sp. + factor(Perc.Canopy) + Per.Veg | 241.2 | 1.7 |
| 3 | log(Horiz..Force..N.) ~ AG.Biomass..g. × Sp. + Per.Veg + Park | 241.5 | 2.0 |
| 4 | log(Horiz..Force..N.) ~ AG.Biomass..g. × Sp. | 241.8 | 2.3 |
References
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Berlin/Heidelberg, Germany, 2009; 216p. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Luther, D.A.; Greenberg, R. Mangroves: A Global Perspective on the Evolution and Conservation of Their Terrestrial Vertebrates. BioScience 2009, 59, 602–612. [Google Scholar] [CrossRef] [Green Version]
- Carugati, L.; Gatto, B.; Rastelli, E.; Martire, M.L.; Coral, C.; Greco, S.; Danovaro, R. Impact of mangrove forests degradation on biodiversity and ecosystem functioning. Sci. Rep. 2018, 8, 13298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClenachan, G.M.; Donnelly, M.J.; Shaffer, M.N.; Sacks, P.E.; Walters, L.J. Does size matter? Quantifying the cumulative impact of small-scale living shoreline and oyster reef restoration projects on shoreline erosion. Restor. Ecol. 2020, 28, 1365–1371. [Google Scholar] [CrossRef]
- Tanaka, N.; Sasaki, Y.; Mowjood, M.I.M.; Jinadasa, K.B.S.N.; Homchuen, S. Coastal vegetation structures and their functions in tsunami protection: Experience of the recent Indian Ocean tsunami. Landsc. Ecol. Eng. 2007, 3, 33–45. [Google Scholar] [CrossRef]
- Mazda, Y.; Wolanski, E.; King, B.; Sase, A.; Ohtsuka, D.; Magi, M. Drag force due to vegetation in mangrove swamps. Mangroves Salt Marshes 1997, 1, 193–199. [Google Scholar] [CrossRef]
- Cheong, S.-M.; Silliman, B.; Wong, P.P.; Van Wesenbeeck, B.; Kim, C.-K.; Guannel, G. Coastal adaptation with ecological engineering. Nat. Clim. Change 2013, 3, 787–791. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The World’s Mangroves 1980–2005: A Thematic Study Prepared in the Framework of the Global Forest Resources Assessment 2005; FAO: Rome, Italy, 2007. [Google Scholar]
- Silva, R.; Martínez, M.; Van Tussenbroek, B.; Guzmán-Rodríguez, L.; Mendoza, E.; López-Portillo, J. A Framework to Manage Coastal Squeeze. Sustainability 2020, 12, 10610. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Musarella, C.M.; Fuentes, J.C.P.; Gomes, C.J.P.; Del Rio, S.; Canas, R.Q.; Cano, E. Analysis of the Conservation of Central American Mangroves Using the Phytosociological Method. In Mangrove Ecosystem Ecology and Function; Intech Publisher: London, UK, 2018; pp. 189–206. [Google Scholar]
- Lewis, R.R. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 2005, 24, 403–418. [Google Scholar] [CrossRef]
- Friess, D.A.; Krauss, K.W.; Horstman, E.; Balke, T.; Bouma, T.J.; Galli, D.; Webb, E. Are all intertidal wetlands naturally created equal? Bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biol. Rev. 2012, 87, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Fillyaw, R.M.; Donnelly, M.J.; Litwak, J.W.; Rifenberg, J.L.; Walters, L.J. Strategies for Successful Mangrove Living Shoreline Stabilizations in Shallow Water Subtropical Estuaries. Sustainability 2021, 13, 11704. [Google Scholar] [CrossRef]
- Kathiresan, K. How do mangrove forests induce sedimentation? Rev. Biol. Trop. 2003, 51, 355–360. [Google Scholar] [PubMed]
- Mazda, Y.; Kobashi, D.; Okada, S. Tidal-Scale Hydrodynamics within Mangrove Swamps. Wetl. Ecol. Manag. 2005, 13, 647–655. [Google Scholar] [CrossRef]
- Cannon, D.; Kibler, K.; Donnelly, M.; McClenachan, G.; Walters, L.; Roddenberry, A.; Phagan, J. Hydrodynamic habitat thresholds for mangrove vegetation on the shorelines of a microtidal estuarine lagoon. Ecol. Eng. 2020, 158, 106070. [Google Scholar] [CrossRef]
- Constance, A.; Haverkamp, P.J.; Bunbury, N.; Schaepman-Strub, G. Extent change of protected mangrove forest and its relation to wave power exposure on Aldabra Atoll. Glob. Ecol. Conserv. 2021, 27, e01564. [Google Scholar] [CrossRef]
- Iii, R.R.L. Ecologically based goal setting in mangrove forest and tidal marsh restoration. Ecol. Eng. 2000, 15, 191–198. [Google Scholar] [CrossRef]
- Balke, T.; Bouma, T.J.; Horstman, E.M.; Webb, E.L.; Erftemeijer, P.L.A.; Herman, P.M.J. Windows of opportunity: Thresholds to mangrove seedling establishment on tidal flats. Mar. Ecol. Prog. Ser. 2011, 440, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Boizard, S.D.; Mitchell, S.J. Resistance of red mangrove (Rhizophora mangle L.) seedlings to deflection and extraction. Trees 2011, 25, 371–381. [Google Scholar] [CrossRef]
- Lima, K.O.D.O.; Tognella, M.M.P.; Cunha, S.R.; de Andrade, H.A. Growth models of Rhizophora mangle L. seedlings in tropical southwestern Atlantic. Estuar. Coast. Shelf Sci. 2018, 207, 154–163. [Google Scholar] [CrossRef]
- Minchinton, T.E. Canopy and substratum heterogeneity influence recruitment of the mangrove Avicennia marina. J. Ecol. 2001, 89, 888–902. [Google Scholar] [CrossRef]
- Edmaier, K.; Burlando, P.; Perona, P. Mechanisms of vegetation uprooting by flow in alluvial non-cohesive sediment. Hydrol. Earth Syst. Sci. 2011, 15, 1615–1627. [Google Scholar] [CrossRef] [Green Version]
- Bywater-Reyes, S.; Wilcox, A.C.; Stella, J.C.; Lightbody, A.F. Flow and scour constraints on uprooting of pioneer woody seedlings. Water Resour. Res. 2015, 51, 9190–9206. [Google Scholar] [CrossRef] [Green Version]
- Calvani, G.; Francalanci, S.; Solari, L. A Physical Model for the Uprooting of Flexible Vegetation on River Bars. J. Geophys. Res. Earth Surf. 2019, 124, 1018–1034. [Google Scholar] [CrossRef]
- Le Minor, M.; Bartzke, G.; Zimmer, M.; Gillis, L.; Helfer, V.; Huhn, K. Numerical modelling of hydraulics and sediment dynamics around mangrove seedlings: Implications for mangrove establishment and reforestation. Estuar. Coast. Shelf Sci. 2019, 217, 81–95. [Google Scholar] [CrossRef]
- Kamali, B.; Hashim, R. Mangrove restoration without planting. Ecol. Eng. 2011, 37, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, D. Dispersal Properties of Mangrove Propagules. Biotropica 1978, 10, 47. [Google Scholar] [CrossRef]
- Cano, E.; Cano-Ortiz, A.; Veloz, A.; Alatorre, J.; Otero, R. Comparative analysis between the mangrove swamps of the Caribbean and those of the State of Guerrero (Mexico). Plant Biosyst.-Int. J. Deal. all Asp. Plant Biol. 2012, 146, 112–130. [Google Scholar] [CrossRef]
- Mckee, K.L. Interspecific Variation in Growth, Biomass Partitioning, and Defensive Characteristics of Neotropical Mangrove Seedlings—Response to Light and Nutrient Availability. Am. J. Bot. 1995, 82, 299–307. [Google Scholar] [CrossRef]
- López-Hoffman, L.; Ackerly, D.D.; Anten, N.P.R.; DeNoyer, J.L.; Martinez-Ramos, M. Gap-dependence in mangrove life-history strategies: A consideration of the entire life cycle and patch dynamics. J. Ecol. 2007, 95, 1222–1233. [Google Scholar] [CrossRef]
- Service, N.P. De Soto National Memorial. 2019. Available online: https://www.nps.gov/deso/index.htm (accessed on 23 May 2022).
- Service, N.P. Canaveral National Seashore Florida. 2019. Available online: http://www.nps.gov/cana/index.htm (accessed on 23 May 2022).
- Phlips, E.J.; Badylak, S.; Lasi, M.A.; Chamberlain, R.; Green, W.C.; Hall, L.M.; Hart, J.A.; Lockwood, J.C.; Miller, J.D.; Morris, L.J.; et al. From Red Tides to Green and Brown Tides: Bloom Dynamics in a Restricted Subtropical Lagoon Under Shifting Climatic Conditions. Estuaries Coasts 2015, 38, 886–904. [Google Scholar] [CrossRef]
- McNulty, J.K.; Lindall, W.N.; Sykes, J.E. Cooperative Gulf of Mexico Estuarine Inventory and Study, Florida: Phase 1, Area Description; US Department of Commerce, National Oceanographic and Atmospheric Association: Seattle, WA, USA, 1972; Volume 368. [CrossRef]
- Caratti, J.F. Line intercept: Sampling Method. 2006. Available online: https://www.fs.fed.us/rm/pubs/rmrs_gtr164/rmrs_gtr164_11_line_inter.pdf (accessed on 23 May 2022).
- Kibler, K.M.; Kitsikoudis, V.; Donnelly, M.; Spiering, D.W.; Walters, L. Flow–Vegetation Interaction in a Living Shoreline Restoration and Potential Effect to Mangrove Recruitment. Sustainability 2019, 11, 3215. [Google Scholar] [CrossRef] [Green Version]
- Spiering, D.W.; Kibler, K.M.; Kitsikoudis, V.; Donnelly, M.J.; Walters, L.J. Detecting hydrodynamic changes after living shoreline restoration and through an extreme event using a Before-After-Control-Impact experiment. Ecol. Eng. 2021, 169, 106306. [Google Scholar] [CrossRef]
- Lightbody, A.F.; Nepf, H.M. Prediction of velocity profiles and longitudinal dispersion in emergent salt marsh vegetation. Limnol. Oceanogr. 2006, 51, 218–228. [Google Scholar] [CrossRef]
- Liu, C.; Evett, J. Soil Properties, Testing, Measurement and Evaluation; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Bolker, B.; Bolker, M.B. Package ‘bbmle’. Tools for General Maximum Likelihood Estimation. 2022. Available online: https://cran.r-project.org/web/packages/bbmle/bbmle.pdf (accessed on 23 May 2022).
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Struve, J.; Falconer, R.; Wu, Y. Influence of model mangrove trees on the hydrodynamics in a flume. Estuar. Coast. Shelf Sci. 2003, 58, 163–171. [Google Scholar] [CrossRef]
- Mullarney, J.C.; Henderson, S.M. Flows Within Marine Vegetation Canopies. Adv. Coast. Hydraul. 2018, 1–46. [Google Scholar] [CrossRef]
- Coutts, M.P. Components of Tree Stability in Sitka Spruce on Peaty Gley Soil. Forestry 1986, 59, 173–197. [Google Scholar] [CrossRef]
- Moore, J.R. Differences in maximum resistive bending moments of Pinus radiata trees grown on a range of soil types. For. Ecol. Manag. 2000, 135, 63–71. [Google Scholar] [CrossRef]
- Peltola, H.; Kellomäki, S.; Hassinen, A.; Granander, M. Mechanical stability of Scots pine, Norway spruce and birch: An analysis of tree-pulling experiments in Finland. For. Ecol. Manag. 2000, 135, 143–153. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Gardiner, B.A.; Rayner, B.; Peace, A.J. Anchorage of coniferous trees in relation to species, soil type, and rooting depth. Can. J. For. Res. -Rev. Can. Rech. For. 2006, 36, 1871–1883. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Roumet, C.; Buisson, E.; Dutoit, T. Linking plant morphological traits to uprooting resistance in eroded marly lands (Southern Alps, France). Plant Soil 2009, 324, 31–42. [Google Scholar] [CrossRef]
- Bankhead, N.L.; Thomas, R.E.; Simon, A. A combined field, laboratory and numerical study of the forces applied to, and the potential for removal of, bar top vegetation in a braided river. Earth Surf. Process. Landf. 2017, 42, 439–459. [Google Scholar] [CrossRef] [Green Version]
- Karrenberg, S.; Blaser, S.; Kollmann, J.; Speck, T.; Edwards, P.J. Root anchorage of saplings and cuttings of woody pioneer species in a riparian environment. Funct. Ecol. 2003, 17, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. The root anchorage ability of Salix alba var. tristis using a pull-out test. Afr. J. Biotechnol. 2011, 10. [Google Scholar] [CrossRef]
- Schutten, J.; Dainty, J.; Davy, A.J. Root anchorage and its significance for submerged plants in shallow lakes. J. Ecol. 2005, 93, 556–571. [Google Scholar] [CrossRef]
- Edmaier, K.; Crouzy, B.; Ennos, R.; Burlando, P.; Perona, P. Influence of root characteristics and soil variables on the uprooting mechanics of Avena sativa and Medicago sativa seedlings. Earth Surf. Process. Landforms 2014, 39, 1354–1364. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef] [Green Version]
- Simpson, L.; Osborne, T.; Feller, I. Establishment and Biomass Allocation of Black and Red Mangroves: Response to Propagule Flotation Duration and Seedling Light Availability. J. Coast. Res. 2017, 335, 1126–1134. [Google Scholar] [CrossRef]
- Oplatka, M.; Sutherland, A. Tests on willow poles used for river bank protection. J. Hydrol. 1995, 33, 35–58. [Google Scholar]
- Donnelly, M.; Shaffer, M.; Connor, S.; Sacks, P.; Walters, L. Using mangroves to stabilize coastal historic sites: Deployment success versus natural recruitment. Hydrobiologia 2017, 803, 389–401. [Google Scholar] [CrossRef]
- Duarte, C.; Geertz-Hansen, O.; Thampanya, U.; Terrados, J.; Fortes, M.D.; Kamp-Nielsen, L.; Borum, J.; Boromthanarath, S. Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient status. Mar. Ecol. Prog. Ser. 1998, 175, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Norris, B.K.; Mullarney, J.C.; Bryan, K.R.; Henderson, S.M. Turbulence Within Natural Mangrove Pneumatophore Canopies. J. Geophys. Res. Oceans 2019, 124, 2263–2288. [Google Scholar] [CrossRef]
- Thampanya, U.; Vermaat, J.; Duarte, C.M. Colonization success of common Thai mangrove species as a function of shelter from water movement. Mar. Ecol. Prog. Ser. 2002, 237, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Bouma, T.J.; Vries, M.B.D.; Low, E.; Kusters, L.; Herman, P.M.J.; Tanczos, I.C.; Temmerman, S.; Hesselink, A.; Meire, P.; Regenmortel, S.V. Flow hydrodynamics on a mudflat and in salt marsh vegetation: Identifying general relationships for habitat characterisations. Hydrobiologia 2005, 540, 259–274. [Google Scholar] [CrossRef]
- Stevens, P.W.; Fox, S.L.; Montague, C.L. The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetl. Ecol. Manag. 2006, 14, 435–444. [Google Scholar] [CrossRef]
- Cannon, D.; Kibler, K.; Walters, L.; Chambers, L. Hydrodynamic and biogeochemical evolution of a restored intertidal oyster (Crassostrea virginica) reef. Sci. Total Environ. 2022, 831, 154879. [Google Scholar] [CrossRef]







| Estimates | Std. Error | t Value | Pr (>|t|) | ||
|---|---|---|---|---|---|
| Field tests: | Intercept | 2.180934 | 0.15793 | 13.809 | <0.001 |
| Above-Ground Biomass | 0.22507 | 0.05033 | 4.472 | <0.001 | |
| Rhizophora mangle L. | 0.52852 | 0.16573 | 3.189 | 0.0017 | |
| Percent Vegetation | 0.00468 | 0.00224 | 2.092 | 0.0379 | |
| Above-Ground Biomass: R. mangle L. | −0.18942 | 0.05067 | −3.739 | <0.001 | |
| Lab tests: | Intercept | 1.74262 | 0.07502 | 24.346 | <0.001 |
| Below-Ground Biomass | 0.19579 | 0.03580 | 5.352 | <0.001 | |
| Rhizophora mangle L. | 0.93614 | 0.14639 | 6.388 | <0.001 | |
| Crushed shell | 0.28001 | 0.05431 | 2.178 | 0.0309 | |
| 3 months | 0.75687 | 0.07717 | 8.796 | <0.001 | |
| 4 months | 1.01866 | 0.07727 | 10.993 | <0.001 | |
| Below-Ground Biomass: R. mangle L. | −0.13226 | 0.03578 | −3.585 | <0.001 | |
| Crushed shell: 3 months | −0.16509 | 0.12749 | −1.295 | 0.197 | |
| Crushed shell: 4 months | −0.35429 | 0.13143 | −2.696 | 0.008 |
| Species | N | Park | Above-Ground Biomass (g) | Below-Ground Biomass (g) | Height (cm) | Leaf Number | Basal Diameter (cm) |
|---|---|---|---|---|---|---|---|
| Rhizophora mangle L. | 95 | CANA | 24.9 ± 1.4 | 6.4 ± 0.4 | 42.8 ± 1.3 | 7.5 ± 0.5 | 1.2 ± 0.0 |
| DSNM | 17.7 ± 0.8 | 6.6 ± 0.4 | 35.5 ± 0.9 | 5.1 ± 0.3 | 1.2 ± 0.0 | ||
| Avicennia germinans (L.) L. | 87 | CANA | 2.1 ± 0.1 | 0.6 ± 0.0 | 21.8 ± 0.6 | 4.7 ± 0.2 | 0.4 ± 0.0 |
| DSNM | 1.4 ± 0.2 | 0.7 ± 0.1 | 19.7 ± 0.9 | 4.5 ± 0.3 | 0.3 ± 0.0 |
| Site/Treatment | D50 (mm) | D16 (mm) | D84 (mm) | Organic Matter (%) |
|---|---|---|---|---|
| Field testing | ||||
| CANA 1 | 0.18 | 0.04 | 0.50 | 29.7 |
| CANA 2 | 0.21 | 0.08 | 0.49 | 16.0 |
| De Soto 1 | 0.32 | 0.16 | 1.88 | 28.5 |
| De Soto 2 | 0.46 | 0.06 | 6.21 | 56.4 |
| De Soto 3 | 0.22 | 0.15 | 0.42 | 10.4 |
| Laboratory testing | ||||
| Crushed shell-sand | 0.38 | 0.19 | 2.28 | –– |
| Whole shell-sand | 0.44 | 0.21 | 19.3 | –– |
| Species | Age (months) | N | Above-Ground Biomass (g) | Below-Ground Biomass (g) | Height (cm) | Force to Removal (N) |
|---|---|---|---|---|---|---|
| Rhizophora mangle L. | 1 | 30 | 16.0 ± 0.7 | 7.8 ± 0.4 | 25.2 ± 0.9 | 27.2 ± 2.0 |
| 3 | 30 | 19.3 ± 0.7 | 10.7 ± 0.7 | 31.8 ± 0.9 | 68.1 ± 3.1 | |
| 4 | 30 | 18.3 ± 1.2 | 10.6 ± 0.6 | 31.2 ± 1.4 | 86.7 ± 4.7 | |
| Avicennia germinans (L.) L. | 1 | 30 | 2.7 ± 0.2 | 0.8 ± 0.1 | 9.5 ± 0.5 | 9.1 ± 0.6 |
| 3 | 30 | 2.4 ± 0.2 | 2.6 ± 0.2 | 15.2 ± 0.6 | 22.7 ± 1.5 | |
| 4 | 30 | 2.6 ± 0.3 | 2.4 ± 0.2 | 15.2 ± 0.8 | 25.0 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kibler, K.M.; Pilato, C.; Walters, L.J.; Donnelly, M.; Taye, J. Hydrodynamic Limitations to Mangrove Seedling Retention in Subtropical Estuaries. Sustainability 2022, 14, 8605. https://doi.org/10.3390/su14148605
Kibler KM, Pilato C, Walters LJ, Donnelly M, Taye J. Hydrodynamic Limitations to Mangrove Seedling Retention in Subtropical Estuaries. Sustainability. 2022; 14(14):8605. https://doi.org/10.3390/su14148605
Chicago/Turabian StyleKibler, Kelly M., Christian Pilato, Linda J. Walters, Melinda Donnelly, and Jyotismita Taye. 2022. "Hydrodynamic Limitations to Mangrove Seedling Retention in Subtropical Estuaries" Sustainability 14, no. 14: 8605. https://doi.org/10.3390/su14148605
APA StyleKibler, K. M., Pilato, C., Walters, L. J., Donnelly, M., & Taye, J. (2022). Hydrodynamic Limitations to Mangrove Seedling Retention in Subtropical Estuaries. Sustainability, 14(14), 8605. https://doi.org/10.3390/su14148605








