Features of Plant Community and Driving Forces of Plant Community Succession in the Typical Desert Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Data Processing and Calculation
3. Results
3.1. Features of Plant Community in the Typical Desert Wetlands
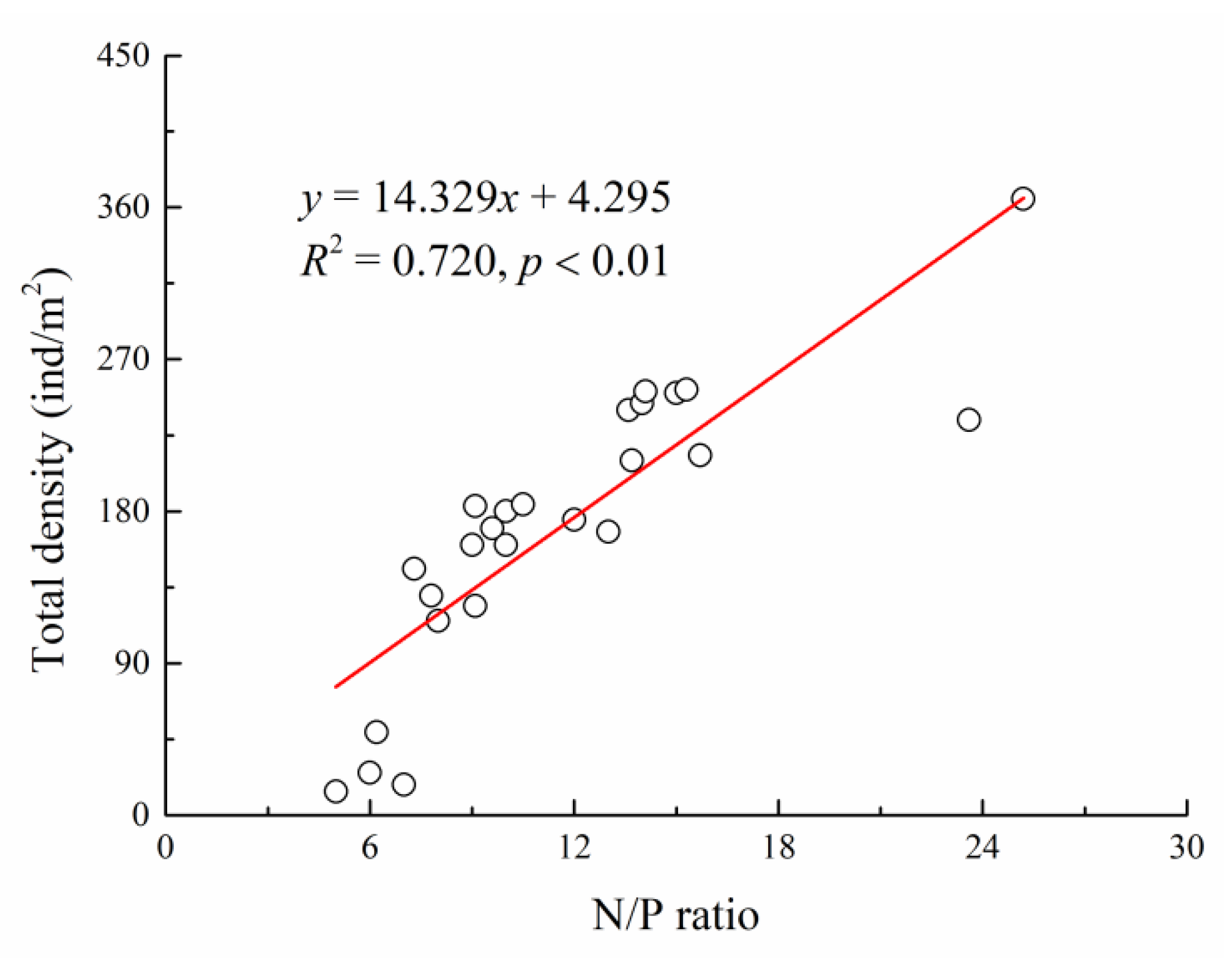
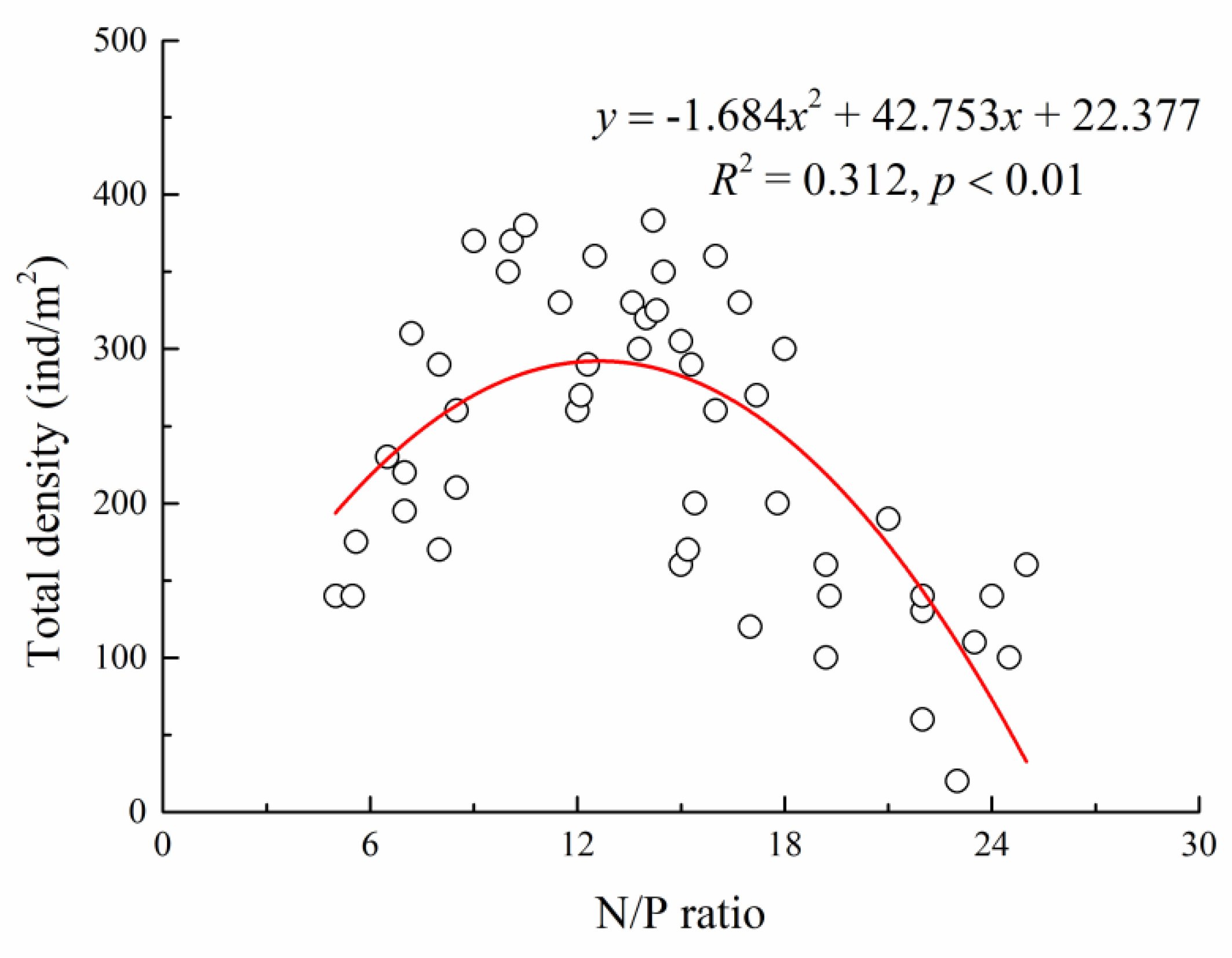
3.2. Driving Force of Plant Community Succession in the Typical Desert Wetlands
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.X.; Wang, W.; Yang, W.T.; Zhang, Q.T. Assessing the health of inland wetland ecosystems over space and time in China. J. Resour. Ecol. 2021, 12, 650–657. [Google Scholar] [CrossRef]
- Ahn, C.; Jones, S. Assessing organic matter and organic carbon contents in soils of created mitigation wetlands in Virginia. Environ. Eng. Res. 2013, 18, 151–156. [Google Scholar] [CrossRef]
- Ouse, A.R.; Thompson, J.R.; Acreman, M.C. Projecting impacts of climate change on hydrological conditions and biotic responses in a chalk valley riparian wetland. J. Hydrol. 2016, 534, 178–192. [Google Scholar] [CrossRef]
- Springer, K.B.; Manker, C.R.; Pigati, J.S. Dynamic response of desert wetlands to abrupt climate change. Proc. Natl. Acad. Sci. USA 2015, 112, 14522–14526. [Google Scholar] [CrossRef] [PubMed]
- Decleer, K.; Wouters, J.; Jacobs, S.; Staes, J.; Spanhove, T.; Meire, P.; Diggelen, R. Mapping wetland loss and restoration potential in Flanders (Belgium) an ecosystem service perspective. Ecol. Soc. 2016, 21, 46–78. [Google Scholar] [CrossRef][Green Version]
- Pattison-Williams, J.K.; Pomeroy, J.W.; Badiou, P.; Gabor, S. Wetlands, flood control and ecosystem services in the Smith Creek Drainage Basin: A case study in Saskatchewan, Canada. Ecol. Econ. 2018, 147, 36–47. [Google Scholar] [CrossRef]
- Sharma, R.C.; Rawat, J.S. Monitoring of aquatic macroinvertebrates as bioindicator for assessing the health of wetlands: A case study in the Central Himalayas, India. Ecol. Indic. 2009, 9, 118–128. [Google Scholar] [CrossRef]
- Mo, M.H.; Wang, X.L.; Wu, H.J.; Cai, S.M.; Zhang, X.Y.; Wang, H.L. Ecosystem health assessment of Honghu Lake Wetland of China using artificial neural network approach. Chin. Geogr. Sci. 2009, 19, 349–356. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, T.; Yan, D.; Yang, M.; Yu, H.; Shi, W. The impact on the ecosystem services value of the ecological shelter zone reconstruction in the upper reaches basin of the Yangtze River in China. Int. J. Environ. Res. Public Health 2018, 15, 2273. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Bastin, G.N.; Chewings, V.H.; Eager, R.W.; Liedloff, A.C. Leakiness: A new index for monitoring the health of arid and semiarid landscapes using remotely sensed vegetation cover and elevation data. Ecol. Indic. 2007, 7, 442–454. [Google Scholar] [CrossRef]
- Sun, T.T.; Lin, W.P.; Chen, G.S.; Guo, P.P.; Zeng, Y. Wetland ecosystem health assessment through integrating remote sensing and inventory data with an assessment model for the Hangzhou Bay, China. Sci. Total Environ. 2016, 566, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.H.; Wang, Z.M.; Du, B.J.; Li, L.; Tian, Y.L.; Jia, M.M.; Zeng, Y.; Song, K.S.; Jiang, M.; Wang, Y.Q. National wetland mapping in China: A new product resulting from object-based and hierarchical classification of Landsat 8 OLI images. ISPRS J. Photogramm. 2020, 164, 11–25. [Google Scholar] [CrossRef]
- Li, X.R.; Tian, F.; Jia, R.L.; Zhang, Z.S.; Liu, L.C. Do biological soil crusts determine vegetation changes in sandy deserts? Implications for managing artificial vegetation. Hydrol. Proc. 2010, 24, 3621–3630. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, X.R.; Liu, L.C.; Jia, R.L.; Zhang, J.G.; Wang, T. Distribution, biomass, and dynamics of roots in a revegetated stand of Caragana korshinskii in the Tengger Desert, Northwestern China. J. Plant Res. 2009, 122, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Liccari, F.; Boscutti, F.; Bacaro, G.; Sigura, M. Connectivity, landscape structure, and plant diversity across agricultural landscapes: Novel insight into effective ecological network planning. J. Environm. Manag. 2022, 317, 115358. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, W.; Wang, H. Effects of vegetation restoration on soil erosion on the Loess Plateau: A case study in the Ansai watershed. Int. J. Environ. Res. Public Health 2021, 18, 6266. [Google Scholar] [CrossRef]
- Berglund, S.; Bosson, E.; Sassner, M. From site data to safety assessment: Analysis of present and future hydrological conditions at a coastal site in Sweden. AMBIO 2013, 4, 425–434. [Google Scholar] [CrossRef]
- Klausmeier, C.A. Regular and irregular patterns in semiarid vegetation. Science 1999, 284, 1826–1828. [Google Scholar] [CrossRef]
- Wang, X.P.; Kang, E.S.; Zhang, J.G.; Li, X.R. Soil moisture dynamics in an artificially re-vegetated desert area. Adv. Water Sci. 2004, 15, 216–222. [Google Scholar] [CrossRef]
- Palmer, M.; Ruhi, A. Linkages between flow regime, biota, and ecosystem processes: Implications for river restoration. Science 2019, 365, e2087. [Google Scholar] [CrossRef]
- Romano, F.; Maneul, D.J.; Andrea, R.; Ignacio, R.I. Hydroperiod regime controls the organization of plant species in wetlands. Proc. Natl. Acad. Sci. USA 2012, 109, 19596–19600. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, L.D.; Pan, X.; Li, W.; Kang, X.M.; Li, J.; Ning, Y.; Zhang, M.X.; Cui, L.J. Hydrological conditions affect the interspecific interaction between two emergent wetland species. Front. Plant Sci. 2017, 8, 2253. [Google Scholar] [CrossRef] [PubMed]
- Mooneye, P.; O’Connell, M. The phytosociology and ccology of the aquatic and wetland plant communities of the lower Corrib basin, County Galway. Proc. R. Ir. Academy. Sect. B Biol. Geol. Chem. Sci. 1990, 90, 57–97. [Google Scholar]
- Kazantseva, T.I.; Adamovich, B.V.; Mikheeva, T.M. Biological interpretation of the third main factor influencing the long-term dynamics of hydrological and ecological parameters of the Three Naroch Lakes under changing nutrient load in 1978–2015. Contemp. Probl. Ecol. 2022, 15, 11–18. [Google Scholar] [CrossRef]
- Pigati, J.S.; Springer, K.B.; Honke, J.S. Desert wetlands record hydrologic variability within the Younger Dryas chronozone, Mojave Desert, USA. Quat. Res. 2019, 91, 51–62. [Google Scholar] [CrossRef]
- Kong, X.Z.; He, Q.S.; Yang, B.; He, W.; Xu, F.L.; Janssen, A.B.G.; Kuiper, J.J.; van Gerven, L.P.A.; Qin, N.; Jiang, Y.J.; et al. Hydrological regulation drives regime shifts: Evidence from paleolimnology and ecosystem modeling of a large shallow Chinese lake. Glob. Change Biol. 2017, 23, 737–754. [Google Scholar] [CrossRef]
- Jiang, H.B.; Wen, Y.; Zou, L.F.; Wang, Z.Q.; He, C.G.; Zou, C.L. The effects of a wetland restoration project on the Siberian crane (Grus leucogeranus) population and stopover habitat in Momoge National Nature Reserve, China. Ecol. Eng. 2016, 96, 170–177. [Google Scholar] [CrossRef]
- Wang, B.Z.; Ji, J.R.; Luo, L.C.; Wang, C.X.; Dan, X.Q.; He, P.; Shi, X.H.; Dong, G.Z. Ecological restoration for the wetland of the Xinjizhou Shoals, Nanjing. Wetland Sci. 2006, 4, 210–218. [Google Scholar] [CrossRef]
- Hu, Y.F.; Peng, J.J.; Deng, L.J.; Xiao, H.H.; Jiang, S.L.; Ma, K.Y. Influences of fencing and planting branchy tamarisk on soil particles composition and mineral nutrients in desertization land in Northwestern Sichuan Province. Chin. J. Soil Sci. 2015, 46, 54–61. [Google Scholar] [CrossRef]
- Niu, Q.C. Restoration construction, management and sustainable utilization of urban wetland in Xi’an City: A case of Chanba ecological zone. J. Anhui Agri. Sci. 2016, 44, 138–139. [Google Scholar] [CrossRef]
- Apajalahti, J.H.A.; Särkilahti, L.K.; Mäki, B.R.E.; Heikkinen, J.P.; Nurminen, P.H.; Holben, W.E. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of Broiler Chickens. Appl. Environ. Microbiol. 1998, 64, 4084–4088. [Google Scholar] [CrossRef]
- Mauchamp, A.; Chauvelon, P.; Grillas, P. Restoration of floodplain wetlands: Opening polders along a coastal river in Mediterranean France, Vistre marshes. Ecol. Eng. 2002, 18, 619–632. [Google Scholar] [CrossRef]
- Liu, L.C.; Li, S.Z.; Duan, Z.H.; Wang, T.; Zhang, Z.S.; Li, X.R. Effects of microbiotic crusts on dew deposition in the restored vegetation area at Shapotou, Northwest China. J. Hydrol. 2006, 328, 331–337. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, L.C.; Li, X.R.; Zhang, J.G.; He, M.Z.; Tan, H.J. Evaporation properties of a revegetated area of the Tengger Desert, North China. J. Arid Environ. 2008, 72, 964–973. [Google Scholar] [CrossRef]
- Wang, X.P.; Berndtsson, R.; Li, X.R.; Kang, E.S. Water balance change for a re-vegetated xerophyte shrub area. Hydrol. Sci. J. 2004, 49, 283–295. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yu, D.; Zhang, W.J.; You, W.X.; He, X.D. Plant community features and ecological water demands of wetlands for the Ningxia Habahu National Nature Reserve. J. Tianjin Norm. Univ. Nat. Sci. 2019, 39, 50–55. [Google Scholar] [CrossRef]
- Song, X.; Hao, X.M.; Song, B.Q.; Zhao, X.Y.; Wu, Z.Z.; Wang, X.L.; Du, J.Y.; Huan, W.G.; Riaz, M.; Li, X.F.; et al. The oxidative damage and morphological changes of Sugar Beet (Beta vulgaris L.) leaves at seedlings stage exposed to Boron Deficiency in hydroponics. Sugar Tech. 2022, 24, 532–541. [Google Scholar] [CrossRef]
- Su, Y.G.; Li, X.R.; Cheng, Y.W.; Tan, H.J.; Jia, R.L. Effects of biological soil crusts on emergence of desert vascular plants in North China. Plant Ecol. 2007, 191, 11–19. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, X.R.; Wang, T.; Wang, X.P.; Xue, Q.W.; Liu, L.C. Distribution and seasonal dynamics of roots in a revegetated stand of Artemisia ordosica Kracsh. in the Tengger Desert (North China). Arid Land Res. Manag. 2008, 22, 195–211. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, J.G.; Wang, X.P.; Liu, L.C.; Xiao, H.L. Study on soil microbiotic crust and its influences on sand fixing vegetation in arid desert region. Acta Bot. Sin. 2000, 42, 965–970. [Google Scholar]
- Zhang, Y.; Liu, T.; Guo, J.; Tan, Z.; Dong, W.; Wang, H. Changes in the understory diversity of secondary Pinus tabulaeformis forests are the result of stand density and soil properties. Glob. Ecol. Conserv. 2021, 28, e01628. [Google Scholar] [CrossRef]
- Xu, M.P.; Wang, J.Y.; Zhu, Y.F.; Han, X.H.; Ren, C.J.; Yang, G.H. Plant biomass and soil nutrients mainly explain the variation of soil microbial communities during secondary succession on the Loess Plateau. Microb. Ecol. 2022, 83, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, X.R.; Song, W.M.; Gao, Y.P.; Zheng, J.G.; Jia, R.L. Effects of crust and shrub patches on runoff, sedimentation, and related nutrient (C, N) redistribution in the desertified steppe zone of the Tengger Desert, Northern China. Geomorphlogy 2008, 96, 221–232. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Zhong, Z.; Guo, S.; Han, X.; Yang, G.; Ren, C.; Chen, Z.; Dai, Y.; Qiao, W. Vegetation restoration alters the diversity and community composition of soil nitrogen-fixing microorganisms in the Loess Hilly Region of China. Soil Sci. Soc. Am. J. 2019, 83, 1378–1386. [Google Scholar] [CrossRef]
- Xu, Y.D.; Wang, T.; Li, H.; Ren, C.J.; Chen, J.W.; Yang, G.H.; Han, X.H.; Feng, Y.Z.; Ren, G.X.; Wang, X.J. Variations of soil nitrogen-fixing microorganism communities and nitrogen fractions in a Robinia pseudoacacia chronosequence on the Loess Plateau of China. Catena 2019, 174, 316–323. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.L.; Zhang, Y.L.; Wang, Z.X.; Zhang, Q.F. Soil nitrogen dynamics following short-term revegetation in the water level fluctuation zone of the Three Gorges Reservoir, China. Ecol. Eng. 2012, 38, 37–44. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Hu, W.F.; Zhang, W.L.; Zhang, L.H.; Chen, X.Y.; Lin, W.; Zeng, C.S.; Tong, C. Stoichiometric characteristics of nitrogen and phosphorus in major wetland vegetation of China. Chin. J. Plant Ecol. 2014, 38, 1041–1052. [Google Scholar] [CrossRef]
- Xiao, Y.; Tao, Y.; Zhang, Y.M. Biomass allocation and leaf stoichiometric characteristics in four desert herbaceous plants during different growth periods in the Gurbantünggüt Desert, China. Chin. J. Plant Ecol. 2014, 38, 929–940. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar] [CrossRef]
- Ellison, A.M. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biol. 2006, 8, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Lei, J.Q.; Xu, X.W.; Tang, Q.L.; Gao, P.; Wang, Y.D. The stoichiometric characteristics of C, N, P for artificial plants and soil in the hinterland of Taklimakan Desert. Acta Ecol. Sin. 2013, 33, 5760–5767. [Google Scholar] [CrossRef]
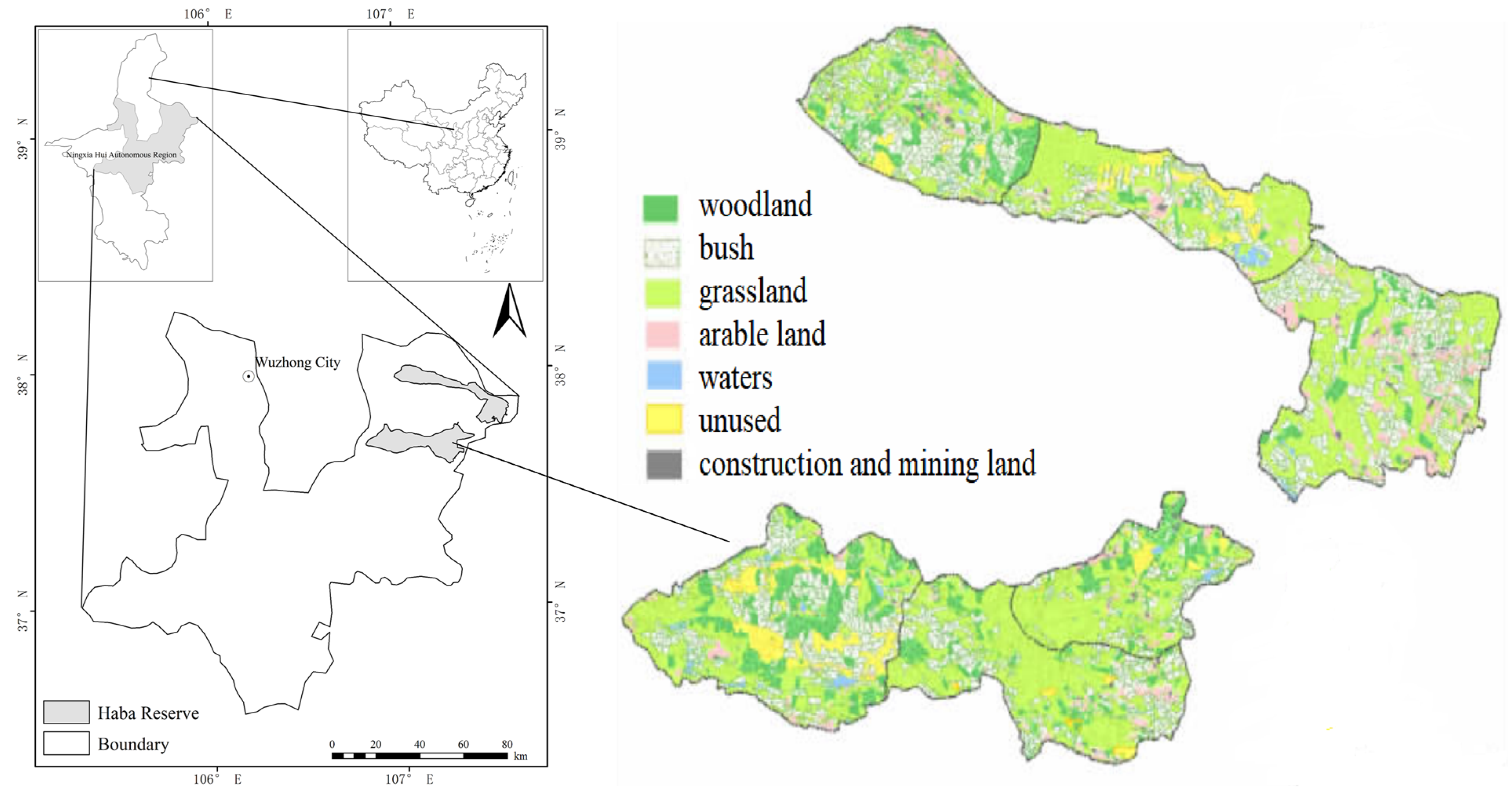





| Name | Location | Altitude (m) | Wetland Type | Composition | Soil Type |
|---|---|---|---|---|---|
| Habahu wetland | 107°7′ E 37°42′ N | 1385–1510 | Seasonal lagoon | Lakes, swamps, dunes, and wetland meadows | Sandy soil, saline soil, and fluvo-aquic soil |
| Huamahu wetland | 107°23′ E 37°44′ N | 1345–1350 | Reservoir | Surface water, swamp, and wetland meadow | Sandy soil, saline soil, and fluvo-aquic soil |
| Distance from the Lake | Species | Density (ind/m2) | Biomass (g) | Height (cm) | Important Value | Niche Breadth |
|---|---|---|---|---|---|---|
| 0 m | Phragmites communis | 415 | 568.5 | 76 | 224.1 | 2.988 |
| Scirpus triqueter | 47 | 42.1 | 27 | 32.5 | 3.829 | |
| Aneurolepidium angustus | 7 | 2.6 | 53 | 32.7 | 2.309 | |
| SmallAcorus calamus | 3 | 5.1 | 16 | 10.8 | 2.987 | |
| 10 m | Aneurolepidium angustus | 277 | 418.3 | 78 | 201.3 | 3.184 |
| Phragmites communis | 19 | 27.4 | 66 | 40.6 | 3.29 | |
| Artemisia scoparia | 23 | 11.9 | 39 | 26.6 | 3.484 | |
| Sonchus brachyotus | 16 | 34.7 | 13 | 17.6 | 3.408 | |
| Setaria viridis | 3 | 2.4 | 28 | 13.9 | 2.255 | |
| 20 m | Heteropappus altaicus | 5 | 12.2 | 16 | 31.3 | 2.953 |
| Aneurolepidium angustus | 16 | 15.5 | 45 | 65.5 | 2.96 | |
| Artemisia scoparia | 23 | 13.6 | 35 | 70.1 | 2.93 | |
| Phragmites communis | 7 | 3.2 | 27 | 26.8 | 2.719 | |
| Setaria viridis | 4 | 2.8 | 24 | 20.2 | 2.581 | |
| Lactuca tatarica | 2 | 4.1 | 11 | 13.4 | 2.897 | |
| Pennisetum centrasiaticum | 3 | 1.2 | 19 | 14.4 | 2.442 | |
| Leonurus japonicus | 1 | 2.9 | 38 | 21.3 | 2.095 | |
| Astragalus laxmannii Jacquin | 1 | 18.2 | 26 | 37.1 | 2.84 |
| Distance from the Lake | Species | Total Density (ind/m2) | Total Biomass (g) | Height (cm) | Important Value | Niche Breadth |
|---|---|---|---|---|---|---|
| 0 m | Phragmites communis | 58 | 224.7 | 39 | 147.5 | 2.854 |
| Leymus secalinus | 44 | 16.8 | 25 | 56.3 | 3.358 | |
| Artemisia scoparia | 27 | 38.4 | 17 | 45.3 | 3.588 | |
| Artemisia argyi | 4 | 7.5 | 35 | 30.7 | 2.661 | |
| Chenopodium glaucum | 2 | 6.3 | 23 | 20.2 | 2.705 | |
| 10 m | Leymus secalinus | 121 | 145.9 | 28 | 140.8 | 3.358 |
| Artemisia scoparia | 29 | 39.1 | 26 | 45.3 | 3.812 | |
| Sophora alopecuroides | 7 | 31.7 | 39 | 36.2 | 3.339 | |
| Phragmites communis | 5 | 4.6 | 31 | 20.8 | 2.619 | |
| Corispermum hyssopifolium | 3 | 11.4 | 7 | 9.6 | 3.43 | |
| Swainsonia salsula | 1 | 11.4 | 28 | 19.4 | 2.769 | |
| Astragalus melilotoides | 1 | 27.2 | 33 | 27.8 | 3.043 | |
| 20 m | Leymus secalinus | 15 | 18.3 | 38 | 46.7 | 3.328 |
| Sophora alopecuroides | 7 | 18.9 | 32 | 35.1 | 3.249 | |
| Artemisia scoparia | 37 | 45.2 | 34 | 89.5 | 3.364 | |
| Phragmites communis | 7 | 8.3 | 42 | 32 | 2.744 | |
| Astragalus laxmannii Jacquin | 3 | 18.3 | 29 | 28.7 | 3.107 | |
| Pennisetum centrasiaticum | 6 | 1.3 | 7 | 11 | 3.085 | |
| Corispermum hyssopifolium | 4 | 10.1 | 13 | 17.3 | 3.362 | |
| Chenopodium glaucum | 2 | 7.4 | 18 | 15.2 | 2.956 | |
| Heteropappus altaicus | 3 | 10.9 | 14 | 17.1 | 3.286 | |
| Clematis urophylla | 1 | 5.3 | 6 | 7.4 | 3.236 |
| Site | Distance away from the Lake | Total Density (ind/m2) | Total Biomass (g) | Magalef Index | Shannon–Wiener Index | Simpson Index | Pielou Evenness Index |
|---|---|---|---|---|---|---|---|
| Huamahu | 0 m | 472 | 618.3 | 0.487 | 0.631 | 0.217 | 1.048 |
| 10 m | 338 | 494.7 | 0.687 | 1.001 | 0.319 | 1.433 | |
| 20 m | 62 | 73.7 | 1.938 | 2.502 | 0.781 | 2.622 | |
| Habahu | 0 m | 135 | 293.7 | 0.815 | 1.756 | 0.673 | 2.512 |
| 10 m | 167 | 271.3 | 1.172 | 1.311 | 0.444 | 1.552 | |
| 20 m | 85 | 144 | 2.026 | 2.578 | 0.764 | 2.578 |
| Plant | Observations | Mean | Min | Max | Extreme | Standard Error | Standard Deviation | Kurtosis | Skewness |
|---|---|---|---|---|---|---|---|---|---|
| Artemisia ordosica | 25 | 11.27 | 4.93 | 25.02 | 20.09 | 0.97 | 4.87 | 2.16 | 1.34 |
| Sophora alopecuroides | 25 | 17.08 | 7.56 | 23.88 | 16.32 | 0.86 | 4.32 | −0.66 | −0.35 |
| Stipa bungeana | 25 | 20.84 | 13.34 | 38.78 | 25.45 | 1.01 | 5.04 | 5.84 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Deng, L.; Sun, T.; Fei, K.; Song, N.; Wang, X. Features of Plant Community and Driving Forces of Plant Community Succession in the Typical Desert Wetlands. Sustainability 2022, 14, 8430. https://doi.org/10.3390/su14148430
Zhang L, Deng L, Sun T, Fei K, Song N, Wang X. Features of Plant Community and Driving Forces of Plant Community Succession in the Typical Desert Wetlands. Sustainability. 2022; 14(14):8430. https://doi.org/10.3390/su14148430
Chicago/Turabian StyleZhang, Liping, Longzhou Deng, Tianyu Sun, Kai Fei, Naiping Song, and Xing Wang. 2022. "Features of Plant Community and Driving Forces of Plant Community Succession in the Typical Desert Wetlands" Sustainability 14, no. 14: 8430. https://doi.org/10.3390/su14148430
APA StyleZhang, L., Deng, L., Sun, T., Fei, K., Song, N., & Wang, X. (2022). Features of Plant Community and Driving Forces of Plant Community Succession in the Typical Desert Wetlands. Sustainability, 14(14), 8430. https://doi.org/10.3390/su14148430






