The Role of NO in the Amelioration of Heavy Metal Stress in Plants by Individual Application or in Combination with Phytohormones, Especially Auxin
Abstract
1. Introduction
2. Origin and Sources of NO
3. Correlation between ROS and NO under HM Stress in Plants
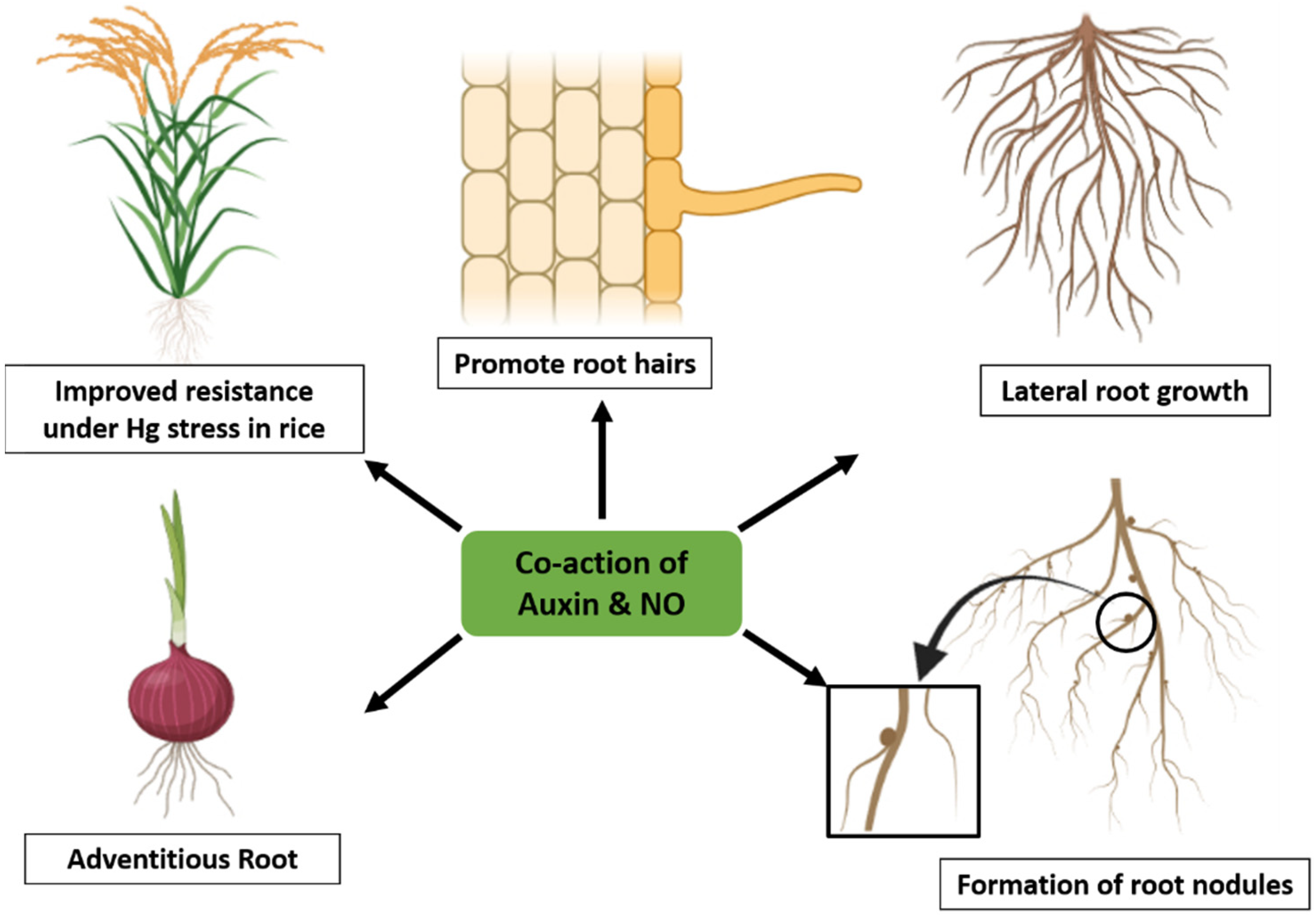
4. The Interplay of NO and Auxin to Reduce Heavy Metal Stress in Plants
5. NO Production in Different Plants under Heavy Metal Toxicity
6. The Role of NO in Overcoming Stress Caused by Different Heavy Metals
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akpor, O.; Muchie, M. Remediation of Heavy Metals in Drinking Water and Wastewater Treatment Systems: Processes and Applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar]
- Keswani, C. Proteomics Studies of Thermotolerant Strain of Trichoderma spp. Ph.D. Thesis, Banaras Hindu University, Varanasi, India, 2015; p. 126. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Mol. Clin. Environ. Toxicol. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing Heavy Metal Sources in Agricultural Soils of an European Mediterranean Area by Multivariate Analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Gautam, S.; Anjani, K.; Srivastava, N. In Vitro Evaluation of Excess Copper Affecting Seedlings and Their Biochemical Characteristics in Carthamus tinctorius L. (Variety PBNS-12). Physiol. Mol. Biol. Plants 2016, 22, 121–129. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Prasad, S.M.; Maurya, J.N. Modulation of Manganese Toxicity in Pisum sativum L. Seedlings by Kinetin. Sci. Hortic. 2010, 4, 467–474. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of Zinc on Scots Pine (Pinus sylvestris L.) Seedlings Grown in Hydroculture. Plant Physiol. Biochem. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of Deleterious Effects of Chromium Phytotoxicity and Photosynthesis in Wheat Plant. Photosynthetica 2016, 54, 185–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, G.H.; Liu, P.; Song, J.M.; Xu, G.D.; Cai, M.Z. Morphological and Physiological Responses of Root Tip Cells to Fe2+ Toxicity in Rice. Acta Physiol. Plant 2011, 33, 683–689. [Google Scholar] [CrossRef]
- Roy, M.; McDonald, L.M. Metal Uptake in Plants and Health Risk Assessments in Metal-Contaminated Smelter Soils. Land Degrad. Dev. 2015, 26, 785–792. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Klavins, M. Transfer of Metals in Food Chain: An Example with Copper and Lettuce. Environ. Clim. Technol. 2012, 10, 21–24. [Google Scholar] [CrossRef][Green Version]
- Sanità di Toppi, L.; Gabbrielli, R. Response to Cadmium in Higher Plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Jaspers, P.; Kangasjärvi, J. Reactive Oxygen Species in Abiotic Stress Signaling. Physiol. Plant 2010, 138, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, e872875. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular Redox Compartmentation and ROS-Related Communication in Regulation and Signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility. A Review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Popova, L.P. Functions and Toxicity of Cadmium in Plants: Recent Advances and Future Prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar] [CrossRef]
- Etim, E.E. Phytoremediation and Its Mechanisms: A Review. Int. J. Environ. Bioeng. 2012, 2, 120–136. [Google Scholar]
- Kaimi, E.; Mukaidani, T.; Tamaki, M. Screening of Twelve Plant Species for Phytoremediation of Petroleum Hydrocarbon-Contaminated Soil. Plant Prod. Sci. 2007, 10, 211–218. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Wahid, A.; Farooq, M.; Maqbool, N.; Arfan, M. Phytoremediation of Soil Cadmium Using Chenopodium Species. Pak. J. Agri. Sci. 2012, 49, 435–445. [Google Scholar]
- Chakraborty, N.; Chandra, S.; Acharya, K. Sublethal Heavy Metal Stress Stimulates Innate Immunity in Tomato. Sci. World J. 2015, 2015, 208649. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Kaur, S.; Batish, D.R.; Sharma, V.P.; Sharma, N.; Kohli, R.K. Nitric Oxide Alleviates Arsenic Toxicity by Reducing Oxidative Damage in the Roots of Oryza Sativa (Rice). Nitric Oxide 2009, 20, 289–297. [Google Scholar] [CrossRef]
- Chakraborty, N.; Acharya, K. “NO Way”! Says the Plant to Abiotic Stress. Plant Gene 2017, 11, 99–105. [Google Scholar] [CrossRef]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric Oxide: A Multitasked Signaling Gas in Plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric Oxide Signaling and Its Crosstalk with Other Plant Growth Regulators in Plant Responses to Abiotic Stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.B.; Corpas, F.J.; Carreras, A.; Sandalio, L.M.; Valderrama, R.; Palma, J.M.; Lupiáñez, J.A.; del Río, L.A. Localization of Nitric-Oxide Synthase in Plant Peroxisomes. J. Biol. Chem. 1999, 274, 36729–36733. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Astier, J.; Rasul, S.; Wawer, I.; Dubreuil-Maurizi, C.; Jeandroz, S.; Wendehenne, D. Current View of Nitric Oxide-Responsive Genes in Plants. Plant Sci. 2009, 177, 302–309. [Google Scholar] [CrossRef]
- Chakraborty, N.; Sarkar, A.; Acharya, K. Elicitor-Mediated Amelioration of Abiotic Stress in Plants. In Molecular Plant Abiotic Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 105–122. ISBN 978-1-119-46366-5. [Google Scholar]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the Origins of Nitric Oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Takahashi, S. An Alternative Pathway for Nitric Oxide Production in Plants: New Features of an Old Enzyme. Trends Plant Sci. 1999, 4, 128–129. [Google Scholar] [CrossRef]
- Campbell, W.H. Structure and Function of Eukaryotic NAD(P)H: Nitrate Reductase. Cell. Mol. Life Sci. 2001, 58, 194–204. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric Oxide in Plants: An Assessment of the Current State of Knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Wilson, I.D.; Neill, S.J.; Hancock, J.T. Nitric Oxide Synthesis and Signalling in Plants. Plant Cell Environ. 2008, 31, 622–631. [Google Scholar] [CrossRef]
- Del Río, L.A. Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Arch. Biochem. Biophys. 2011, 506, 1–11. [Google Scholar] [CrossRef]
- Acharya, K.; Chandra, S.; Chakraborty, N.; Acharya, R. Nitric oxide functions as a signal in induced systemic resistance. Arch Phytopathol. Plant Protect. 2011, 44, 1335–1342. [Google Scholar] [CrossRef]
- Chakraborty, N. Salicylic acid and nitric oxide cross-talks to improve innate immunity and plant vigor in tomato against Fusarium oxysporum stress. Plant Cell Rep. 2021, 40, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Chitosan nanoparticles mitigate Alternaria leaf spot disease of chilli in nitric oxide dependent way. Plant Physiol. Biochem. 2022, 180, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Galatro, A.; Puntarulo, S. An Update to the Understanding of Nitric Oxide Metabolism in Plants. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Khan, M.N., Mohammad, M.M.F., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 3–16. [Google Scholar]
- Chakraborty, N.; Ghosh, S.; Chandra, S.; Sengupta, S.; Acharya, K. Abiotic elicitors mediated elicitation of innate immunity in tomato: An ex vivo comparison. Physiol. Mol. Biol. Plants 2016, 22, 307–320. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Panda, K.; Acharya, K. Chitosan induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol. Biochem. 2017, 115, 298–307. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Unraveling the role of nitric oxide in regulation of defense responses in chilli against Alternaria leaf spot disease. Physiol. Mol. Plant Pathol. 2021, 114, 101621. [Google Scholar] [CrossRef]
- Rasul, S.; Wendehenne, D.; Jeandroz, S. Study of oligogalacturonides-triggered nitric oxide (NO) production provokes new questioning about the origin of NO biosynthesis in plants. Plant Signal. Behav. 2012, 7, 1031–1033. [Google Scholar] [CrossRef]
- Chakraborty, N.; Chandra, S.; Acharya, K. Boosting of innate immunity in chilli. Res. J. Pharm. Technol. 2015, 8, 885–892. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Fernández-Ocaña, A.; Chaki, M.; Luque, F.; Gómez-Rodríguez, M.V.; Colmenero-Varea, P.; Luis, A.; Barroso, J.B. Nitrosative stress in plants. FEBS Lett. 2007, 581, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Rusterucci, C.; Espunya, M.C.; Díaz, M.; Chabannes, M.; Martínez, M.C. S-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol. 2007, 143, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Sarkar, A.; Acharya, K. Biotic elicitor induced nitric oxide production in mitigation of Fusarium wilt of tomato. J. Plant Biochem. Biotechnol. 2021, 30, 960–972. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 2012, 6, 1314–1323. [Google Scholar]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Verma, K.; Mehta, S.K.; Shekhawat, G.S. Nitric Oxide (NO) Counteracts Cadmium Induced Cytotoxic Processes Mediated by Reactive Oxygen Species (ROS) in Brassica Juncea: Cross-Talk between ROS, NO and Antioxidant Responses. Biometals 2013, 26, 255–269. [Google Scholar] [CrossRef]
- Piacentini, D.; Della Rovere, F.; Sofo, A.; Fattorini, L.; Falasca, G.; Altamura, M.M. Nitric Oxide Cooperates with Auxin to Mitigate the Alterations in the Root System Caused by Cadmium and Arsenic. Front. Plant Sci. 2020, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Demecsová, L.; Tamás, L. Reactive Oxygen Species, Auxin and Nitric Oxide in Metal-Stressed Roots: Toxicity or Defence. Biometals 2019, 32, 717–744. [Google Scholar] [CrossRef]
- Freschi, L. Nitric Oxide and Phytohormone Interactions: Current Status and Perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chao, Y.-Y.; Hsu, Y.Y.; Hong, C.-Y.; Kao, C.H. Heme Oxygenase Is Involved in Nitric Oxide- and Auxin-Induced Lateral Root Formation in Rice. Plant Cell Rep. 2012, 31, 1085–1091. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pagnussat, G.C.; Lanteri, M.L.; Lombardo, M.C.; Lamattina, L. Nitric Oxide Mediates the Indole Acetic Acid Induction Activation of a Mitogen-Activated Protein Kinase Cascade Involved in Adventitious Root Development. Plant Physiol. 2004, 135, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Crimi, M.; Cremonese, G.; Spena, A.; Pandolfini, T. Auxin and Nitric Oxide Control Indeterminate Nodule Formation. BMC Plant Biol. 2007, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, F.; Liu, J.; Zhao, Q. Nitric Oxide Affects Rice Root Growth by Regulating Auxin Transport under Nitrate Supply. Front. Plant Sci. 2018, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-M.; Huang, X. Inhibition of Root Meristem Growth by Cadmium Involves Nitric Oxide-Mediated Repression of Auxin Accumulation and Signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Zhu, C. Exogenous Nitric Oxide Mediates Alleviation of Mercury Toxicity by Promoting Auxin Transport in Roots or Preventing Oxidative Stress in Leaves of Rice Seedlings. Acta Physiol. Plant 2015, 37, 194. [Google Scholar] [CrossRef]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Della Rovere, F.; Eiche, E.; Riemann, M.; Altamura, M.M.; Falasca, G. Cadmium and Arsenic Affect Root Development in Oryza Sativa L. Negatively Interacting with Auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Piacentini, D.; Ronzan, M.; Fattorini, L.; Della Rovere, F.; Massimi, L.; Altamura, M.M.; Falasca, G. Nitric Oxide Alleviates Cadmium- But Not Arsenic-Induced Damages in Rice Roots. Plant Physiol. Biochem. 2020, 151, 729–742. [Google Scholar] [CrossRef]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous Nitric Oxide Enhances Cadmium Tolerance of Rice by Increasing Pectin and Hemicellulose Contents in Root Cell Wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric Oxide: The Versatility of an Extensive Signal Molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef]
- Delledonne, M. NO News Is Good News for Plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Planchet, E.; Kaiser, W.M. Nitric Oxide Production in Plants. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan Nanoparticles: A Positive Modulator of Innate Immune Responses in Plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Lead-Induced Stress, Which Triggers the Production of Nitric Oxide (NO) and Superoxide Anion (O2·-) in Arabidopsis Peroxisomes, Affects Catalase Activity. Nitric Oxide 2017, 68, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Planchet, E.; Kaiser, W.M. Nitric Oxide (NO) Detection by DAF Fluorescence and Chemiluminescence: A Comparison Using Abiotic and Biotic NO Sources. J. Exp. Bot. 2006, 57, 3043–3055. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Del Río, L.A. Imaging of Reactive Oxygen Species and Nitric Oxide In Vivo in Plant Tissues. Methods Enzymol. 2008, 440, 397–409. [Google Scholar] [CrossRef]
- Xiong, J.; Fu, G.; Tao, L.; Zhu, C. Roles of Nitric Oxide in Alleviating Heavy Metal Toxicity in Plants. Arch. Biochem. Biophys. 2010, 497, 13–20. [Google Scholar] [CrossRef]
- Illés, P.; Schlicht, M.; Pavlovkin, J.; Lichtscheidl, I.; Baluska, F.; Ovecka, M. Aluminium Toxicity in Plants: Internalization of Aluminium into Cells of the Transition Zone in Arabidopsis Root Apices Related to Changes in Plasma Membrane Potential, Endosomal Behaviour, and Nitric Oxide Production. J. Exp. Bot. 2006, 57, 4201–4213. [Google Scholar] [CrossRef]
- De Michele, R.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; Di Valentin, M.; Careri, M.; Zottini, M.; Sanità di Toppi, L.; Lo Schiavo, F. Nitric Oxide Is Involved in Cadmium-Induced Programmed Cell Death in Arabidopsis Suspension Cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.-P.; Pugin, A.; Wendehenne, D. Nitric Oxide Contributes to Cadmium Toxicity in Arabidopsis by Promoting Cadmium Accumulation in Roots and by Up-Regulating Genes Related to Iron Uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef]
- Arnaud, N.; Murgia, I.; Boucherez, J.; Briat, J.-F.; Cellier, F.; Gaymard, F. An Iron-Induced Nitric Oxide Burst Precedes Ubiquitin-Dependent Protein Degradation for Arabidopsis AtFer1 Ferritin Gene Expression. J. Biol. Chem. 2006, 281, 23579–23588. [Google Scholar] [CrossRef]
- Bartha, B.; Kolbert, Z.; Erdei, L. Nitric Oxide Production Induced by Heavy Metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol. Szeged. 2005, 49, 9–12. [Google Scholar]
- Kopyra, M.; Stachoń-Wilk, M.; Gwóźdź, E.A. Effects of Exogenous Nitric Oxide on the Antioxidant Capacity of Cadmium-Treated Soybean Cell Suspension. Acta Physiol. Plant 2006, 28, 525–536. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Freshour, G.; Dikalova, A.; Griendling, K.; Baylis, C. Vitamin E Reduces Glomerulosclerosis, Restores Renal Neuronal NOS, and Suppresses Oxidative Stress in the 5/6 Nephrectomized Rat. Am. J. Physiol.-Renal Physiol. 2007, 292, F1404–F1410. [Google Scholar] [CrossRef] [PubMed]
- Valentovicová, K.; Halusková, L.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of Cadmium on Diaphorase Activity and Nitric Oxide Production in Barley Root Tips. J. Plant Physiol. 2010, 167, 10–14. [Google Scholar] [CrossRef]
- Xu, J.; Yin, H.; Li, Y.; Liu, X. Nitric Oxide Is Associated with Long-Term Zinc Tolerance in Solanum nigrum. Plant Physiol. 2010, 154, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, W.; Xu, H.; Chen, Y.; He, Z.; Ma, M. Nitric Oxide Modulates Cadmium Influx during Cadmium-Induced Programmed Cell Death in Tobacco BY-2 Cells. Planta 2010, 232, 325–335. [Google Scholar] [CrossRef]
- Groppa, M.D.; Rosales, E.P.; Iannone, M.F.; Benavides, M.P. Nitric Oxide, Polyamines and Cd-Induced Phytotoxicity in Wheat Roots. Phytochemistry 2008, 69, 2609–2615. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of Abscisic Acid (ABA) on Growth, Water Relations and Glycinebetaine Metabolism in Two Maize (Zea mays L.) Cultivars under Drought Stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef]
- Tewari, R.K.; Hahn, E.-J.; Paek, K.-Y. Modulation of Copper Toxicity-Induced Oxidative Damage by Nitric Oxide Supply in the Adventitious Roots of Panax Ginseng. Plant Cell Rep. 2008, 27, 171–181. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.; Corpas, F.J.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium Effect on Oxidative Metabolism of Pea (Pisum sativum L.) Roots. Imaging of Reactive Oxygen Species and Nitric Oxide Accumulation In Vivo. Plant Cell Environ. 2006, 29, 1532–1544. [Google Scholar] [CrossRef]
- Barroso, J.B.; Corpas, F.J.; Carreras, A.; Rodríguez-Serrano, M.; Esteban, F.J.; Fernández-Ocaña, A.; Chaki, M.; Romero-Puertas, M.C.; Valderrama, R.; Sandalio, L.M.; et al. Localization of S-Nitrosoglutathione and Expression of S-Nitrosoglutathione Reductase in Pea Plants under Cadmium Stress. J. Exp. Bot. 2006, 57, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular Response of Pea Plants to Cadmium Toxicity: Cross Talk between Reactive Oxygen Species, Nitric Oxide, and Calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yun, B.-W.; Spoel, S.H.; Loake, G.J. A Sleigh Ride through the SNO: Regulation of Plant Immune Function by Protein S-Nitrosylation. Curr. Opin. Plant Biol. 2012, 15, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Yin, H.; Liu, X.; Sun, H.; Mi, Q. Exogenous Nitric Oxide Improves Antioxidative Capacity and Reduces Auxin Degradation in Roots of Medicago truncatula Seedlings under Cadmium Stress. Plant Soil 2009, 326, 321. [Google Scholar] [CrossRef]
- Mahmood, T.; Gupta, K.J.; Kaiser, W.M. Cadmium stress stimulates nitric oxide production by wheat roots. Pak. J. Bot. 2009, 41, 6. [Google Scholar]
- Chen, F.; Wang, F.; Sun, H.; Cai, Y.; Mao, W.; Zhang, G.; Vincze, E.; Wu, F. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare). J. Plant Growth Regul. 2010, 29, 394–408. [Google Scholar] [CrossRef]
- Meng, Y.; Jing, H.; Huang, J.; Shen, R.; Zhu, X. The Role of Nitric Oxide Signaling in Plant Responses to Cadmium Stress. Int. J. Mol. Sci. 2022, 23, 6901. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Chakraborty, A.; Rai, R.; Bera, B.; Acharya, K. Abiotic elicitor-mediated improvement of innate immunity in Camellia sinensis. J. Plant Growth Regul. 2014, 33, 849–859. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric Oxide Regulates Plant Responses to Drought, Salinity, and Heavy Metal Stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Ekmekci, Y.; Bohms, A.; Thomson, J.A.; Mundree, S.G. Photochemical and Antioxidant Responses in the Leaves of Xerophyta viscosa Baker and Digitaria sanguinalis L. under Water Deficit. Z. Naturforsch. C J. Biosci. 2005, 60, 435–443. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Esposito, E. Biotechnological Strategies Applied to the Decontamination of Soils Polluted with Heavy Metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef]
- Paul, A.; Sarkar, A.; Acharya, K.; Chakraborty, N. Fungal Elicitor-Mediated Induction of Innate Immunity in Catharanthus roseus Against Leaf Blight Disease Caused by Alternaria alternata. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Wang, H.-H.; Huang, J.-J.; Bi, Y.-R. Nitrate Reductase-Dependent Nitric Oxide Production Is Involved in Aluminum Tolerance in Red Kidney Bean Roots. Plant Sci. 2010, 179, 281–288. [Google Scholar] [CrossRef]
- He, H.-Y.; He, L.-F.; Gu, M.-H.; Li, X.-F. Nitric Oxide Improves Aluminum Tolerance by Regulating Hormonal Equilibrium in the Root Apices of Rye and Wheat. Plant Sci. 2012, 183, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric Oxide Mediated Transcriptional Modulation Enhances Plant Adaptive Responses to Arsenic Stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, Q.; Wang, Y.; Chu, L.; Li, X.; Xu, Y. Effects of Nitric Oxide on Alleviating Cadmium Stress in Typha angustifolia. Plant Growth Regul. 2016, 78, 243–251. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Ma, Z.; Wang, J. Bacillus amyloliquefaciens SAY09 Increases Cadmium Resistance in Plants by Activation of Auxin-Mediated Signaling Pathways. Genes 2017, 8, 173. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous Sodium Nitroprusside and Glutathione Alleviate Copper Toxicity by Reducing Copper Uptake and Oxidative Damage in Rice (Oryza sativa L.) Seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Chakraborty, A.; Rai, R.; Bera, B.; Acharya, K. Induction of defence response against blister blight by calcium chloride in tea. Arch. Phytopathol. Plant Prot. 2014, 47, 2400–2409. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Ramakrishnan, M.; Liu, G.; Li, Y. Nitric Oxide Ameliorates Plant Metal Toxicity by Increasing Antioxidant Capacity and Reducing Pb and Cd Translocation. Antioxidants 2021, 10, 1981. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, G.; Arora, K.; Kohli, R.K. Nitric Oxide (as Sodium Nitroprusside) Supplementation Ameliorates Cd Toxicity in Hydroponically Grown Wheat Roots. Environ. Exp. Bot. 2008, 63, 158–167. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, W.; Xu, L.; Kong, J.; Liu, S.; He, Z. Nitric Oxide Can Induce Tolerance to Oxidative Stress of Peanut Seedlings under Cadmium Toxicity. Plant Growth Regul. 2016, 79, 19–28. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Lombardo, C.; Lamattina, L. Nitric Oxide: An Active Nitrogen Molecule That Modulates Cellulose Synthesis in Tomato Roots. New Phytol. 2008, 179, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Ghosh, S.; Paul, S.; Mazumdar, S.; Das, G.; Das, S. Pre-treatment of seeds with salicylic acid attenuates cadmium chloride-induced oxidative damages in the seedlings of mungbean (Vigna radiata L. Wilczek). Acta Physiol. Plant. 2016, 38, 11. [Google Scholar] [CrossRef]

| Name of Species | HM Stress | Type of Tissue Exposed to HMs | Duration of Exposure to HMs | Level of NO Content in the Tissue | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Al | Root | 1 h | Fall | [81] |
| Al | Root | 3 days | Both rise and fall | [81] | |
| Cd | Cell suspension | 72 h | Rise | [82] | |
| Cd | Leaf | 96 h | Rise | [35,83] | |
| Fe | Cell suspension | 30 min | Rise | [84] | |
| Pb | Seedling | 14 days | Rise | [77] | |
| Brassica juncea | Cu | Root | 7 days | Rise | [85] |
| Zn | Root | 7 days | Rise | [85] | |
| Glycine max | Cd | Cell suspension | 72 h | Rise | [86] |
| Hibiscus moscheutos | Al | Root | 20 min | Fall | [87] |
| Hordeum vulgare | Cd | Root | 24 h | Rise | [88] |
| Medicago truncatula | Cd | Root | 48 h | Fall | [89] |
| Nicotiana tabacum | Cd | Cell suspension | 12 h | Rise | [90] |
| Triticum aestivum | Cd | Root | 5 days | Rise | [91] |
| Cd | Root | 3 h | Rise | [91] | |
| Oryza sativa | Cd | Root | 24 h | Fall | [72] |
| Cd | Root and shoot | 7 days | Fall | [92] | |
| Panax ginseng | Cu | Root | 24 h | Rise | [93] |
| Pisum sativum | Cd | Root | 7 days | Rise | [85] |
| Cd | Root | 15 days | Fall | [94] | |
| Cd | Leaf | 14 days | Fall | [95,96] | |
| Cu | Root | 7 days | Rise | [85] | |
| Zn | Root | 7 days | Rise | [85] | |
| Pogonatherum crinitum | Pb | Root | 24 h | Rise | [97] |
| Solanum nigrum | Zn; Zn + Fe | Root | 0–10 days | First rose up to Day 2–3, then began to fall | [89] |
| Application of NO or NO Donor Individually or in Combination with Other Phytohormones | Name of Heavy Metal Causing Stress | Plant Species under HM Stress | Role of NO in Alleviating HM Stress | References |
|---|---|---|---|---|
| Indirect application of NO | Al | Phaseolus vulgaris | Reducing oxidative stress in the roots | [103] |
| Exogenous NO application | Al | Secale cereale and Triticum aestivum seedlings | Reducing Al accumulation in the apical zone of roots to promote Al tolerance | [104,108] |
| NO individually | As | Oryza sativa | Minimizing the levels of ROS and malondialdehyde (MDA) | [29] |
| NO individually | As | Oryza sativa | Modulating regulatory networks involved in JA biosynthesis. | [105,109] |
| NO individually | Cd | Typha angustifolia | Improvement in the plant growth and development, total yield of biomass by suppressing Cd stress | [106,110] |
| NO individually | Cd | Oryza sativa | Reducing alterations in the root system | [73] |
| NO individually | Cd | Oryza sativa | Stopping Cd accumulation by enhancing the pectin and hemicelluloses content in the cell wall of the root system | [72] |
| Indirect application of NO downstream of auxin, in presence of a bacterium, Bacillus amyloliquefaciens SAY09 | Cd | Arabidopsis sp. | Activating auxin-mediated signaling pathway to bring Cd toxicity under control | [111,112] |
| SNP at low concentrations | Cd | Oryza sativa | Promoting cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall | [72] |
| SNP along with glutathione | Cu | Oryza sativa | Reducing Cu uptake and oxidative damage | [113] |
| Indirect application of NO | Cu | Panax ginseng | Reducing oxidative damage in the adventitious roots | [114] |
| NO donor | Cd and Pb | Bamboo species (Arundinaria pygmaea) | Increasing antioxidant activity, protein content, photosynthetic properties, plant biomass, and plant limiting metal translocation from roots to shoots, and diminishing metal accumulation in the roots, shoots, and stems | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, R.; Sarkar, A.; Acharya, K.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Sushkova, S.; Chakraborty, N. The Role of NO in the Amelioration of Heavy Metal Stress in Plants by Individual Application or in Combination with Phytohormones, Especially Auxin. Sustainability 2022, 14, 8400. https://doi.org/10.3390/su14148400
Ganguly R, Sarkar A, Acharya K, Keswani C, Minkina T, Mandzhieva S, Sushkova S, Chakraborty N. The Role of NO in the Amelioration of Heavy Metal Stress in Plants by Individual Application or in Combination with Phytohormones, Especially Auxin. Sustainability. 2022; 14(14):8400. https://doi.org/10.3390/su14148400
Chicago/Turabian StyleGanguly, Retwika, Anik Sarkar, Krishnendu Acharya, Chetan Keswani, Tatiana Minkina, Saglara Mandzhieva, Svetlana Sushkova, and Nilanjan Chakraborty. 2022. "The Role of NO in the Amelioration of Heavy Metal Stress in Plants by Individual Application or in Combination with Phytohormones, Especially Auxin" Sustainability 14, no. 14: 8400. https://doi.org/10.3390/su14148400
APA StyleGanguly, R., Sarkar, A., Acharya, K., Keswani, C., Minkina, T., Mandzhieva, S., Sushkova, S., & Chakraborty, N. (2022). The Role of NO in the Amelioration of Heavy Metal Stress in Plants by Individual Application or in Combination with Phytohormones, Especially Auxin. Sustainability, 14(14), 8400. https://doi.org/10.3390/su14148400










