Dynamic Modelling of Enzymatic Hydrolysis of Oil Using Lipase Immobilized on Zeolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Enzymes
2.2. Enzyme Immobilization
2.3. Enzymatic Reaction Rate
2.4. Characterization of L-Zeolite
3. Results and Discussion
3.1. Characteristics of L-Zeolite
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. Surface Morphology
3.1.4. Porosity and Surface Area
3.2. Lipase Immobilization
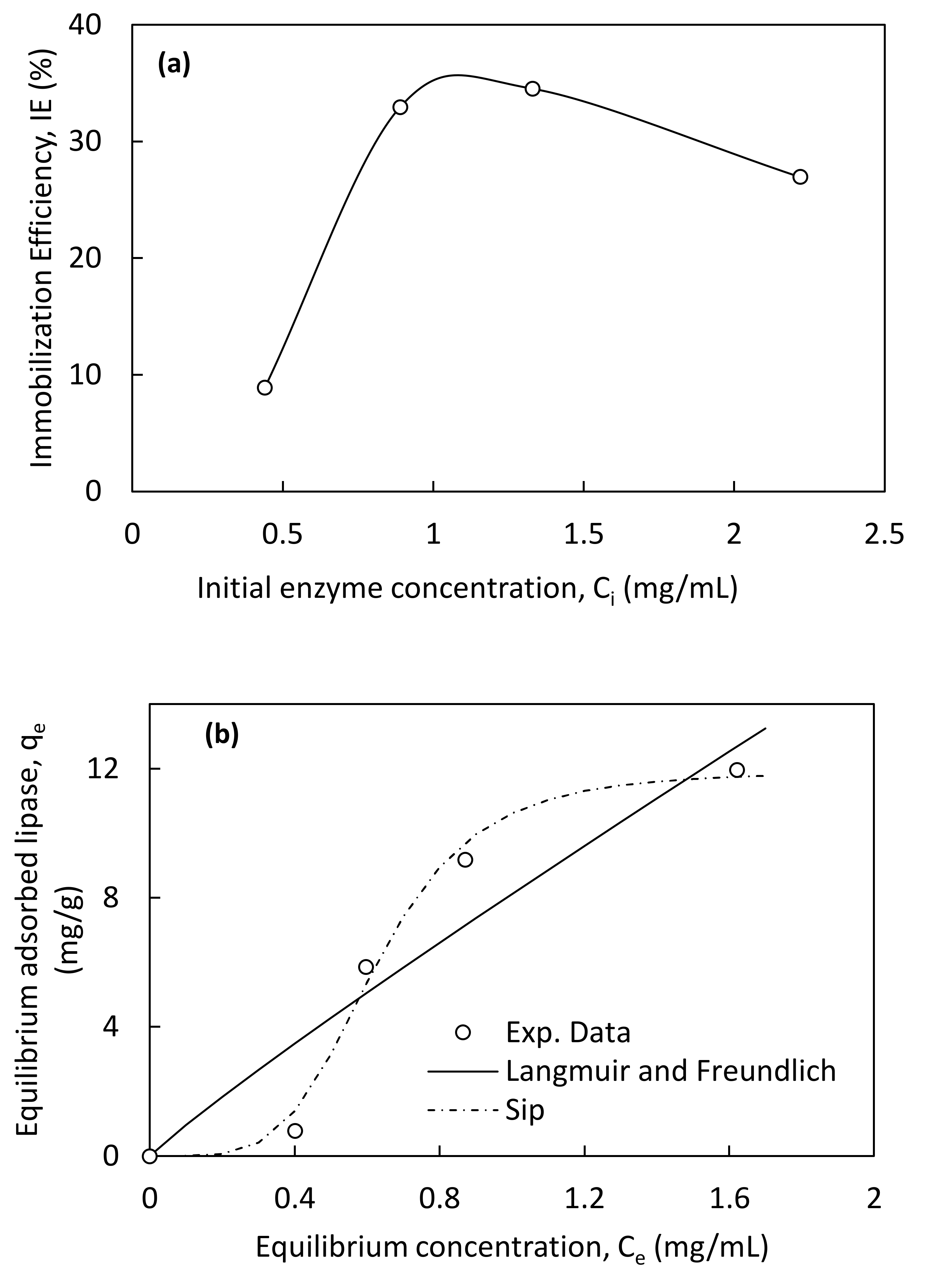
3.2.1. Immobilization Efficiency
3.2.2. Adsorption Isotherms
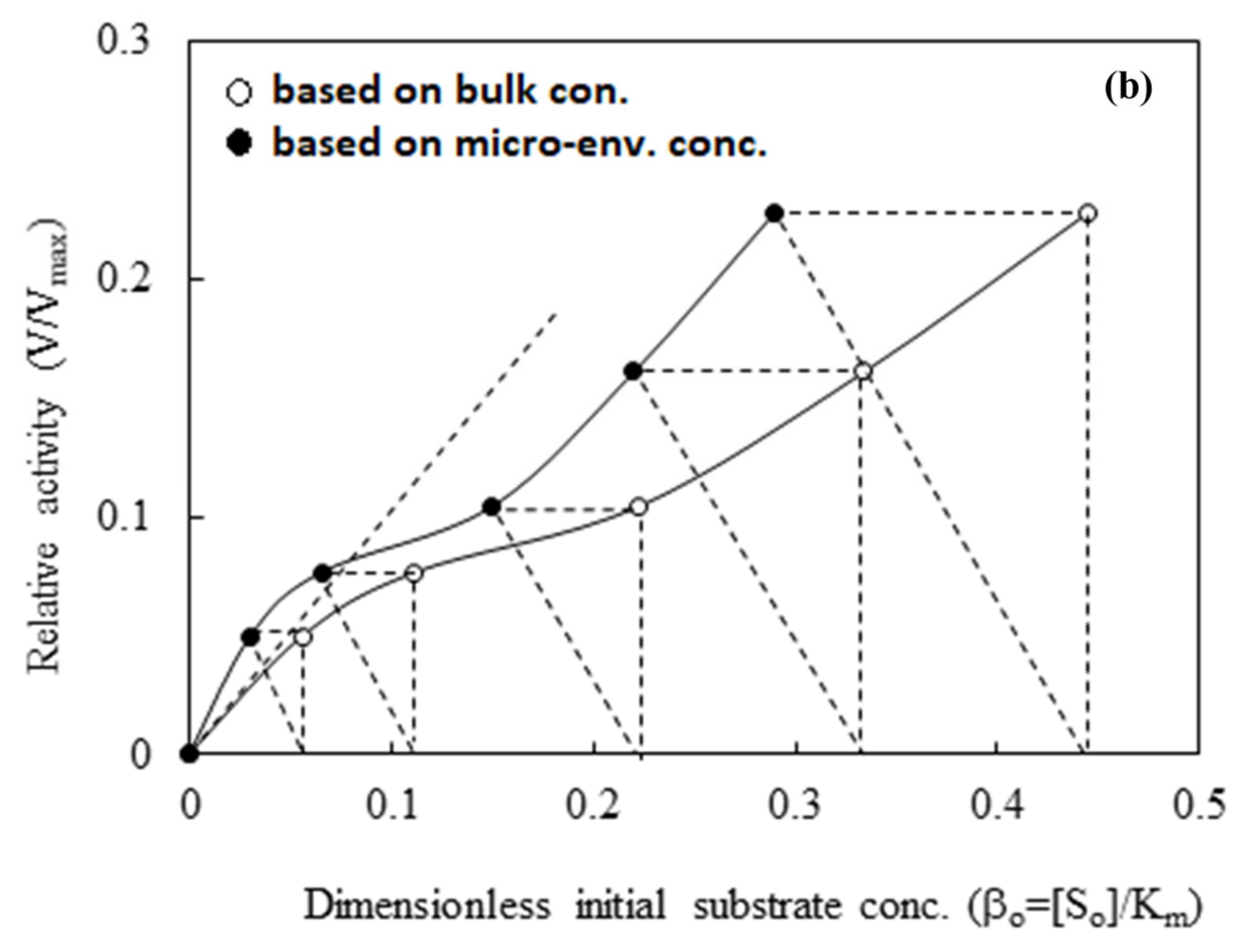
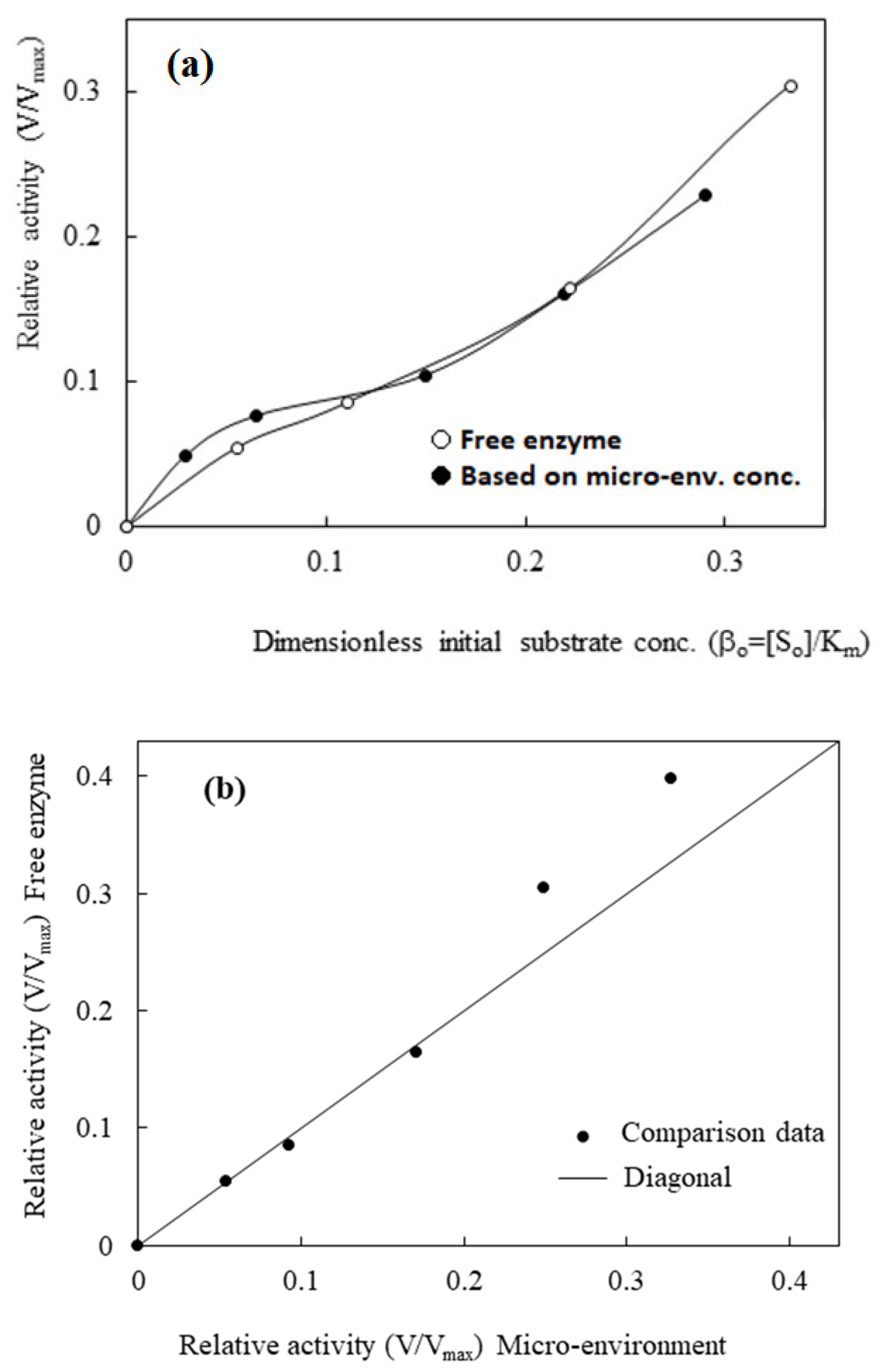
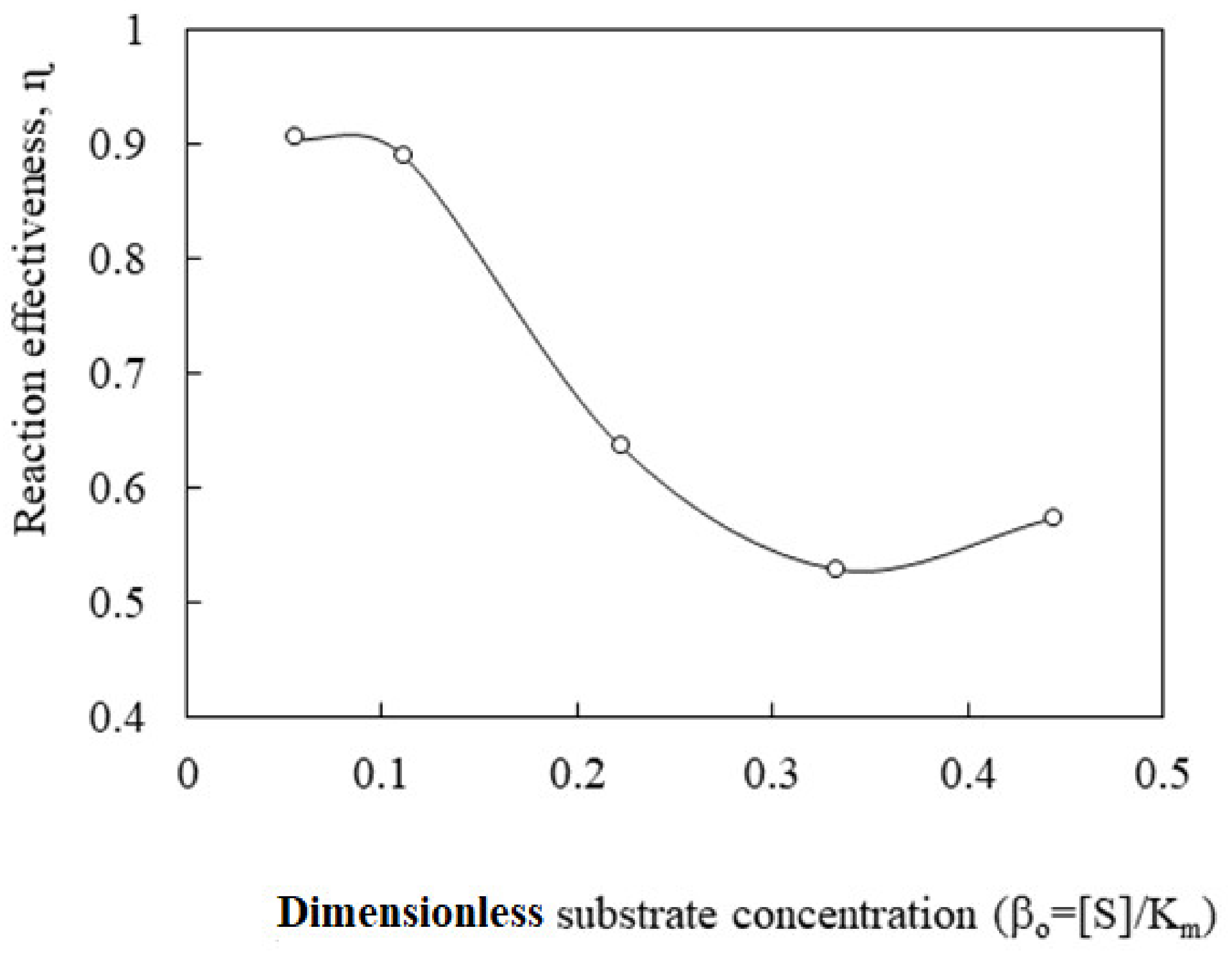
3.2.3. Diffusion-Reaction Kinetics Model
| Lipase Source | Vmax (mg/h·mg·Protein) | Km (g/mL) | Temp (oC) | pH | Ref |
|---|---|---|---|---|---|
| Eversa Transform 2.0 | 15.2 | 0.036 | 40 | 7.0 | This work |
| Rhizomucor miehie | 2.44 | 0.0553 | 37 | 7.0 | [46] |
| Porcine pancreas | 5.91 | 0.0042 | 37 | 6.9 | [42] |
| Pseudomonas gessardii | 0.01 | 0.0006 | 30 | 3.5 | [43] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Contesini, F.J.; Davanço, M.G.; Borin, G.P.; Vanegas, K.G.; Cirino, J.P.G.; Melo, R.R.D.; Mortensen, U.H.; Hildén, K.; Campos, D.R.; Carvalho, P.D.O. Advances in recombinant lipases: Application in the pharmaceutical industry. Catalysts 2020, 10, 1032. [Google Scholar] [CrossRef]
- Kirana, S.; Arshada, Z.; Nosheenb, S.; Kamala, S.; Gulzara, T.; Majeeda, M.S.; Jannata, M.; Rafiquec, M.A. Microbial Lipases: Production and Applications: A Review. J. Biochem. Biotechnol. Biomater. 2016, 1, 7–20. [Google Scholar]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Show, P.-L.; Ling, T.-C.; Lan, J.C.-W.; Tey, B.-T.; Ramanan, R.N.; Yong, S.-T.; Ooi, C.-W. Review of Microbial Lipase Purification Using Aqueous Two-phase Systems. Curr. Org. Chem. 2015, 19, 19–29. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Yap, Y.; Lam, H.L.; Ling, T.C.; Pan, G.-T.; Yang, T.C.-K. Sustainable approach in recycling of phase components of large scale aqueous two-phase flotation for lipase recovery. J. Clean. Prod. 2018, 184, 938–948. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Javed, S.S.; Hameed, A. Enzymes used in detergents: Lipases. Afr. J. Biotechnol. 2010, 9, 4836–4844. [Google Scholar]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Grela, A.; Kuc, J.; Bajda, T. A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials 2021, 14, 4994. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Alfutimie, A. Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column. Energies 2022, 15, 347. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Sankaran, R.; Show, P.L.; Ooi, C.W.; Liu, B.-L.; Chai, W.S.; Chang, Y.-K. Removal of protein wastes by cylinder-shaped NaY zeolite adsorbents decorated with heavy metal wastes. Int. J. Biol. Macromol. 2021, 185, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized Biocatalysts of Eversa® Transform 2.0 and Lipase from Thermomyces Lanuginosus: Comparison of Some Properties and Performance in Biodiesel Production. Catalysts 2020, 19, 738. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Rub, F.A.; Ashour, I.; al Marzouqi, M. Effect of competitive interference on the biosorption of lead (II) by Chlorella vulgaris. Chem. Eng. Process. 2007, 46, 1391–1399. [Google Scholar] [CrossRef]
- Subramanyam, B.; Das, A. Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J. Environ. Health Sci. Eng. 2014, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Gurses, A.; Doğar, Ç.; Yalcin, M.; Açıkyıldız, M.; Bayrak, R.; Karaca, S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. J. Hazard. Mater. 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shafiullah, A.Z.; Pal, A.; Islam, M.A.; Jahan, I.; Saha, B.B. Study on optimum IUPAC adsorption isotherm models employing sensitivity of parameters for rigorous adsorption system performance evaluation. Energies 2021, 14, 7478. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X. Novel Silica-Based Hybrid Adsorbents: Lead(II) Adsorption Isotherms. Sci. World J. 2013, 2013, 897159. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Baskar, R. Adsorption of Basic Magenta II onto H2SO4 activated immature Gossypium hirsutum seeds: Kinetics, isotherms, mass transfer, thermodynamics and process design. Arab. J. Chem. 2019, 12, 1322–1337. [Google Scholar] [CrossRef] [Green Version]
- Mailer, A.R. Chemistry and quality of olive oil. Prime Facts 2006, 227, 1–4. [Google Scholar]
- Khanday, W.A.; Majid, S.A.; Shekar, S.C.; Tomar, R. Dynamic adsorption of DMMP over synthetic zeolite-Alpha. Arab. J. Chem. 2014, 7, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Byrappa, K.; Kumar, B.V.S. Characterization of zeolites by infrared spectroscopy. Asian J. Chem. 2007, 19, 4933–4935. [Google Scholar]
- Asefa, M.T.; Feyisa, C.B. Comparative Investigation on Two Synthesizing Methods of Zeolites for Removal of Methylene Blue from Aqueous Solution. Int. J. Chem. Eng. 2022, 2022, 9378712. [Google Scholar] [CrossRef]
- Jamalluddin, N.A.; Abdullah, A.Z. Low frequency sonocatalytic degradation of Azo dye in water using Fe-doped zeolite Y catalyst. Ultrason. Sonochem. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Farhan, S.N.; Khadom, A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int. J. Ind. Chem. 2015, 6, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-estupiñán, P.; Giraldo, L.; Carlos, J. Adsorption calorimetry onto activated carbons prepared from corncobs waste from toluene solutions. J. Therm. Anal. Calorim. 2019, 2, 2577–2595. [Google Scholar] [CrossRef]
- Tzabar, N.; ter Brake, H.J.M. Adsorption isotherms and Sips models of nitrogen, methane, ethane, and propane on commercial activated carbons and polyvinylidene chloride. Adsorption 2016, 22, 901–914. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Liu, Y.; Zheng, S.; Xu, Y. High removal efficiency of diatomite-based X zeolite for Cu2+ and Zn2+. Materials 2021, 14, 6525. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Raisi, A.; Aroujalian, A.; Dabir, B.; Irani, M. Synthesis of Nano-NaX Zeolite by Microwave Heating Method for Removal of Lead, Copper, and Cobalt Ions from Aqueous Solution. J. Environ. Eng. 2015, 141, 04014088. [Google Scholar] [CrossRef]
- Tutar, H.; Yilmaz, E.; Pehlivan, E.; Yilmaz, M. Immobilization of Candida rugosa lipase on sporopollenin from Lycopodium clavatum. Int. J. Biol. Macromol. 2009, 45, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-Y.; Liu, X.-Y.; He, P.; Peng, D.-H.; Fan, B.; Xia, K. Lipase adsorption on woven nylon-6 membrane: Optimization, kinetic and thermodynamic analyses. Biocatal. Biotransform. 2014, 32, 188–197. [Google Scholar] [CrossRef]
- Wang, F.; Nie, T.T.; Shao, L.L.; Cui, Z. Comparison of physical and covalent immobilization of lipase from Candida antarctica on polyamine microspheres of alkylamine matrix. Biocatal. Biotransform. 2014, 32, 314–326. [Google Scholar] [CrossRef]
- Shomal, R.; Du, W.; Al-Zuhair, S. Immobilization of Lipase on Metal-Organic frameworks for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107265. [Google Scholar] [CrossRef]
- Gilani, S.L.; Najafpour, G.D.; Moghadamnia, A.; Kamaruddin, A.H. Kinetics and isotherm studies of the immobilized lipase on chitosan support. Int. J. Eng. Trans. A Basics 2016, 29, 1319–1331. [Google Scholar]
- Rodrigues, A.R.; Cabral, J.M.; Taipa, M. Immobilization of Chromobacterium viscosum lipase on Eudragit S-100: Coupling, characterization and kinetic application in organic and biphasic media. Enzyme Microb. Technol. 2002, 31, 133–141. [Google Scholar] [CrossRef]
- Chen, G.-J.; Kuo, C.-H.; Chen, C.-I.; Yu, C.-C.; Shieh, C.-J.; Liu, Y.-C. Effect of membranes with various hydrophobic/hydrophilic properties on lipase immobilized activity and stability. J. Biosci. Bioeng. 2012, 113, 166–172. [Google Scholar] [CrossRef]
- Atyaksheva, L.F.; Kasyanov, I.A.; Ivanova, I.I. Adsorptive Immobilization of Proteins on Mesoporous Molecular Sieves and Zeolites. Pet. Chem. 2019, 59, 327–337. [Google Scholar] [CrossRef]
- Rodhi, M.N.M.; Hamzah, F.; Hamid, K.H.K. Kinetic Behaviour of Pancreatic Lipase Inhibition by Ultrasonicated A. malaccensis and A. subintegra Leaves of Different Particle Sizes. Bull. Chem. React. Eng. Catal. 2020, 15, 818–828. [Google Scholar] [CrossRef]
- Bai, Y.-X.; Li, Y.-F.; Yang, Y.; Yi, L.-X. Covalent immobilization of triacylglycerol lipase onto functionalized novel mesoporous silica supports. J. Biotechnol. 2006, 125, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Kennedy, L.J.; Vidya, C.; Boopathy, R.; Sekaran, G. Immobilization of acidic lipase derived from Pseudomonas gessardii onto mesoporous activated carbon for the hydrolysis of olive oil. J. Mol. Catal. B Enzym. 2010, 62, 58–65. [Google Scholar] [CrossRef]
- Dossat, V.; Combes, D.; Marty, A. Efficient lipase catalyzed production of a lubricant and surfactant formulation using a continuous solvent-free process. J. Biotechnol. 2002, 9, 117–124. [Google Scholar] [CrossRef]
- Maceiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- Del Pozo, D.F.; van Hauwermeiren, D. Effect of Mass Transfer Limitations on Enzymatic Reactions in Microreactors: A Model-Based Analysis. Master’s Thesis, Ghent University, Ghent, Belgium, 2015. [Google Scholar]
- Knezevic, Z.; Mojovic, L.; Adnadjevic, B. Palm oil hydrolysis by lipase from Candida cylindracea immobilized on zeolite type Y. Enzyme Microb. Technol. 1998, 22, 275–280. [Google Scholar] [CrossRef]
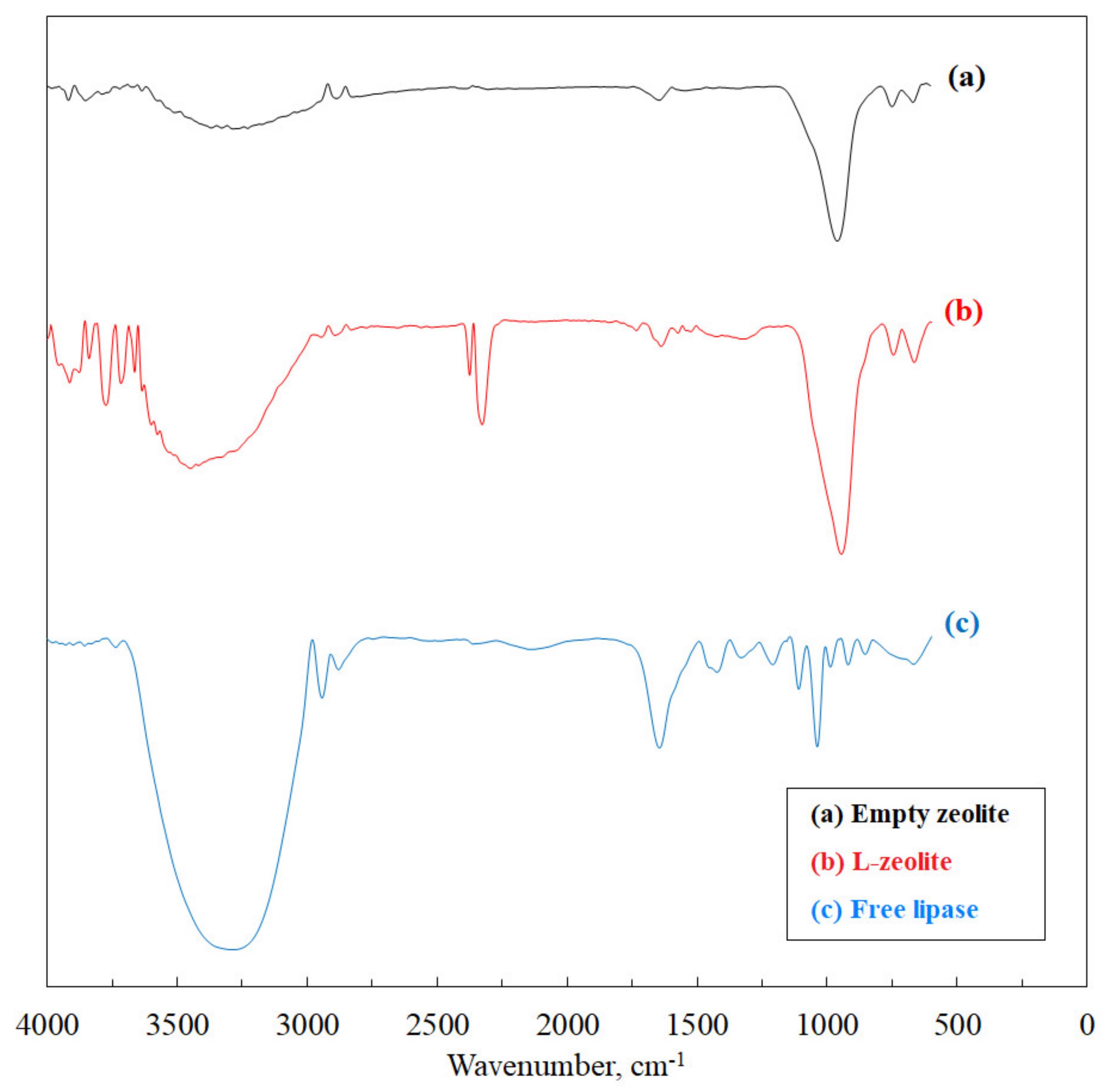
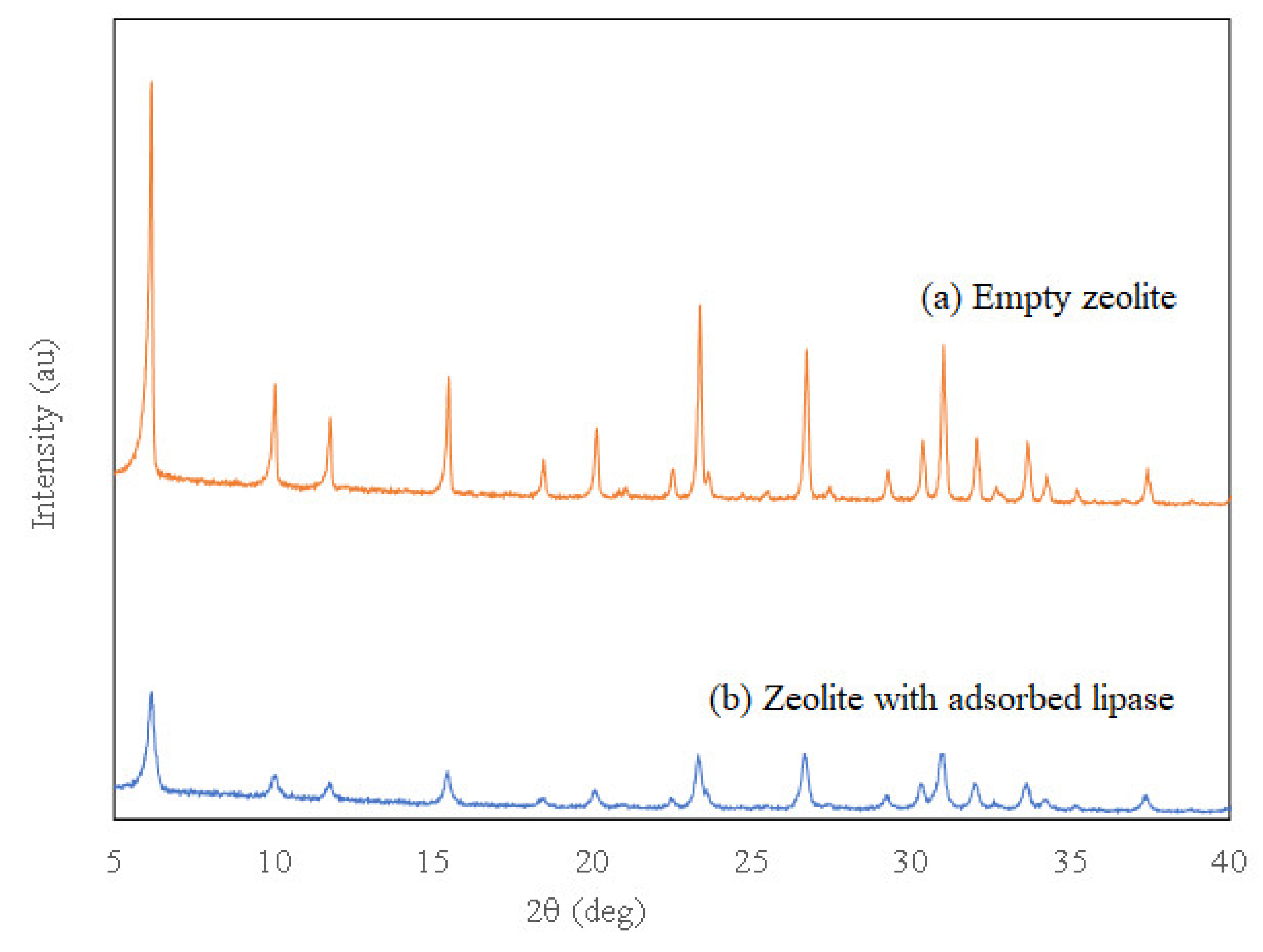
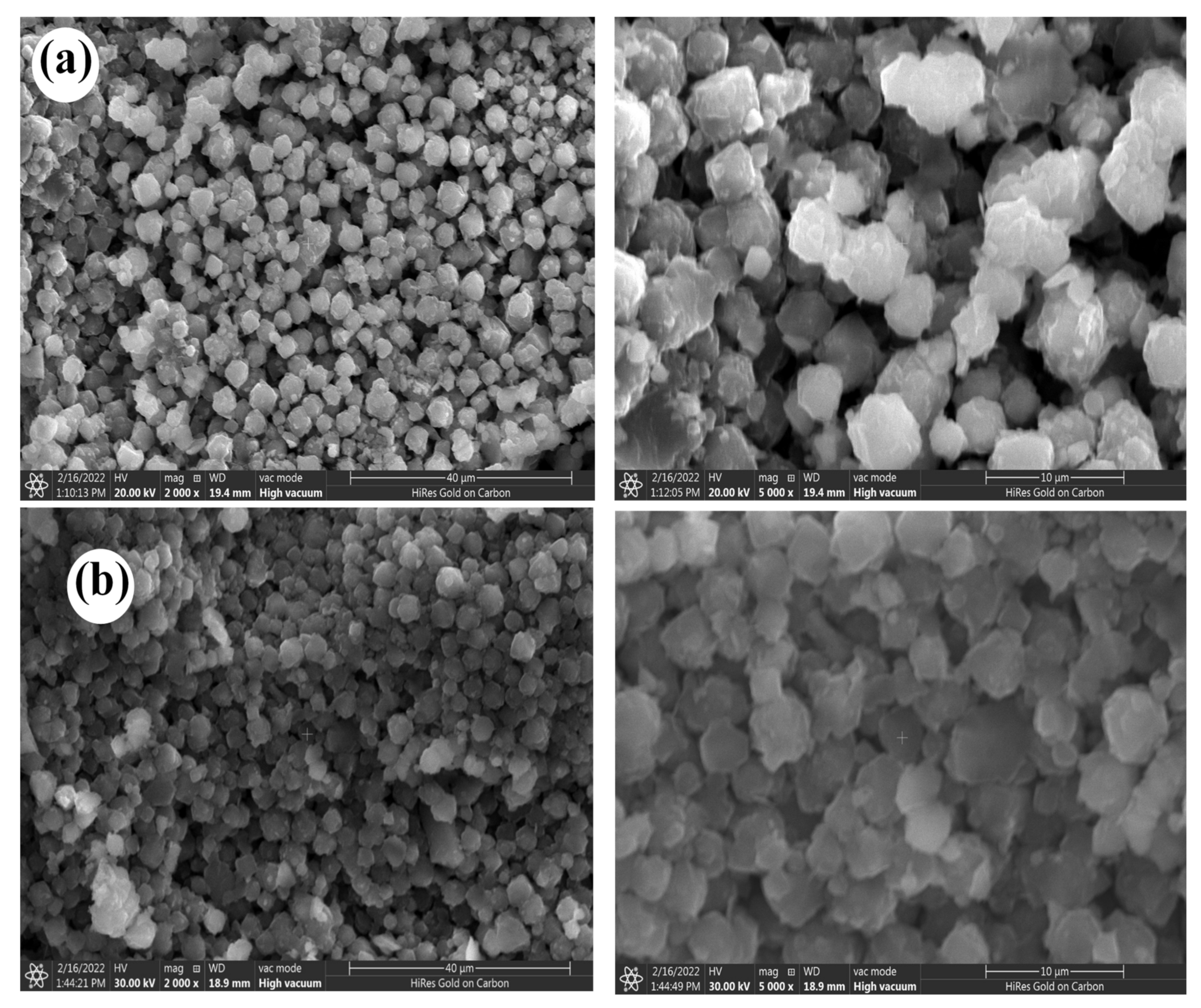

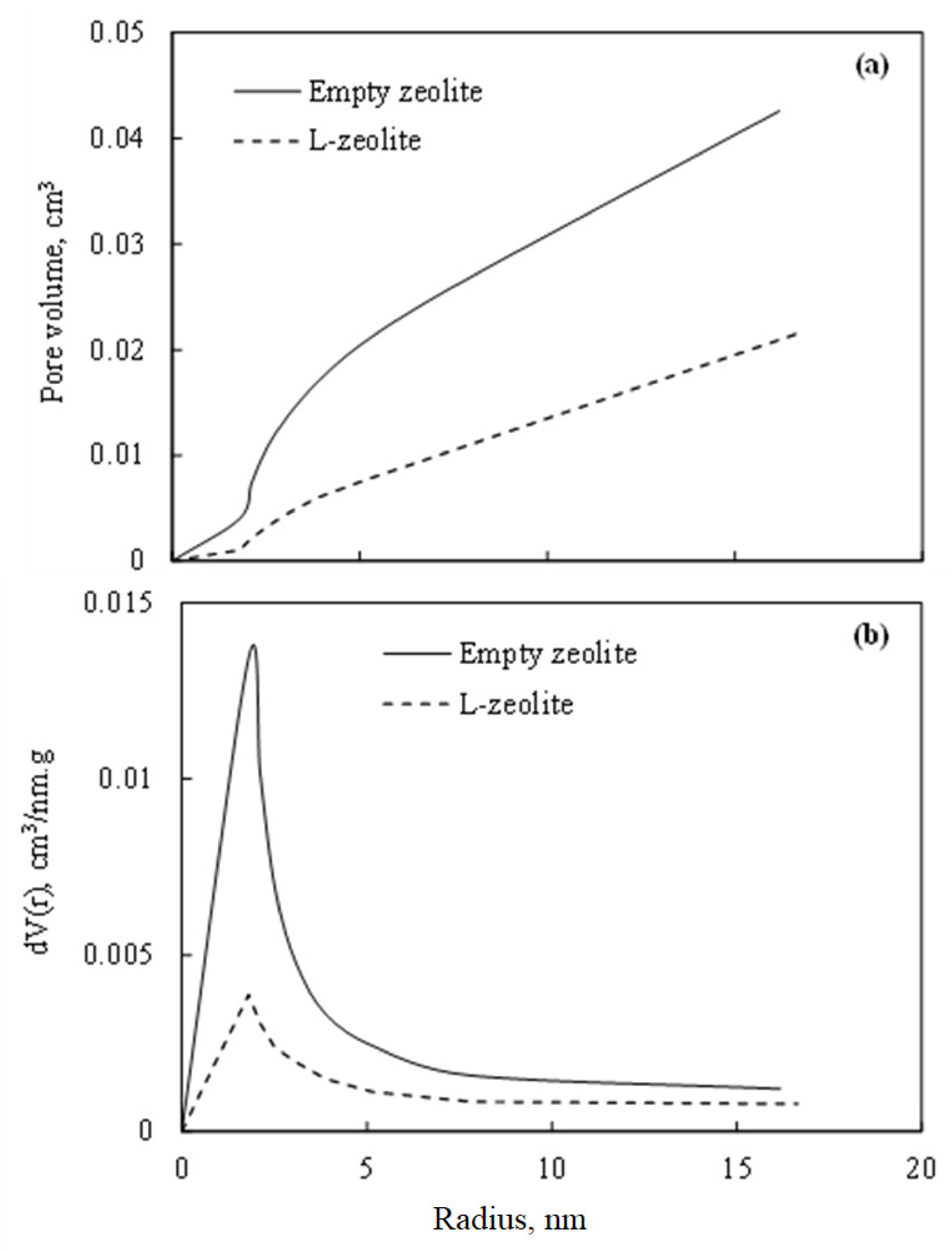

| Composite | SBET (m2 g−1) | Total Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Empty zeolite | 361.37 | 0.227 | 1.805 |
| L-zeolite | 107.93 | 0.081 | 1.800 |
| Isotherm | Parameters | Values | R2 |
|---|---|---|---|
| Langmuir | b | 0.15 | 0.83 |
| qm (mg/g) | 62.58 | ||
| Freundlich | aF | 8.11 | 0.82 |
| n | 1.08 | ||
| Sips (L-F) | aLF | 1.60 | 0.98 |
| KLF | 98.23 | ||
| nLF | 4.51 |
| Support | Temp | Model Best Fit | Parameters | Values | R2 | Ref. |
|---|---|---|---|---|---|---|
| Sporopollenin | 40 °C | Langmuir | b (mL/mg) qm (mg/g) | 0.76 13.47 | 0.952 | [33] |
| Nylon-6 | 30 °C | Langmuir | b (mL/mg) qm (mg/g) | 11.641 1.793 | 0.997 | [34] |
| PA-M | 35 °C | Langmuir | KL (mL/mg) qm (mg/g) | 21.2 253.2 | 0.994 | [35] |
| ZIF-8 | 35 °C | Langmuir | KL (L/mg) qm (mg/g) | 0.22 33.06 | 0.990 | [36] |
| ZIF-67 | 30 °C | Langmuir | KL (L/mg) qm (mg/g) | 6.76 34.22 | 0.873 | [36] |
| HKUST-1 | 35 °C | Langmuir | KL (L/mg) qm (mg/g) | 4.12 18.74 | 1.000 | [36] |
| Chitosan beads | 30 °C | Langmuir | b (mL/mg) qm (mg/g) | 797 0.158 | 0.946 | [37] |
| Activated chitosan beads | 30 °C | Freundlich | KF (mL/mg)1/n 1/n | 0.379 4.975 | 0.995 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Qayoudi, A.; Al-Zuhair, S. Dynamic Modelling of Enzymatic Hydrolysis of Oil Using Lipase Immobilized on Zeolite. Sustainability 2022, 14, 8399. https://doi.org/10.3390/su14148399
Al Qayoudi A, Al-Zuhair S. Dynamic Modelling of Enzymatic Hydrolysis of Oil Using Lipase Immobilized on Zeolite. Sustainability. 2022; 14(14):8399. https://doi.org/10.3390/su14148399
Chicago/Turabian StyleAl Qayoudi, Aysha, and Sulaiman Al-Zuhair. 2022. "Dynamic Modelling of Enzymatic Hydrolysis of Oil Using Lipase Immobilized on Zeolite" Sustainability 14, no. 14: 8399. https://doi.org/10.3390/su14148399
APA StyleAl Qayoudi, A., & Al-Zuhair, S. (2022). Dynamic Modelling of Enzymatic Hydrolysis of Oil Using Lipase Immobilized on Zeolite. Sustainability, 14(14), 8399. https://doi.org/10.3390/su14148399







