A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China
Abstract
:1. Introduction
2. Materials and Methods
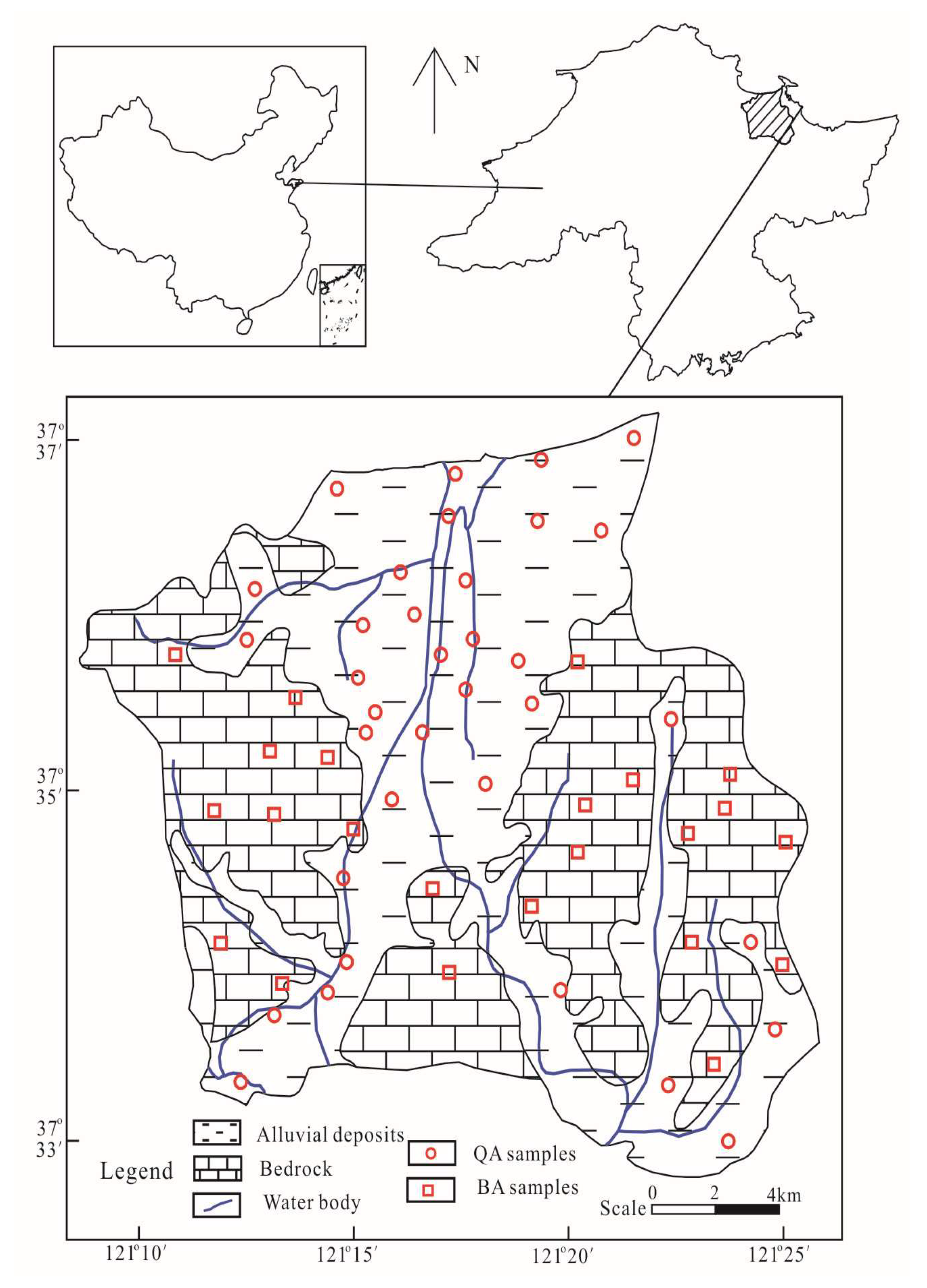
2.1. Study Area
2.2. Methodology
2.2.1. Sample Collection and Analyses
2.2.2. Multivariate Analysis
2.2.3. Graphical Illustration
3. Results and Discussion
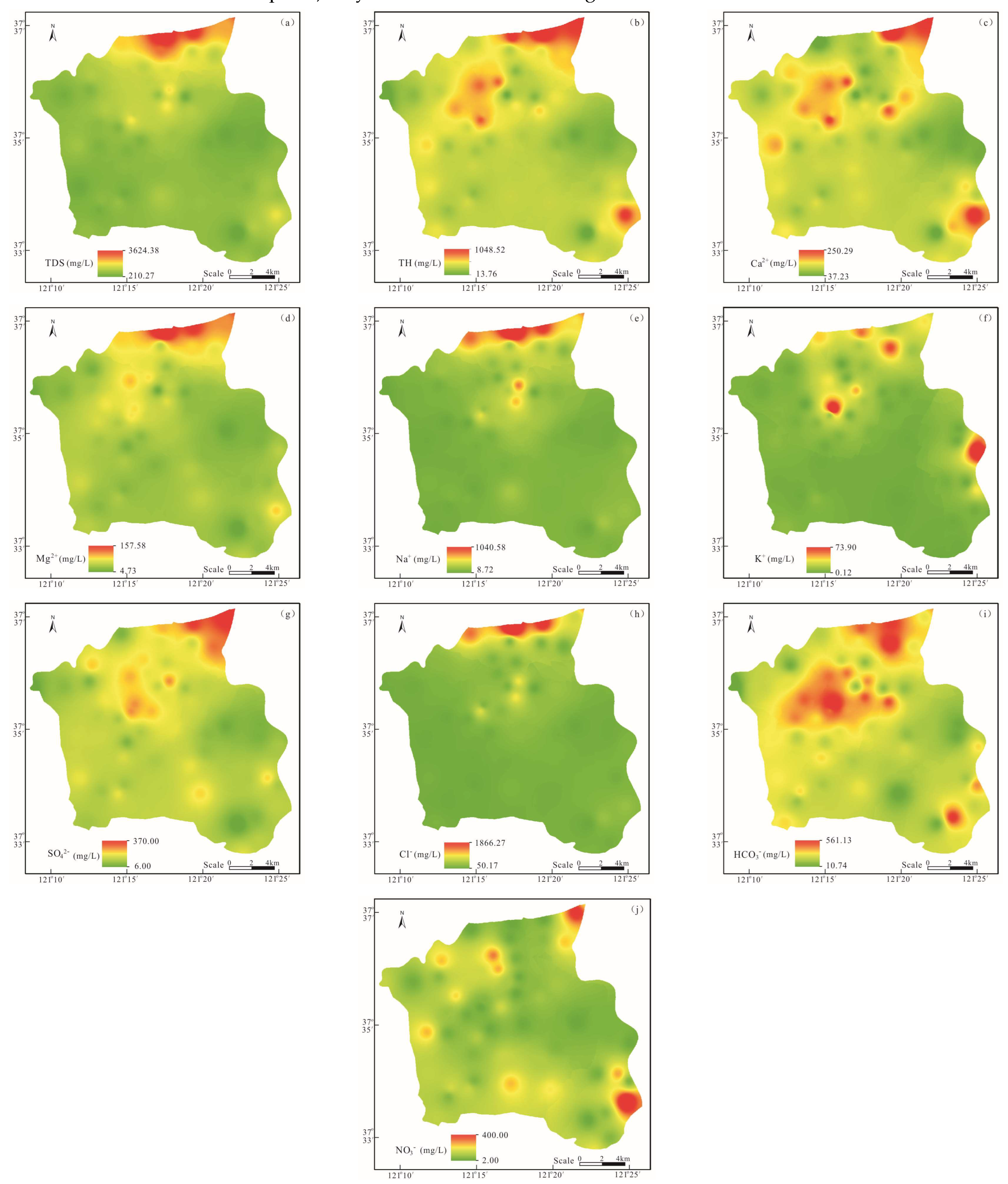
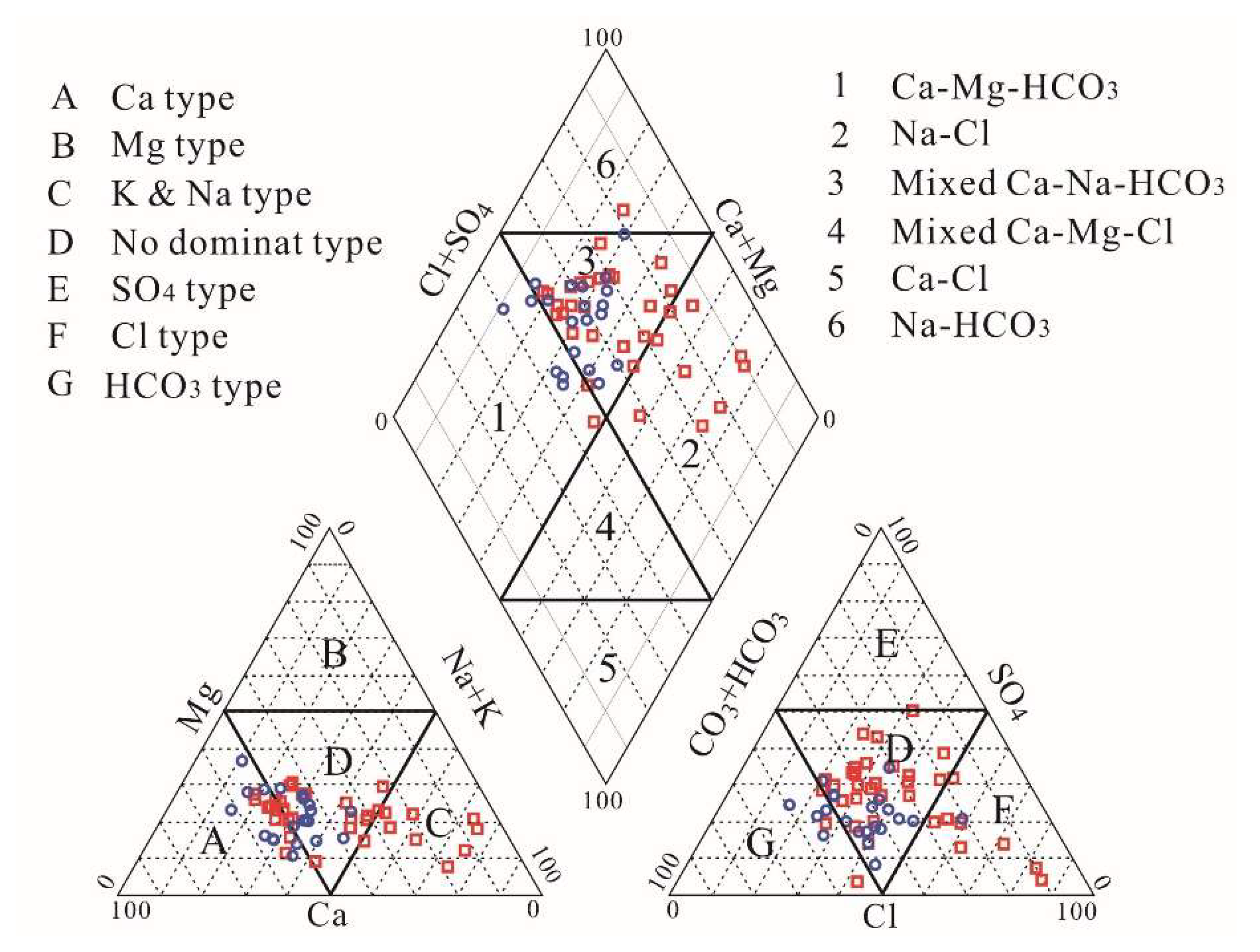
3.1. Hydrochemical Characteristics
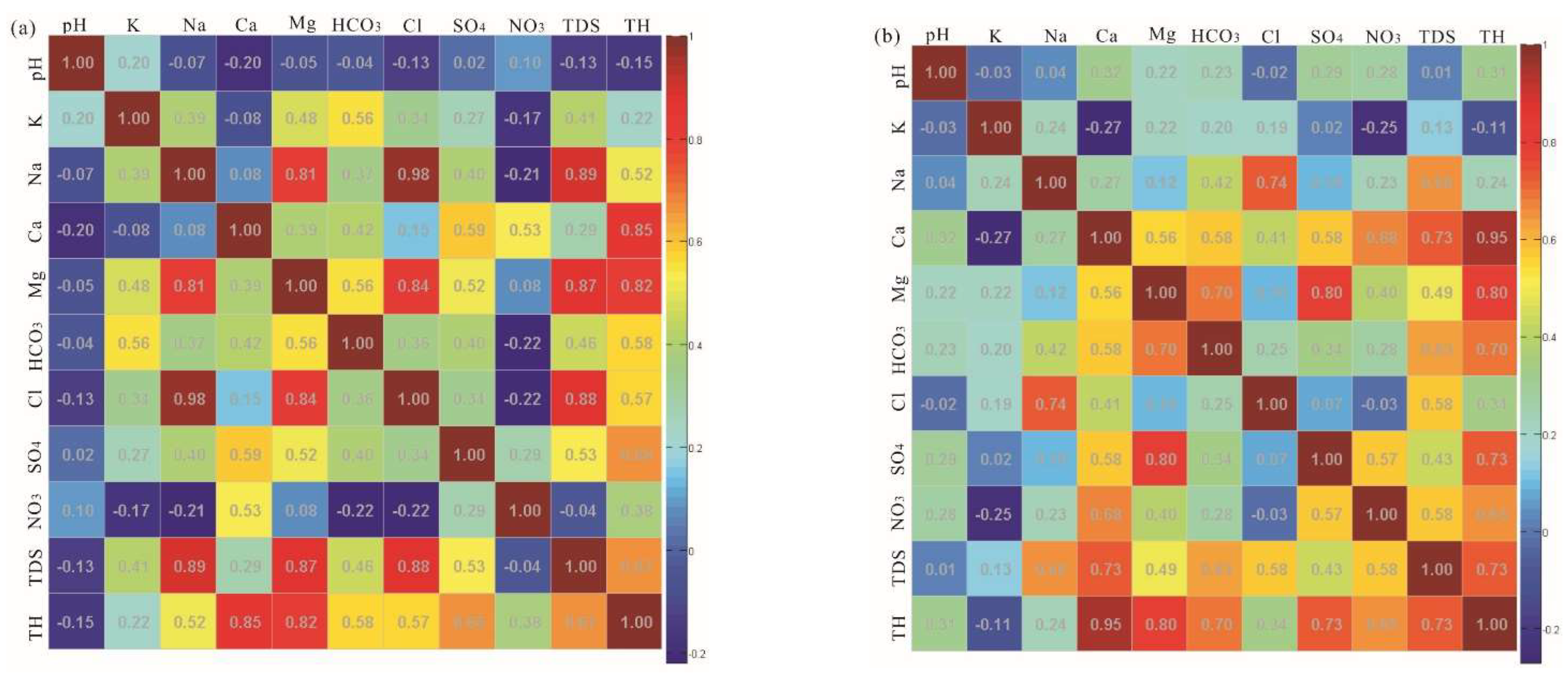
3.2. Correlation Relationship
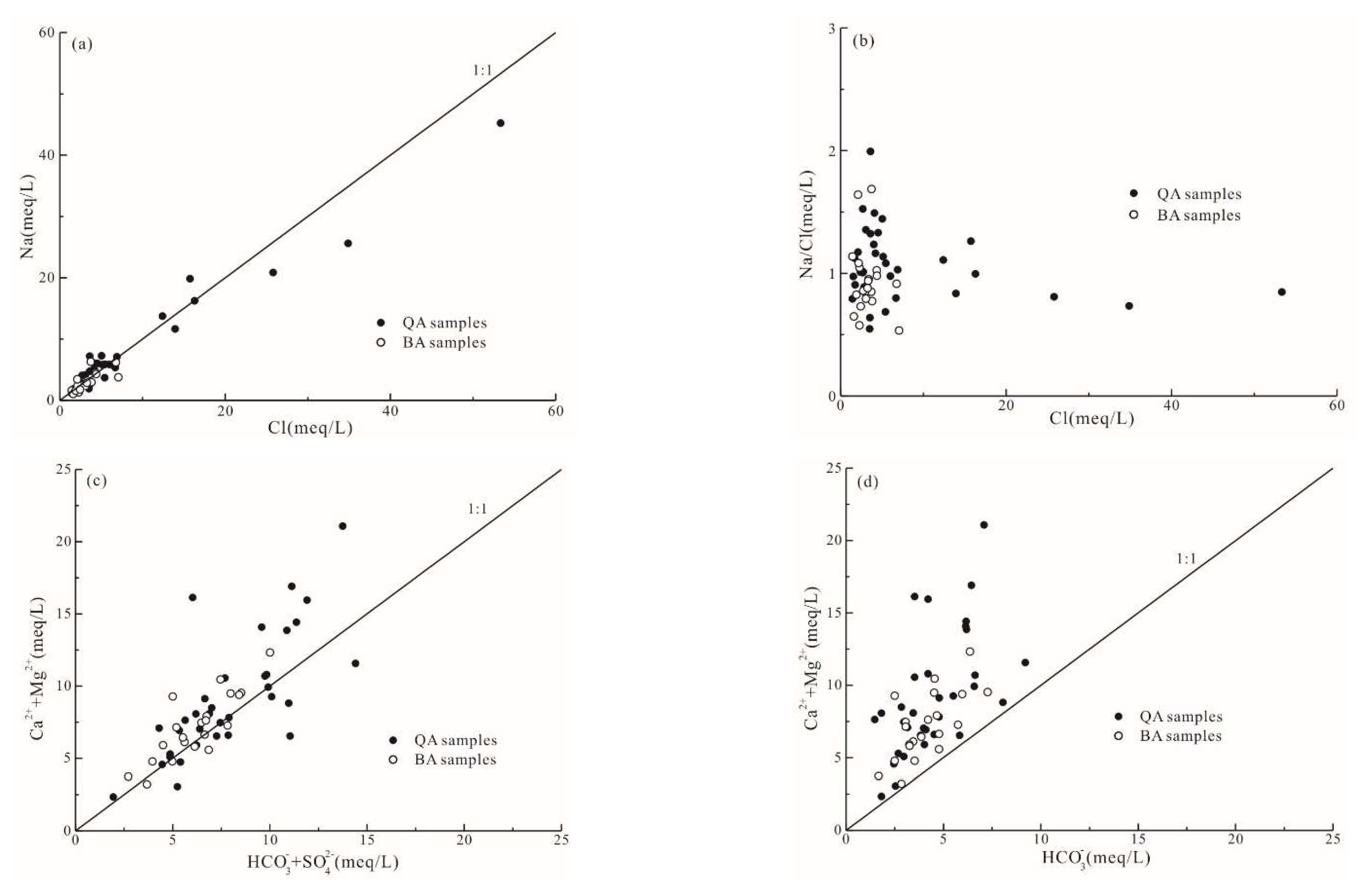
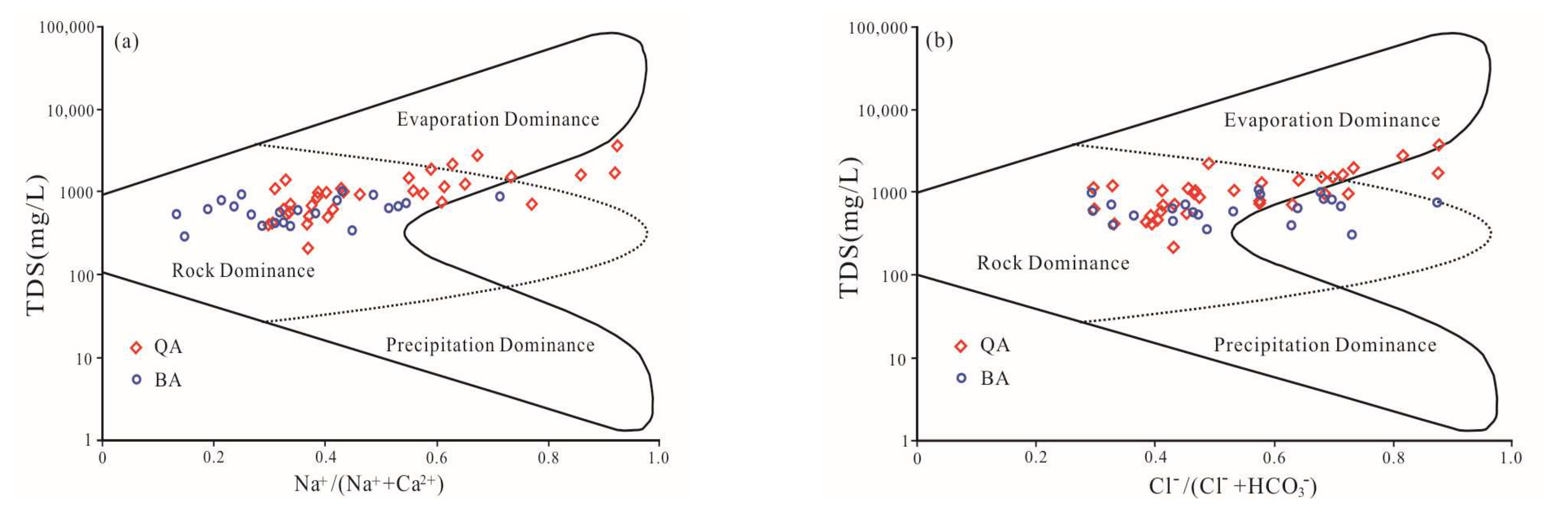
3.3. Hydrochemical Processes
3.4. Principal Components Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminiyan, M.M.; Aminiyan, F.M. Comprehensive integrated index–based geochemistry and hydrochemical analyses of groundwater resources for multiple consumptions under coastal conditions. Environ. Sci. Pollut. Res. 2020, 27, 21386–21406. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, Z.; Gao, M.; Hou, G. Evolutionary process of saline-water intrusion in Holocene and Late Pleistocene groundwater in southern Laizhou Bay. Sci. Total Environ. 2017, 607, 586–599. [Google Scholar] [CrossRef]
- De Graaf, I.E.; Gleeson, T.; Sutanudjaja, E.H.; Bierkens, M.F. Environmental flow limits to global groundwater pumping. Nature 2019, 574, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ni, J.; Song, Q.; Li, C.; Wang, F.; Cao, Y. Analysis of coastal groundwater hydrochemistry evolution based on groundwater flow system division. J. Hydrol. 2021, 601, 126631. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, W. Study on the groundwater quality and its influencing factor in Songyuan City, northeast China, using integrated hydrogeochemical method. Sci. Total Environ. 2021, 773, 144958. [Google Scholar] [CrossRef]
- Al-Barakah, F.N.; Al-jassas, A.M.; Aly, A.A. Water quality assessment and hydrochemical characterization of Zamzam groundwater, Saudi Arabia. Appl. Water Sci. 2017, 7, 3985–3996. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, P.; Somashekar, R.K. Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl. Water Sci. 2017, 7, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, Z.; Zhang, Y.; Sun, Z.; Sun, T.; Fan, H.; Wu, B.; Li, M.; Qian, L. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 129164. [Google Scholar] [CrossRef]
- Kenniche, S.; Bekkoussa, B.; M’nassri, S.; Teffahi, M.; Taupin, J.D.; Patris, N.; Zaagane, M.; Majdoub, R. Hydrochemical characterization, physicochemical and bacteriological quality of groundwater in Sidi Kada Mountains, northwest of Algeria. Arab. J. Geosci. 2022, 15, 1061. [Google Scholar] [CrossRef]
- Bastianoni, A.; Guastaldi, E.; Barbagli, A.; Bernardinetti, S.; Zirulia, A.; Brancale, M.; Colonna, T. Multivariate analysis applied to aquifer hydrogeochemical evaluation: A case study in the coastal significant subterranean water body between “Cecina River and San Vincenzo”, Tuscany (Italy). Appl. Sci. 2021, 11, 7595. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.B.; Subba Rao, N. Applications of geochemical and multivariate statistical approaches for the evaluation of groundwater quality and human health risks in a semi-arid region of eastern Maharashtra, India. Environ. Geochem. Health 2021, 43, 683–703. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.H.; Thao, N.T.; Batsaikhan, B.; Yun, S.T. Hydrochemical assessment of freshening saline groundwater using multiple end-members mixing modeling: A study of Red River delta aquifer, Vietnam. J. Hydrol. 2017, 549, 703–714. [Google Scholar] [CrossRef]
- Perera, T.A.N.T.; Herath, H.M.M.S.D.; Piyadasa, R.U.K.; Jianhui, L.; Bing, L. Spatial and physicochemical assessment of groundwater quality in the urban coastal region of Sri Lanka. Environ. Sci. Pollut. Res. 2022, 29, 16250–16264. [Google Scholar] [CrossRef]
- Wen, X.; Lu, J.; Wu, J.; Lin, Y.; Luo, Y. Influence of coastal groundwater salinization on the distribution and risks of heavy metals. Sci. Total Environ. 2019, 652, 267–277. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, J.; Song, T.; Feng, H.; Liu, Z.; Liu, H.; Ren, X. Influences of natural and anthropogenic processes on the groundwater quality in the Dagujia River Basin in Yantai, China. J. Water Supply Res. Technol. 2020, 69, 184–196. [Google Scholar] [CrossRef]
- Hou, X.; Ying, L.; Chang, Y.; Qian, S.S.; Zhang, Y. Modeling of non-point source nitrogen pollution from 1979 to 2008 in Jiaodong Peninsula, China. Hydrol. Process. 2014, 28, 3264–3275. [Google Scholar] [CrossRef]
- Gao, Z.; Han, C.; Xu, Y.; Zhao, Z.; Luo, Z.; Liu, J. Assessment of the water quality of groundwater in Bohai Rim and the controlling factors—a case study of northern Shandong Peninsula, north China. Environ. Pollut. 2021, 285, 117482. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, G.; Lin, J.; Xu, X.; Yu, H.; Liu, W.; Fu, T.; Su, Q.; Wang, Y.; Dai, Y.; et al. Elucidating the pollution sources and groundwater evolution in typical seawater intrusion areas using hydrochemical and environmental stable isotope technique: A case study for Shandong Province, China. Lithosphere 2021, 2021, 4227303. [Google Scholar] [CrossRef]
- Chen, R.; Liu, L.; Li, Y.; Zhai, Y.; Chen, H.; Hu, B.; Zhang, Q.; Teng, Y. Characteristics of hydro-geochemistry and groundwater pollution in Songnen Plain in northeastern China. Sustainability 2022, 14, 6527. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A.; Khan, R.; Li, P. Spatial analysis of groundwater quality and human health risk assessment in parts of Raebareli district, India. Environ. Earth Sci. 2021, 80, 800. [Google Scholar] [CrossRef]
- Aravinthasamy, P.; Karunanidhi, D.; Subramani, T.; Roy, P.D. Demarcation of groundwater quality domains using GIS for best agricultural practices in the drought-prone Shanmuganadhi River basin of South India. Environ. Sci. Pollut. Res. 2021, 28, 18423–18435. [Google Scholar] [CrossRef] [PubMed]
- Wali, S.U.; Alias, N.; Harun, S.B. Reevaluating the hydrochemistry of groundwater in basement complex aquifers of Kaduna Basin, NW Nigeria using multivariate statistical analysis. Environ. Earth Sci. 2021, 80, 208. [Google Scholar] [CrossRef]
- Şehnaz, Ş.; Şener, E.; Davraz, A.; Varol, S. Hydrogeological and hydrochemical investigation in the Burdur Saline Lake Basin, southwest Turkey. Geochemistry 2020, 80, 125592. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Paramasivam, S.K.; Karuppannan, S.; Ravichandran, N.; Selvaraj, P.A. GIS-based evaluation of hydrochemical characterisation of groundwater in hard rock region, South Tamil Nadu, India. Arab. J. Geosci. 2020, 13, 837. [Google Scholar] [CrossRef]
- Yidana, S.; Banoeng-Yakubo, B.; Sakyi, P.A. Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J. Earth Syst. Sci. 2012, 121, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Atikul Islam, M.; Zahid, A.; Rahman, M.; Islam, M.J.; Akter, Y.; Shammi, M.; Bodrud-Doza, M.; Roy, B. Investigation of groundwater quality and its suitability for drinking and agricultural use in the south central part of the coastal region in Bangladesh. Expos. Health 2017, 9, 27–41. [Google Scholar] [CrossRef]
- Singh, V.B.; Ramanathan, A.L.; Sharma, P.; Pottakkal, J.G. Dissolved ion chemistry and suspended sediment characteristics of meltwater draining from Chhota Shigri Glacier, western Himalaya, India. Arab. J. Geosci. 2015, 8, 281–293. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pisciotta, A.; De Maio, M. Evaluation of groundwater salinization and pollution level on Favignana Island, Italy. Environ. Pollut. 2019, 249, 969–981. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Raja, P.; Krishnaraj, S.; Selvaraj, G.; Kumar, S.; Francis, V. Hydrogeochemical investigations to assess groundwater and saline water interaction in coastal aquifers of the southeast coast, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2021, 28, 5495–5519. [Google Scholar] [CrossRef]
- Rosen, M.; Jones, S. Controls on the chemical composition of groundwater from alluvial aquifers in the Wanaka and Wakatipu basins, Central Otago, New Zealand. Hydrogeol. J. 1998, 6, 264–281. [Google Scholar]
- Ruiz-Pico, Á.; Pérez-Cuenca, Á.; Serrano-Agila, R.; Maza-Criollo, D.; Leiva-Piedra, J.; Salazar-Campos, J. Hydrochemical characterization of groundwater in the Loja Basin (Ecuador). Appl. Geochem. 2019, 104, 1–9. [Google Scholar] [CrossRef]
- Ma, F.; Wei, A.; Deng, Q.; Zhao, H. Hydrochemical characteristics and the suitability of groundwater in the coastal region of Tangshan, China. J. Earth Sci. 2014, 25, 1067–1075. [Google Scholar] [CrossRef]
- Simsek, C.; Elci, A.; Gunduz, O.; Erdogan, B. Hydrogeological and hydrogeochemical characterization of a karstic mountain region. Environ. Geol. 2008, 54, 291–308. [Google Scholar] [CrossRef]
- Romshoo, S.A.; Dar, R.A.; Murtaza, K.O.; Rashid, I.; Dar, F.A. Hydrochemical characterization and pollution assessment of groundwater in Jammu Siwaliks, India. Environ. Monit. Assess. 2017, 189, 122. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Raza, M.; Lee, J.Y.; Kim, H.; Jeon, C.; Kim, B.; Kim, J.W.; Kim, R.H. Factors controlling the spatial distribution and temporal trend of nationwide groundwater quality in Korea. Sustainability 2020, 12, 9971. [Google Scholar] [CrossRef]
- Thimonier, A.; Schmitt, M.; Waldner, P.; Schleppi, P. Seasonality of the Na/Cl ratio in precipitation and implications of canopy leaching in validating chemical analyses of throughfall samples. Atmos. Environ. 2008, 42, 9106–9117. [Google Scholar] [CrossRef]
- Gaury, P.K.; Meena, N.K.; Mahajan, A.K. Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environ. Monit. Assess. 2018, 190, 84. [Google Scholar] [CrossRef]
- Prusty, P.; Farooq, S.H.; Swain, D.; Chandrasekharam, D. Association of geomorphic features with groundwater quality and freshwater availability in coastal regions. Int. J. Environ. Sci. Technol. 2020, 17, 3313–3328. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K.; Kumar, K. Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River Basin, western India. Environ. Earth Sci. 2012, 65, 1231–1250. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, M.; Zhang, G.; Zhao, L.; Hou, X.; Yang, Y. Analysis of coastal zone data of northern Yantai collected by remote sensing from 1990 to 2018. Appl. Sci. 2019, 9, 4466. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Gupta, N.C.; Kaur, A.; Singh, K. Application of principal component analysis and correlation for assessing groundwater contamination in and around municipal solid waste landfill of Ghazipur, Delhi. J. Geol. Soc. India 2019, 94, 595–604. [Google Scholar] [CrossRef]
| Parameter | QA Samples (n = 34) | BA Samples (n = 22) | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| pH | 7.17 | 8.42 | 7.79 | 0.34 | 6.96 | 8.25 | 7.56 | 0.34 |
| K+ (mg/L) | 0.14 | 73.80 | 12.41 | 16.48 | 0.12 | 73.90 | 6.71 | 15.90 |
| Na+ (mg/L) | 26.14 | 1040.60 | 177.29 | 205.54 | 23.87 | 144.60 | 64.48 | 32.22 |
| Ca2+ (mg/L) | 37.23 | 250.29 | 109.28 | 52.86 | 47.70 | 157.88 | 100.41 | 34.21 |
| Mg2+ (mg/L) | 4.73 | 157.58 | 41.86 | 29.29 | 8.27 | 53.19 | 28.69 | 11.07 |
| HCO3− (mg/L) | 90.15 | 561.14 | 263.81 | 115.06 | 101.90 | 443.81 | 250.57 | 89.86 |
| Cl− (mg/L) | 50.17 | 1866.31 | 279.23 | 377.46 | 50.17 | 247.50 | 115.09 | 50.83 |
| SO42− (mg/L) | 6.00 | 370.00 | 169.06 | 76.90 | 40.00 | 175.00 | 102.00 | 39.25 |
| NO3− (mg/L) | 2.00 | 400.00 | 76.721 | 94.10 | 2.00 | 180.00 | 68.75 | 54.81 |
| TDS (mg/L) | 210.27 | 3624.42 | 1093.63 | 700.21 | 293.44 | 945.24 | 625.66 | 188.51 |
| TH (mg/L) | 116.81 | 1048.52 | 445.25 | 210.89 | 160.61 | 613.23 | 368.86 | 117.18 |
| Parameter | QA Samples (n = 34) | BA Samples (n = 22) | ||||
|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 1 | PC 2 | PC 3 | |
| pH | −0.123 | −0.045 | 0.683 | 0.319 | −0.481 | −0.482 |
| K+ | 0.342 | −0.421 | 0.696 | 0.098 | 0.321 | 0.816 |
| Na+ | 0.753 | −0.478 | −0.266 | 0.500 | 0.709 | −0.243 |
| Ca2+ | 0.743 | 0.566 | −0.063 | 0.930 | −0.030 | −0.151 |
| Mg2+ | 0.925 | 0.038 | 0.116 | 0.791 | −0.337 | 0.394 |
| Cl− | 0.773 | −0.421 | −0.361 | 0.453 | 0.734 | −0.258 |
| SO42− | 0.737 | 0.222 | 0.160 | 0.728 | −0.414 | 0.154 |
| HCO3− | 0.638 | −0.223 | 0.398 | 0.755 | 0.081 | 0.275 |
| NO3− | 0.212 | 0.843 | 0.108 | 0.701 | −0.272 | −0.244 |
| TDS | 0.869 | −0.212 | −0.158 | 0.852 | 0.359 | −0.061 |
| TH | 0.894 | 0.387 | 0.009 | 0.964 | −0.152 | 0.045 |
| Eigenvalues | 5.270 | 1.912 | 1.389 | 5.303 | 1.894 | 1.366 |
| Variability (%) | 47.907 | 17.381 | 12.630 | 48.206 | 17.217 | 12.417 |
| Cumulative (%) | 47.907 | 65.288 | 77.918 | 48.206 | 65.423 | 77.840 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, A.; Chen, Y.; Deng, Q.; Li, D.; Wang, R.; Jiao, Z. A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China. Sustainability 2022, 14, 8358. https://doi.org/10.3390/su14148358
Wei A, Chen Y, Deng Q, Li D, Wang R, Jiao Z. A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China. Sustainability. 2022; 14(14):8358. https://doi.org/10.3390/su14148358
Chicago/Turabian StyleWei, Aihua, Yuanyao Chen, Qinghai Deng, Duo Li, Rui Wang, and Zhen Jiao. 2022. "A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China" Sustainability 14, no. 14: 8358. https://doi.org/10.3390/su14148358
APA StyleWei, A., Chen, Y., Deng, Q., Li, D., Wang, R., & Jiao, Z. (2022). A Study on Hydrochemical Characteristics and Evolution Processes of Groundwater in the Coastal Area of the Dagujia River Basin, China. Sustainability, 14(14), 8358. https://doi.org/10.3390/su14148358





