Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis and Characterisation of Nanoparticles
2.3. Exposure of V. radiata to Nanoparticles and Treatment Design
2.4. Chlorophyll Content
2.5. Gas Exchange and Chlorophyll Fluorescence
2.6. Carbohydrate and Protein Content
2.7. Lipid Peroxidation
2.8. Antioxidant Status
2.9. Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Nanoparticles
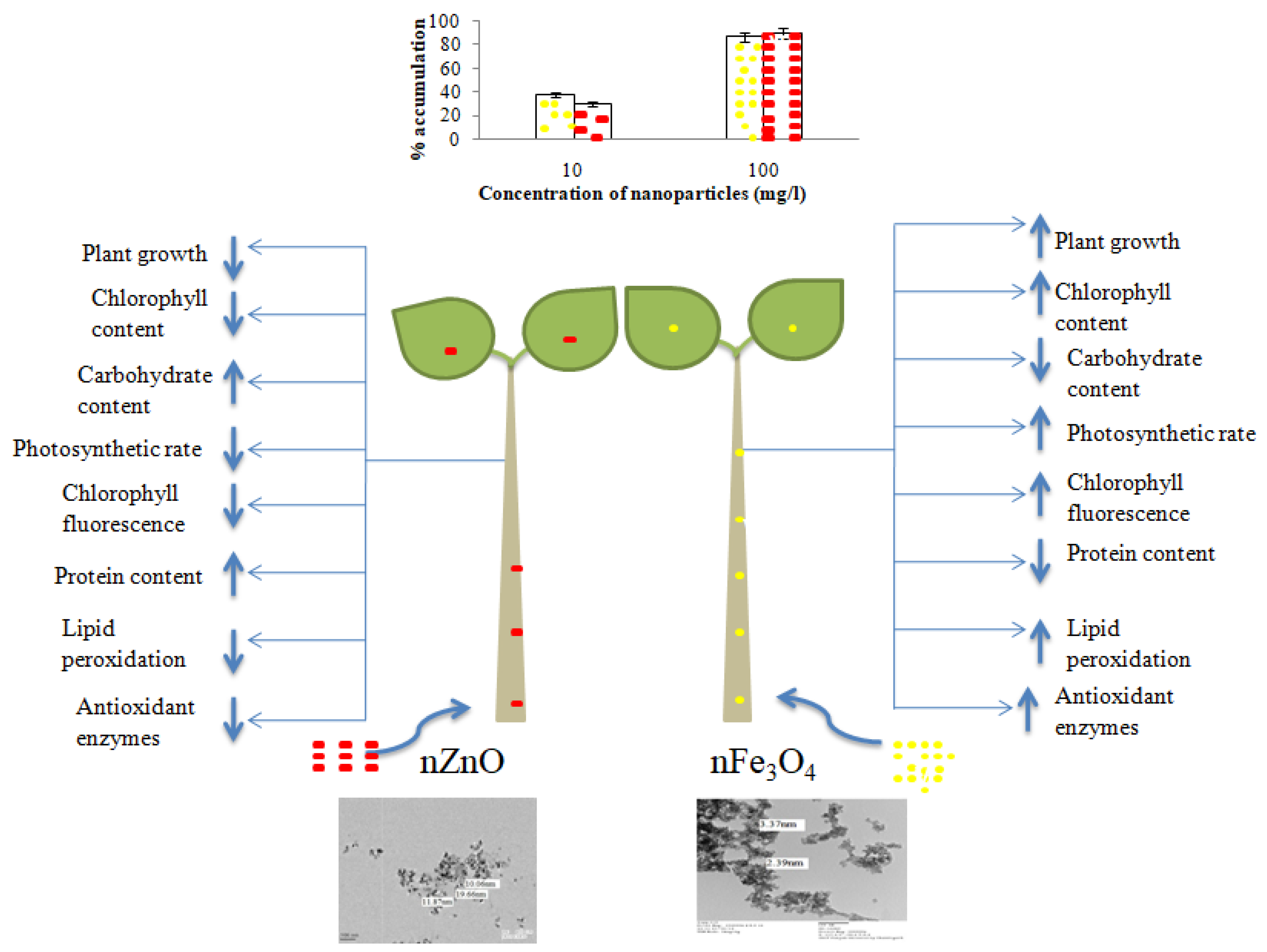
3.2. Bioaccumulation of nFe3O4 and nZnO
3.3. Seed Germination and Plant Growth
3.4. Chlorophyll Content
3.5. Protein Content
3.6. Gaseous Exchange, Chlorophyll Fluorescence and Carbohydrate Content
3.7. Lipid Peroxidation
3.8. Activities of Antioxidant Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, Z.M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Teske, S.S.; Detweiler, C.S. The biomechanisms of metal and metal oxide nanoparticles interactions with cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, K.V.; Anugraga, A.R.; Kannan, M.; Singaravelu, G.; Govindraju, K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour in green gram (Vigna radiata L.). Mater. Lett. 2020, 271, 127792. [Google Scholar] [CrossRef]
- Ramesh, R.; Catherine, G.; Sundaram, S.J.; Khan, F.L.A.; Kaviyarasu, K. Synthesis of Mn3O4 nano complex using aqueous extract of Helianthus annuus seed cake and its effect on biological growth of Vigna radiata. Mater. Today Proc. 2020, 36, 184–191. [Google Scholar] [CrossRef]
- Najafi, S.; Jamei, R. Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of Mung bean (Vigna radiata). J. Stress Physiol. Biochem. 2014, 10, 316–325. [Google Scholar]
- Prakash, M.; Gopalakrishnan, N.; Deung-Hyun, K.; Ill, M.C. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: Physiological and molecular level responses of in vitro grown plants. Acta Physiol. Plant. 2014, 36, 2947–2958. [Google Scholar]
- Jahagirdar, A.S.; Shende, S.; Gade, A.; Rai, M. Bioinspired synthesis of copper nanoparticles and its efficacy on seed viability and seedling growth in mung bean (Vigna radiata L.). Curr. Nanosci. 2020, 16, 246–252. [Google Scholar] [CrossRef]
- Piccino, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Pramod, M.; Dhoke, S.K.; Khanna, A.S.; Tarafdar, J.C. Effect of nano-ZnO on growth of mung bean (Vigna radiata) and chickpea (Cicer arietinum) seedlings using plant agar method. Appl. Biol. Res. 2011, 13, 54–61. [Google Scholar]
- Rani, P.; Kaur, G.; Rao, K.V.; Singh, J.; Rawat, M. Impact of green synthesized metal oxide nanoparticles on seed germination and seedling growth of Vigna radiata (Mung Bean) and Cajanus cajan (Red Gram). J. Inorg. Organomet. Polym. Mater. 2020, 30, 4053–4062. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Norouzi, M.; Saeri, M. Impact of zinc and zinc oxide nanoparticles on the physiological and biochemical processes in tomato and wheat. Can. Sci. Publ. Bot. 2016, 95, 441–455. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, R.; Zheng, F.; Zhang, S.; Jiao, W.; Wang, F. Evaluating phytotoxicity of bare and starch-stabilized zero-valent iron nanoparticles in mung bean. Chemosphere 2019, 236, 124336. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, W.; Zheng, F.; Zhang, S.; Wang, F.; Liu, S. Phytotoxicity of iron-based materials in mung bean: Seed germination tests. Chemosphere 2020, 251, 12643. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, H.; Prasanna, R.; Kaur, S.; Thapa, S.; Kanchan, A.; Singh, R.; Kaushik, S.C.; Singh, S.; Nain, L. Deciphering the mode of interactions of nanoparticles with mung bean (Vigna radiata L.). Isr. J. Plant Sci. 2018, 65, 74–82. [Google Scholar] [CrossRef]
- Khare, T.; Kumar, V.; Kishor, P.B.K. Na+ and Cl- ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 2014, 252, 1149–1165. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Hoque, T.S.; Hasan, M.M.; Burritt, D.J.; Hossain, M.A. Glycinebetaine-mediated abiotic oxidative-stress tolerance in plants: Physiological and biochemical mechanisms. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Sarwat, M., Ed.; Springer International: Cham, Switzerland, 2017; Volume 2, pp. 111–133. [Google Scholar]
- Maity, D.; Aggarwal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Nazari, T.; Badraghi, J.; Kazemzad, M. Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 2009, 4, 247–257. [Google Scholar]
- Siva, G.V.; Benita, L.F.J. Iron oxide nanoparticles promote agronomic traits of ginger (Zingiber officinale Rosc.). Int. J. Adv. Res. Biol. Sci. 2016, 3, 230–237. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhem, Germany, 1983; Volume 3, pp. 273–286. [Google Scholar]
- David, M.; Richard, J.S. Glutathione reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Rani, P.; Unni, M.K.; Karthikeyan, J. Evaluation of antioxidant properties of berries. Indian J. Clin. Biochem. 2004, 19, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habig, W.H.; Pabst, M.J.; Jocoby, W.B. Glutathione-S-transferase: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7310–7339. [Google Scholar]
- Lori, N.; Eydokia, P.; Vassilis, Z.; Anna, P.; Eleana, H.; Christos, N.P. Magnetic nanoparticles in medical diagnostic applications: Synthesis, characterization and proteins conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar]
- Deepa, R.; Muthuraj, D.; Kumar, E.; Veeraputhiran, V. Microwave assisted synthesis of zinc oxide nanoparticles and its antimicrobial efficiency. J. Nanosci. Technol. 2019, 5, 640–641. [Google Scholar]
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; Jose-Yacaman, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515, 60–69. [Google Scholar] [CrossRef]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhu, S. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef] [Green Version]
- Elfeky, S.A.; Mohammed, M.A.; Khater, M.S.; Osman, Y.A.; Elsherbini, E. Effect of magnetite nano-fertilizer on growth and yield of Ocimumbasilicum L. Int. J. Indig. Med. Plants 2013, 46, 1286–1293. [Google Scholar]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, X. Physiological role of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar]
- Plaksenkova, I.; Jermalonoka, M.; Bankovska, L.; Gavarane, I.; Gerbreders, V.; Sledevskis, J.; Kokina, I. Effects of Fe3O4 nanoparticles stress on the growth and development of rocket Eruca sativa. J. Nanomater. 2019, 2019, 2678247. [Google Scholar] [CrossRef] [Green Version]
- Pawar, V.A.; Ambekar, J.D.; Kale, B.B.; Apte, S.K.; Laware, S.L. Response in chickpea (Cicer arietinum L.) seedling growth to seed priming with iron oxide nanoparticles. Int. J. Biosci. 2019, 14, 82–91. [Google Scholar]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Chung, H.; Kim, S.; Lee, I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut. 2013, 224, 1668. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.; Kim, E.J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; Chang, Y.S. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452, 321–332. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, S.; Li, Q.; Wang, W.; Hou, C.; Gao, X.; Wang, L.; Wang, S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 12–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.; Miller, G.W. Copro-porphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem. J. 1969, 117, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, S.C.; Castelfranco, A.; Castelfranco, P. Effects of iron and oxygen on chlorophyll biosynthesis. I. In vivo observations of iron and oxygen-deficient plants. Plant Physiol. 1982, 69, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupper, H.; Kupper, F.C.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6, 12–42. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef]
- Ali, G.; Srivastava, P.S.; Iqbal, M. Influence of cadmium and zinc on growth and photosynthesis of Bacopa monniera cultivated in vitro. Biol. Plant. 2000, 43, 599–601. [Google Scholar] [CrossRef]
- Bonnet, M. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar]
- Kisan, B.; Shruthi, H.; Sharanagouda, H.; Revanappa, S.B.; Pramod, N.K. Effect of nano-zinc oxide on the leaf physical and nutritional quality of spinach. Agrotechnology 2015, 5, 135. [Google Scholar]
- Patra, P.; Choudhury, S.R.; Mandal, S.; Basu, A.; Goswami, A.; Gogoi, R.; Gopal, M. Effect of sulfur and ZnO nanoparticles on stress physiology and plant (Vigna radiata) nutrition. In Advanced Nanomaterials and Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 301–309. [Google Scholar]
- Bona, E.; Marsano, F.; Cavaletto, M.; Berta, G. Proteomic characterization of copper stress response in Cannabis sativa roots. Proteomics 2007, 7, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to Cd exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits and antioxidative enzymes in tomato plants. Biol. Plantarium 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Krasavina, M.S.; Burmistrova, N.A.; Raldugina, G.N. Chapter 11—The Role of Carbohydrates in Plant Resistance to Abiotic Stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Parviz, A., Saiema, R., Eds.; Academic Press: New York, NY, USA, 2014; pp. 229–270. [Google Scholar]
- Souza, L.R.R.; Bernardes, L.E.; Barbetta, M.F.S.; Veiga, M.A.M.S. Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: Effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Pollut. Res. 2019, 26, 24121–24131. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Yauger, Y.J.; Bermudez, S.; Moritz, K.E.; Glaser, E.; Stoica, B.; Byrnes, K.R. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J. Neuro Inflamm. 2019, 16, 41. [Google Scholar] [CrossRef]
- Chvapil, M. Zinc and NADPH-oxidation-dependent lipid peroxidation. In Oxygen Free Radicals and Tissue Damage; CIBA Foundation Symposium (New Series) No. 65; Excerpta Medica: Amsterdam, The Netherlands, 1979; pp. 163–166. [Google Scholar]
- Morrissey, J.; Guerinot, M.L. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [Green Version]
- Fine, P.L.; Frasch, W.D. The oxygen-evolving complex requires chloride to prevent hydrogen peroxide formation. Biochemistry 1992, 31, 12204–12210. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van, M.M.; Inze, D.; Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Foyer, C.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signaling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Xiao, L.; Wang, S.; Yang, D.; Zou, Z.; Li, J. Physiological effects of MgO and ZnO nanoparticles on the Citrus maxima. J. Wuhan Univ. Technol. (Mater. Sci.) Ed. 2019, 34, 243–253. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; Sousa, M.E.; Raap, M.B.F.; Benavides, M.P. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot. 2016, 131, 77–88. [Google Scholar] [CrossRef]
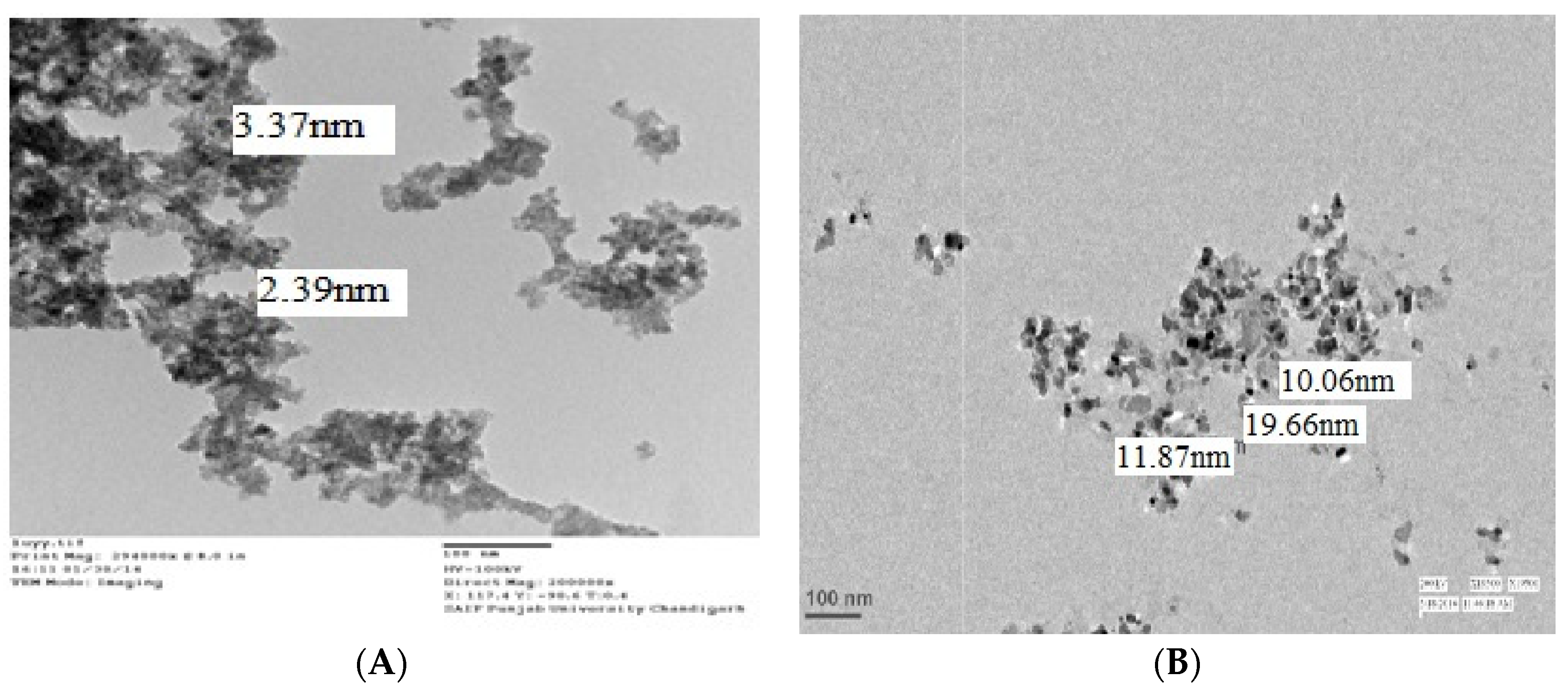
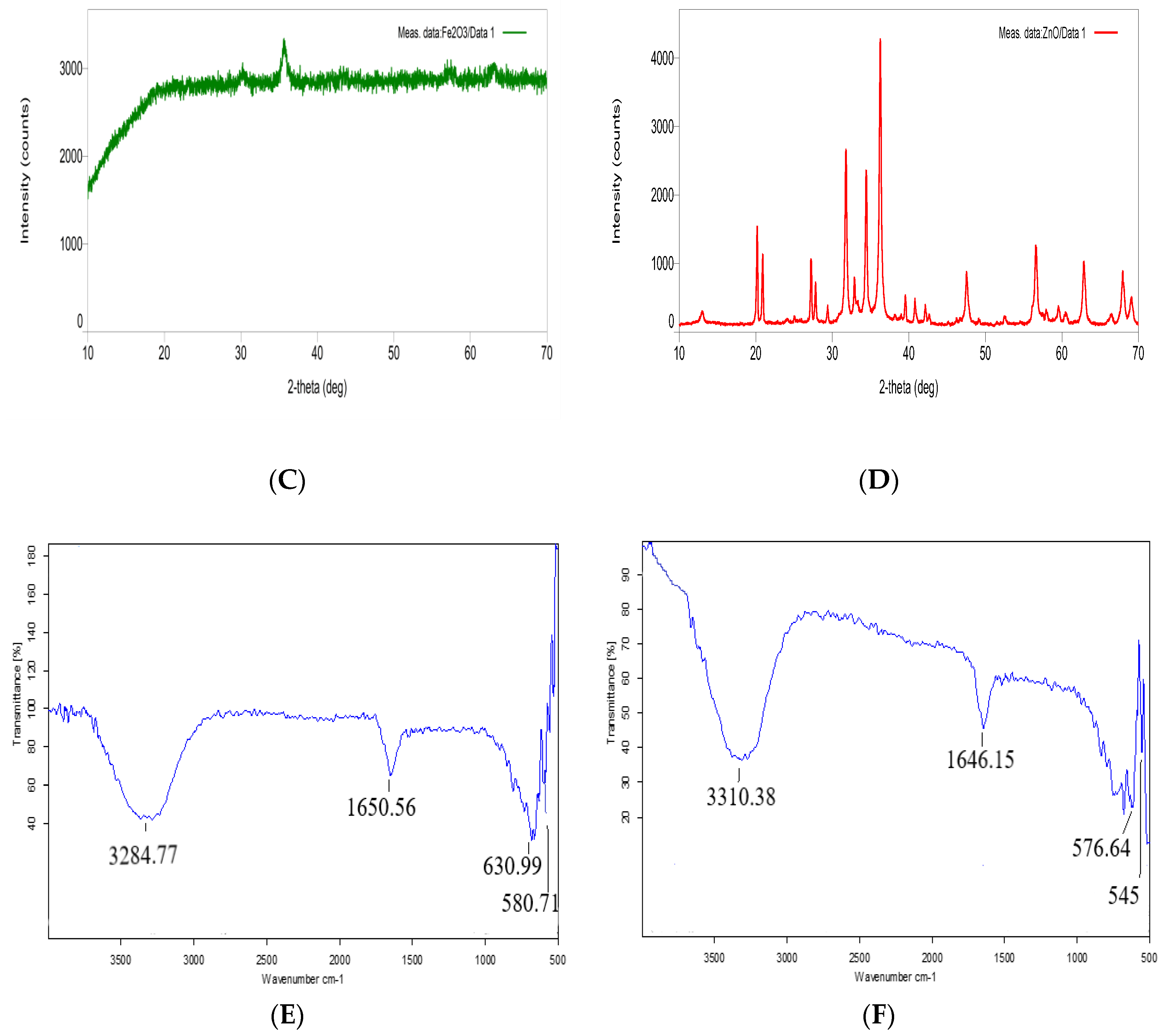
 )- and nZnO (
)- and nZnO (  )-treated and untreated14-day-old V. radiata plants. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
)-treated and untreated14-day-old V. radiata plants. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
 )- and nZnO (
)- and nZnO (  )-treated and untreated14-day-old V. radiata plants. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
)-treated and untreated14-day-old V. radiata plants. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.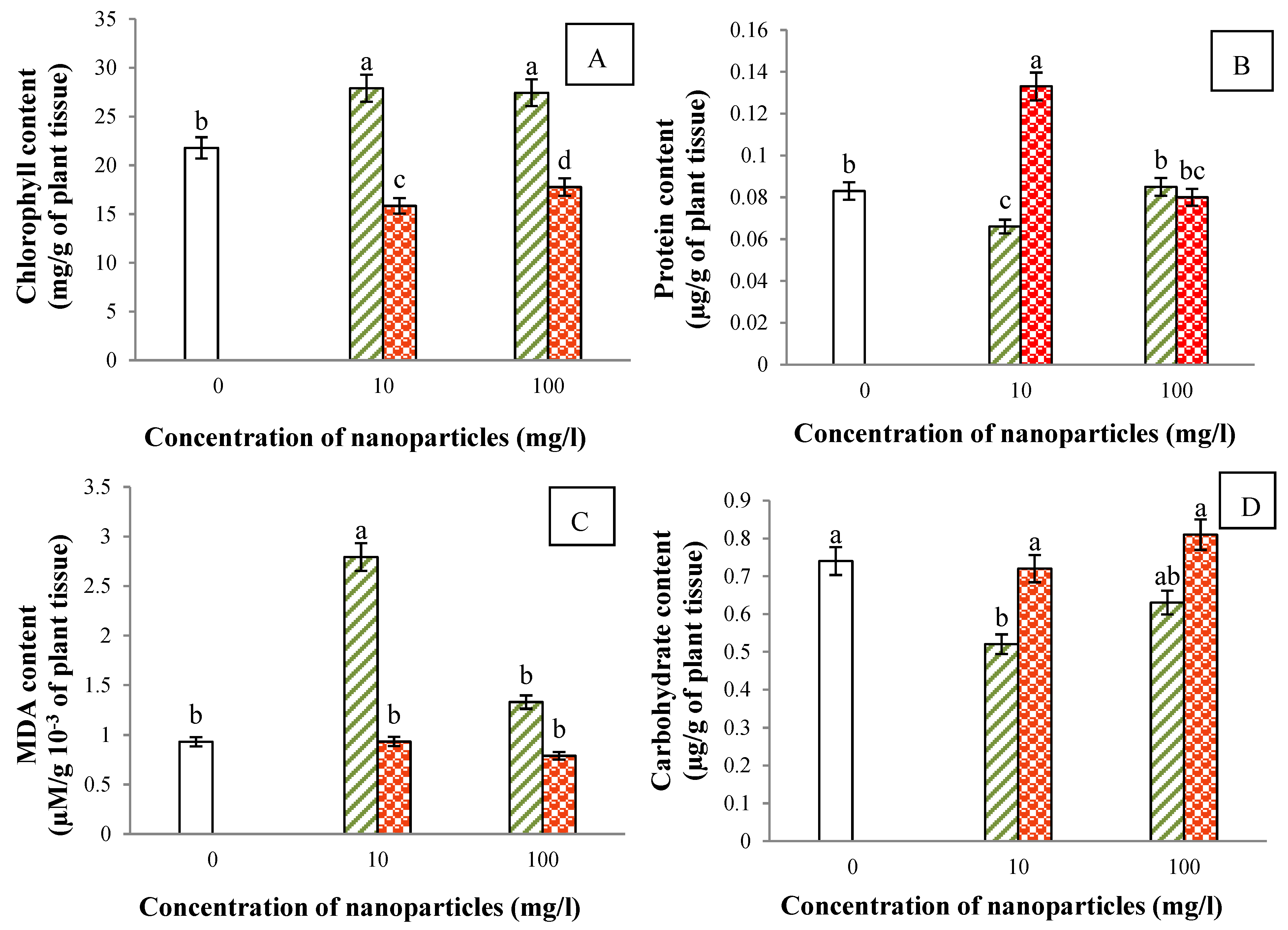
 )- and nZnO (
)- and nZnO (  )-spiked Hoagland solution. Column with label “0” on the x-axis represents control. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
)-spiked Hoagland solution. Column with label “0” on the x-axis represents control. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
 )- and nZnO (
)- and nZnO (  )-spiked Hoagland solution. Column with label “0” on the x-axis represents control. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.
)-spiked Hoagland solution. Column with label “0” on the x-axis represents control. The values are mean of five different replicates, error bars represent standard error and different letters indicate significant differences at p < 0.05.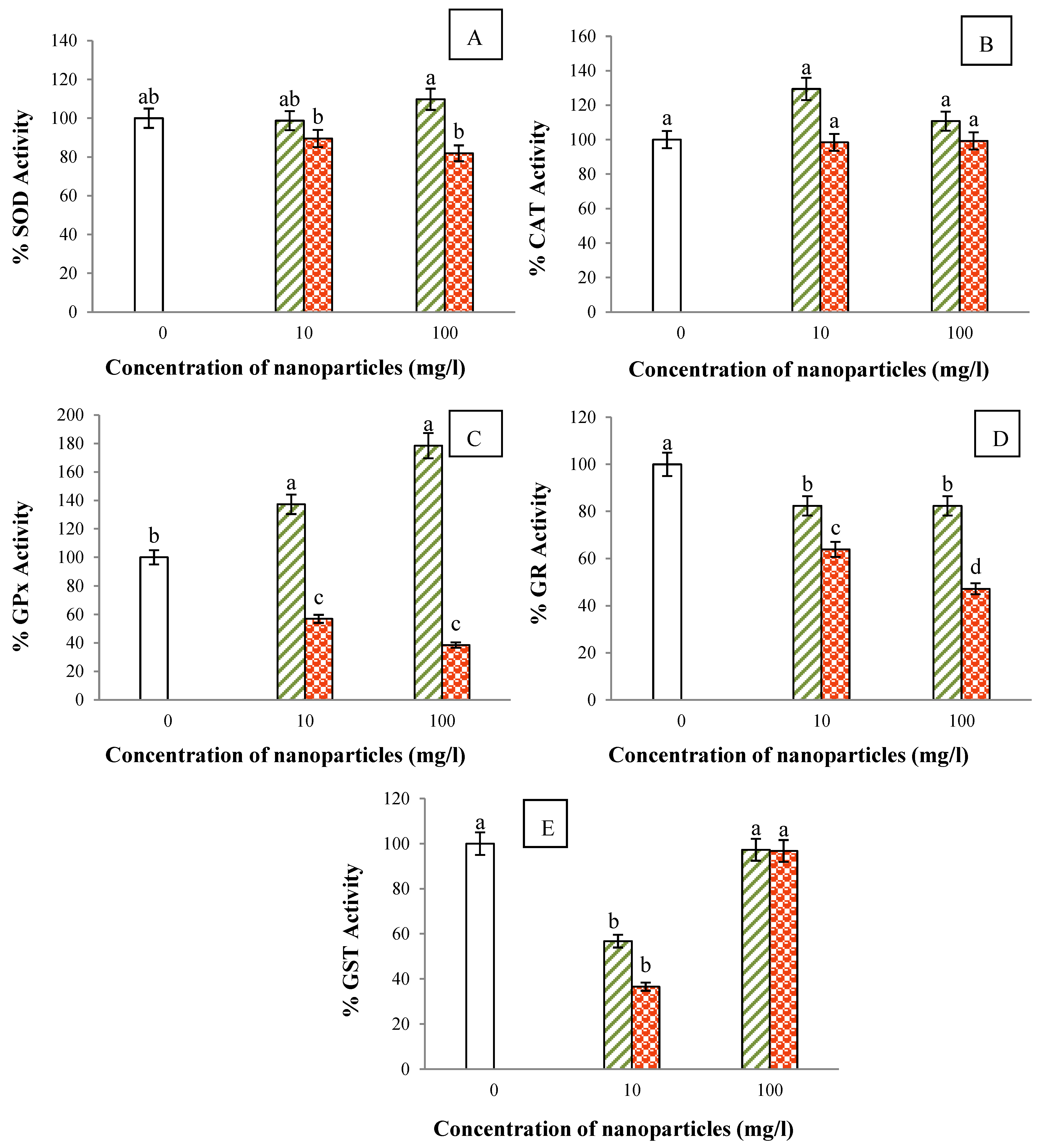
| Concentration of Nanoparticles (mg/L) | Elemental Content in Shoot (mg/kg) Mean ± S.E. | |
|---|---|---|
| Zn | Fe | |
| Control | 0.26 ± 0.01 c | 3.81 ± 0.01 b |
| 10 | 3.0 ± 0.01 b | 3.82 ± 0.01 b |
| 100 | 90.1 ± 0.45 a | 86.4 ± 0.16 a |
| S. No. | Growth Parameters | Concentration of Nanoparticles (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| Control | Fe3O4 | ZnO | |||||
| 0 | 10 | 100 | 10 | 100 | |||
| 1. | Shoot length (cm) | 22.46 ± 1.98 b | 25.37 ± 0.92 b | 28.88 ± 0.90 a | 23.18 ± 1.92 b | 22.24 ± 2.18 b | |
| 2. | Root length (cm) | 17.46 ± 1.99 a | 19.17 ± 0.95 a | 22.25 ± 1.06 a | 18.94 ± 2.8 a | 16.84 ± 2.05 a | |
| 3. | Plant biomass (g) | Fresh weight | 0.61 ± 0.25 c | 0.83 ± 0.15 b | 0.97 ± 0.28 a | 0.70 ± 0.09 c | 0.57 ± 0.15 d |
| Dry weight | 0.049 ± 0.02 a | 0.080 ± 0.01 a | 0.087 ± 0.03 a | 0.058 ± 0.01 a | 0.045 ± 0.03 a | ||
| S. No. | Parameters Tested | Concentration of Nanoparticles (mg/L) | ||||
|---|---|---|---|---|---|---|
| Control | Fe3O4 | ZnO | ||||
| 0 | 10 | 100 | 10 | 100 | ||
| 1. | Photosynthetic rate (µmol CO2 m−2s−1) | 4.77 ± 0.17 a | 5.283 ± 0.41 a | 5.203 ± 0.44 a | 4.373 ± 0.81 a | 4.283 ± 0.41 a |
| 2. | Stomatal conductance (µmol CO2 m−2s−1) | 0.056 ± 0.006 b | 0.173 ± 0.040 a | 0.113 ± 0.021 ab | 0.116 ± 0.015 ab | 0.116 ± 0.015 ab |
| 3. | Transpiration rate (mmol H2O m−2s−1) | 1.01 ± 0.197 b | 2.53 ± 0.153 a | 2.1 ± 0.137 a | 2.67 ± 0.397 a | 2.15 ± 0.555 a |
| 4. | Chlorophyll fluorescence (Fv/Fm) | 0.581 ± 0.026 b | 0.678 ± 0.020 a | 0.668 ± 0.012 a | 0.558 ± 0.011 b | 0.521 ± 0.013 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, N.; Kumari, K.; Sangwan, P.; Barala, P.; Yadav, J.; Vijeta; Rahul; Hooda, V. Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata. Sustainability 2022, 14, 8251. https://doi.org/10.3390/su14148251
Rani N, Kumari K, Sangwan P, Barala P, Yadav J, Vijeta, Rahul, Hooda V. Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata. Sustainability. 2022; 14(14):8251. https://doi.org/10.3390/su14148251
Chicago/Turabian StyleRani, Neelam, Kusum Kumari, Parul Sangwan, Poonam Barala, Jyoti Yadav, Vijeta, Rahul, and Vinita Hooda. 2022. "Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata" Sustainability 14, no. 14: 8251. https://doi.org/10.3390/su14148251
APA StyleRani, N., Kumari, K., Sangwan, P., Barala, P., Yadav, J., Vijeta, Rahul, & Hooda, V. (2022). Nano-Iron and Nano-Zinc Induced Growth and Metabolic Changes in Vigna radiata. Sustainability, 14(14), 8251. https://doi.org/10.3390/su14148251





