Photosynthetic Characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with Special Reference to the Effects of Light Quality
Abstract
:1. Introduction
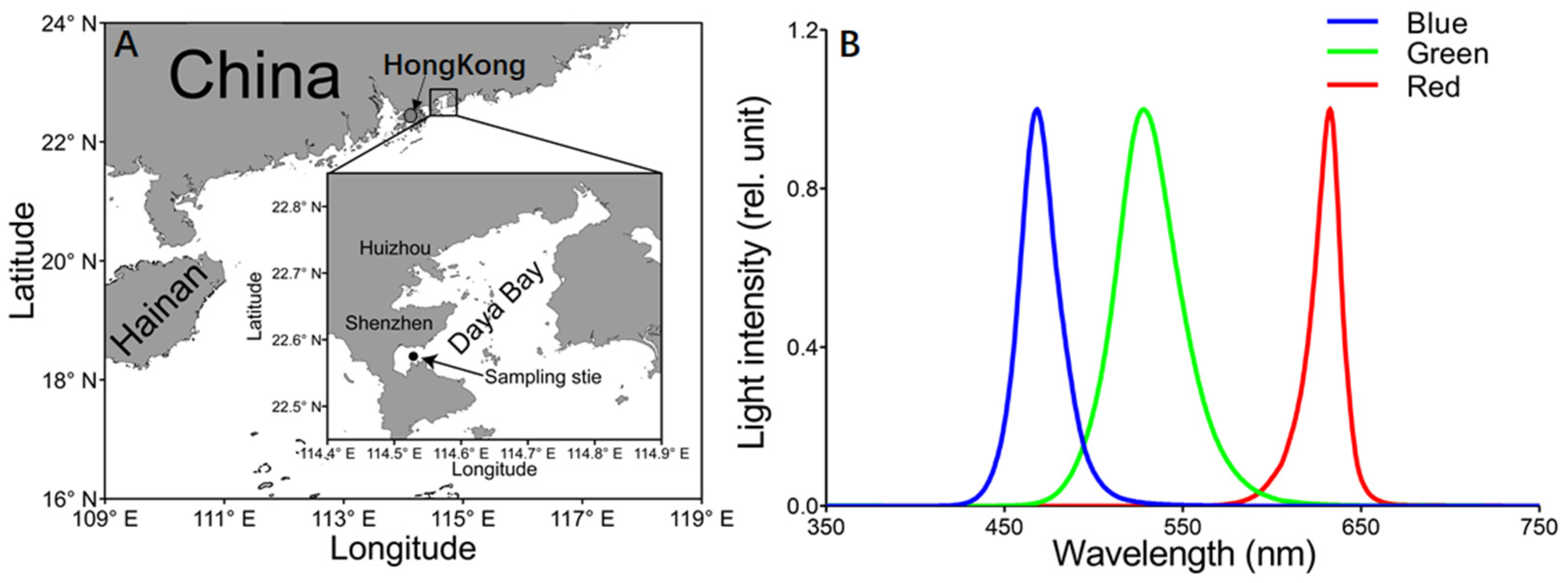
2. Materials and Methods
2.1. Experimental Protocol
2.2. Diel Photosynthetic O2 Evolution and Dark Respiration
2.3. Diel Chlorophyll Fluorescence
2.4. P vs. E Curves under Different Light Qualities
2.5. Environmental Factors and Pigment Light Absorption
2.6. Data Analysis
3. Results
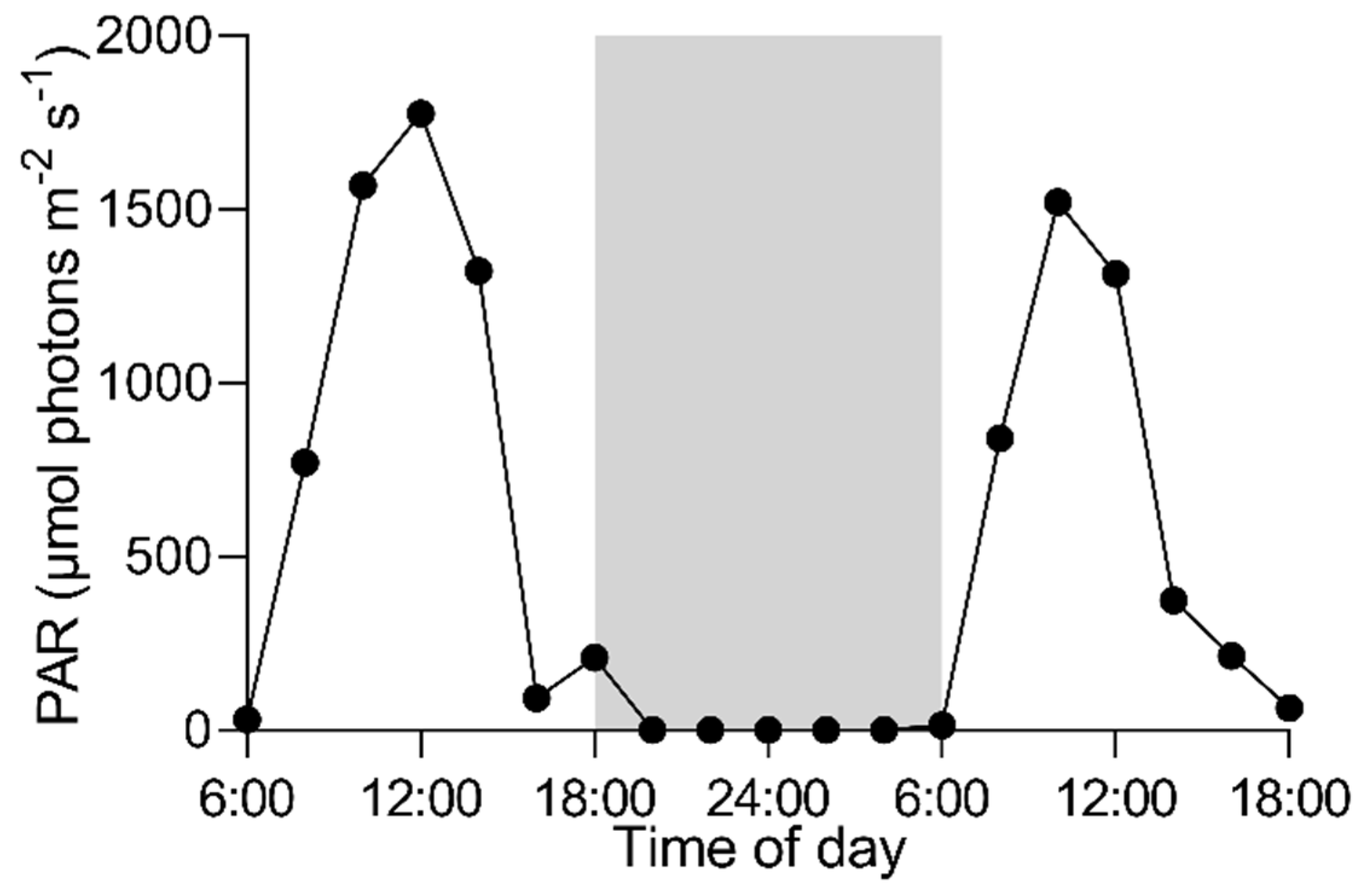
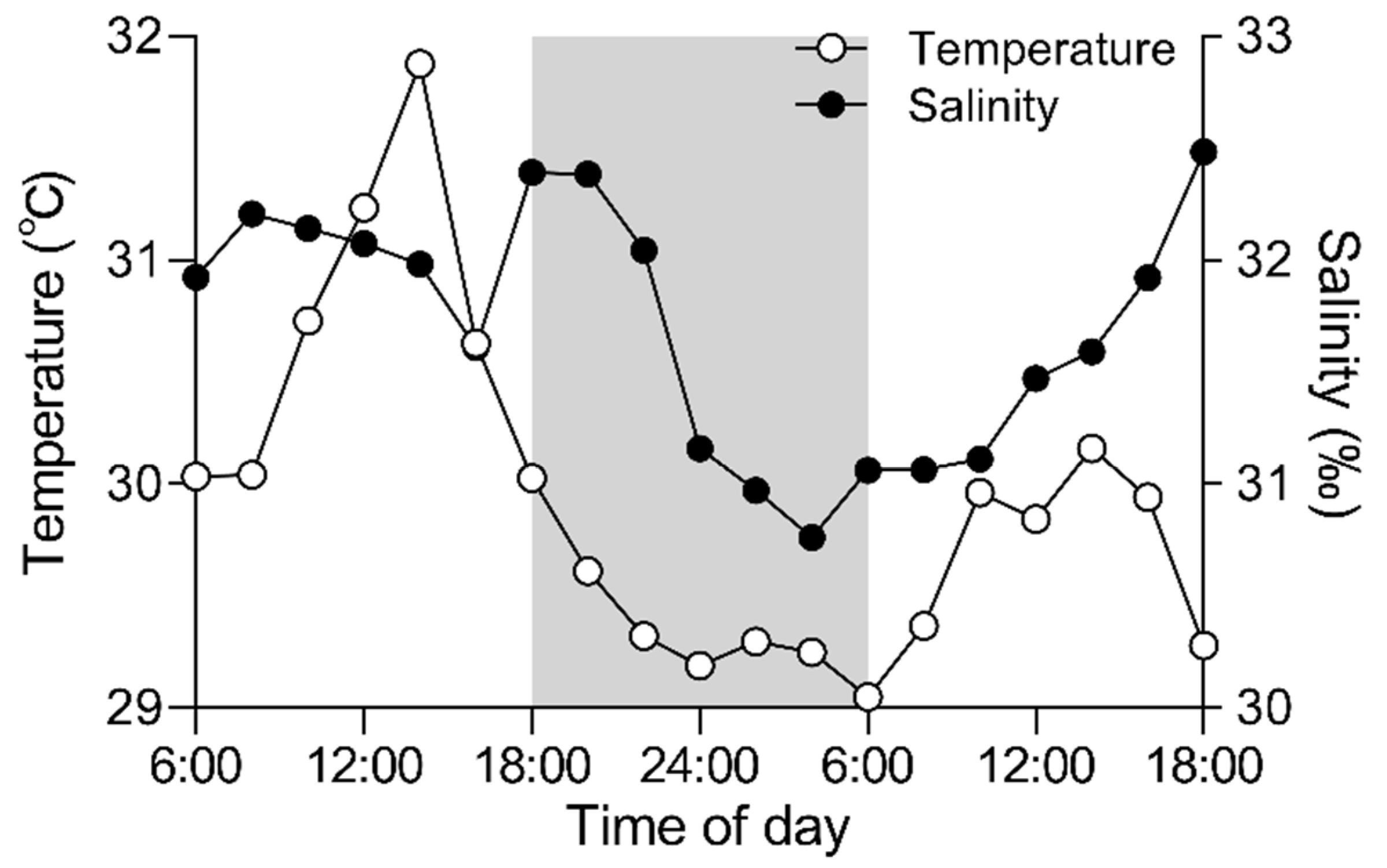
3.1. Daily Field Environmental Changes
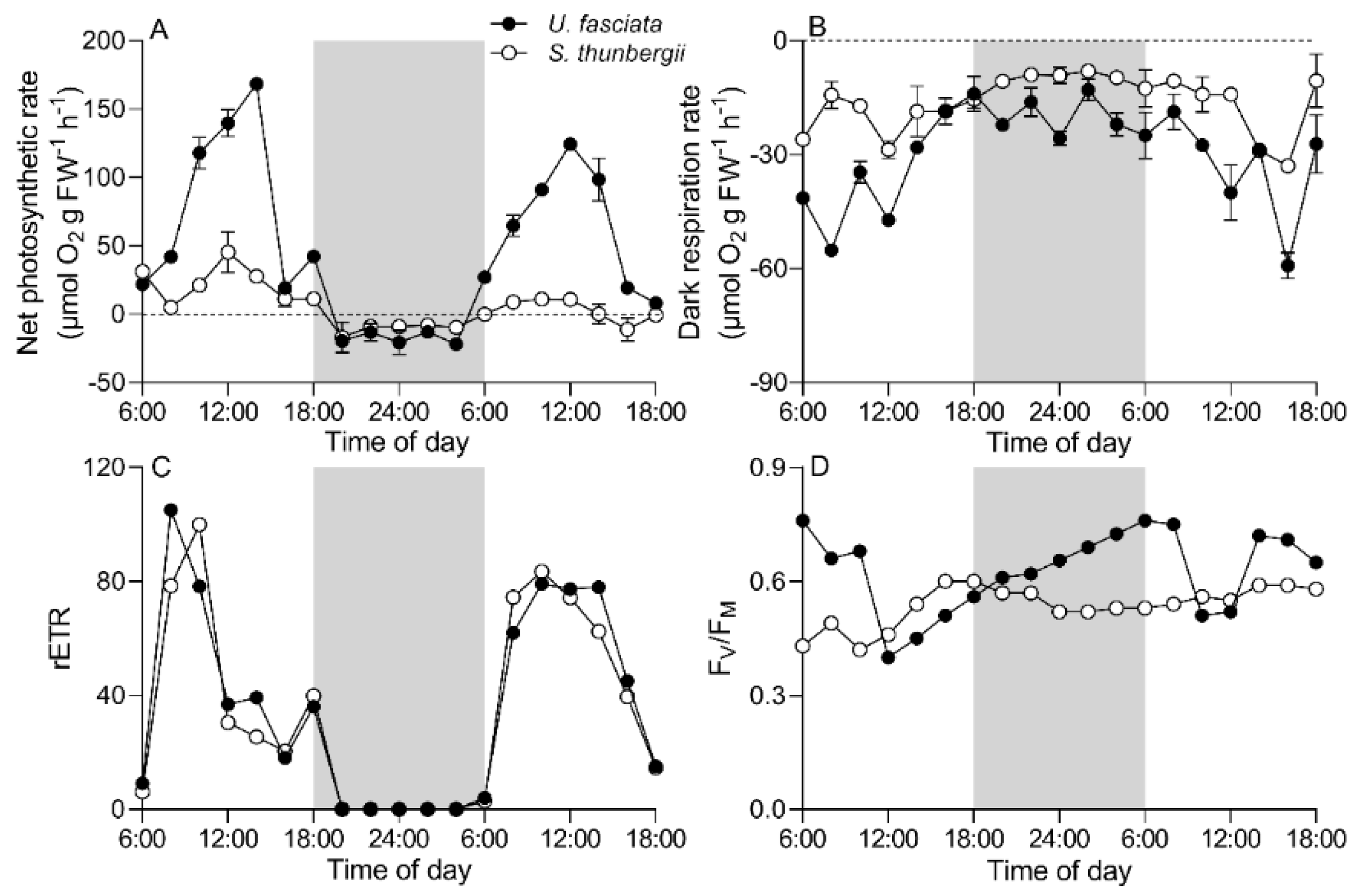
3.2. Daily Photosynthesis and Respiration Changes
3.3. Photosynthetic Characteristics under Different Light Qualities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Ji, Y.; Gao, K. Effects of climate change factors on marine macroalgae: A review. Adv. Mar. Biol. 2021, 88, 91–135. [Google Scholar] [PubMed]
- Smith, S.V. Marine macrophytes as a global carbon sink. Nature 1981, 211, 838–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, C.; Dijkstra, J.A.; Mello, K.; Stevens, A.; O’Brien, B.; Ikedo, W. A novel three-dimensional analysis of functional architecture that describes the properties of macroalgae as a refuge. Mar. Ecol. Progr. Ser. 2019, 608, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Zollmann, M.; Rubinsky, B.; Liberzon, A.; Golberg, A. Multi-scale modeling of intensive macroalgae cultivation and marine nitrogen sequestration. Commun. Biol. 2021, 4, 848. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Q.; Brodie, J. A review of the bladed Bangiales (Rhodophyta) in China: History, culture, and taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Gao, G.; Burgess, G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–371. [Google Scholar] [CrossRef]
- Falkenberg, M.; Nakano, E.; Zambotti-Villela, L.; Zatelli, G.A.; Philippus, A.C.; Imamura, K.B.; Velasquez, A.M.A.; Freitas, R.F.; Tallarico, L.F.; Colepicolo, P.; et al. Bioactive compounds against neglected diseases isolated from macroalgae: A review. J. Appl. Phycol. 2019, 31, 797–823. [Google Scholar] [CrossRef] [Green Version]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Tait, L.W.; Hawes, I.; Schiel, D.R. Shining light on benthic macroalgae mechanisms of complementarity in layered macroalgal assemblages. PLoS ONE 2014, 9, e114146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Li, G.; Helbling, E.W.; Villafañe, E.V. Variability of UVR effects on photosynthesis of summer phytoplankton assemblages from a tropical coastal area of the South China Sea. Photochem. Photobiol. 2007, 83, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Xu, J.; Zhang, X.; Fu, Q.; Gao, G.; Gao, K. Water depth-dependent photosynthetic and growth rates of Gracilaria lemaneiformis, with special reference to effects of solar UV radiation. Aquaculture 2018, 484, 28–31. [Google Scholar] [CrossRef]
- Wu, H.; Gao, G.; Zhong, Z.; Li, X.; Xu, J. Physiological acclimation of the green tidal alga Ulva prolifera to a fast-changing environment. Mar. Environ. Res. 2018, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mai, G.; Zhang, J.; Ni, G.; Shi, X.; Tan, Y.; Zou, D. Rising pCO2 interacts with algal density to reversely alter photosynthetic responses of Gracilaria lemaneiformis and Ulva conglobata. Algal Res. 2021, 54, 102231. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Weyrauch, S.K. Photosynthetic action spectra and light-harvesting in Griffithsia monilis (Rhodophyta). Photochem. Photobiol. 1977, 25, 65–72. [Google Scholar] [CrossRef]
- Haxo, F.T.; Blinks, L.R. Photosynthetic action spectra of marine algae. J. Gen. Physiol. 1950, 33, 389–442. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.; Cruz, S.; Calado, R.; Lillebø, A.; Abreu, H.; Pereira, R.; Pitarma, B.; da Silva, J.M.; Cartaxana, P. Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum. J. Appl. Phycol. 2021, 33, 471–480. [Google Scholar] [CrossRef]
- Lüning, K.; Dring, M.J. Action spectra and spectral quantum yield of photosynthesis in marine macroalgae with thin and thick thalli. Mar. Biol. 1985, 87, 119–129. [Google Scholar] [CrossRef]
- Talarico, L.; Maranzana, G. Light and adaptive responses in red macroalgae: An overview. J. Photochem. Photobiol. B Biol. 2000, 56, 1–11. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, K. NH4+ enrichment and UV radiation interact to affect the photosynthesis and nitrogen uptake of Gracilaria lemaneiformis (Rhodophyta). Mar. Pollut. Bull. 2012, 64, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zou, D.; Hu, S.; Mai, G.; Ma, Z.; Li, G. Photosynthetic characteristics of three cohabitated macroalgae in the Daya Bay, and their responses to temperature rise. Plants 2021, 10, 2441. [Google Scholar] [CrossRef] [PubMed]
- Scarabel, L.; Camuccio, P.; Foltran, A.; Maranzana, G.; Talarico, L.; Rascio, N.; Andreoli, C. Thalli of Plocamium cartilagineum (L.) Dixon (Gigartinales, Rhodophyta) growing at two different depths. Gior. Bot. It. 1996, 130, 690–693. [Google Scholar]
- Dring, M.J. Blue light effects in marine macroalgae. In Blue Light Effects in Biological Systems; Serger, H., Ed.; Springer: Berlin, Germany, 1984; pp. 509–516. [Google Scholar]
- Huang, H.; Lin, Q.; Lin, Y.; Jia, X.; Li, C.; Wang, W. Spatial-temporal variation of large macrobenthic animals in cage culture sea area in Daya Bay. Chin. Environ. Sci. 2005, 25, 412–416, (In Chinese with English abstract). [Google Scholar]
- Xu, G.; Liu, J.; Song, X.; Tan, M.; Ren, H.; Li, D.; Tan, Y.; Huang, L.; Li, G. Diel rhythm in photosynthetic performance of phytoplankton assemblages is predicted to be light-dependent from in situ and mesocosm chlorophyll fluorescence. J. Coast. Res. 2020, 104, 445–454. [Google Scholar] [CrossRef]
- Yu, J.; Tang, D.; Wang, S.; Lian, J.; Wang, Y. Changes of water temperature and harmful algal bloom in the Daya Bay in the northern South China Sea. Mar. Sci. Bull. 2007, 9, 25–33. [Google Scholar]
- Xu, G. Environments and Resources of Daya Bay; Anhui Press of Science and Technology: Hefei, China, 1989. (In Chinese) [Google Scholar]
- Wang, Y.; Lou, Z.; Sun, C.; Sun, S. Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar. Pollut. Bull. 2008, 56, 1871–1879. [Google Scholar] [CrossRef]
- Li, T.; Liu, S.; Huang, L.; Huang, H.; Lian, J.; Yan, Y.; Lin, S.-J. Diatom to dinoflagellate shift in the summer phytoplankton community in a bay impacted by nuclear power plant thermal effluent. Mar. Ecol. Progr. Ser. 2011, 424, 75–85. [Google Scholar] [CrossRef]
- Mai, G.; Song, X.; Xia, X.; Ma, Z.; Tan, Y.; Li, G. Photosynthetic characteristics of smaller and larger cell size-fractioned phytoplankton assemblies in the Daya Bay, northern South China Sea. Microorganisms 2022, 10, 16. [Google Scholar] [CrossRef]
- Yang, Y. Coastal Environmental Bioremediation and Seaweed Resource Utilization; Science Press: Beijing, China, 2016; pp. 1–364. (In Chinese) [Google Scholar]
- Luo, D.; Chen, X.; Li, T.; Yi, J. Production of TM satellite image distribution map of macroalgae in Daya Bay. Remote Sens. Inf. 1990, 4, 30–32, (In Chinese with English abstract). [Google Scholar]
- Li, G.; Wan, M.; Shi, X.; Qin, G.; Mai, G.; Huang, L.; Tan, Y.; Zou, D. Comparative study on photophysiology of four macroalgae from the Zhongsha Atoll, with special reference to the effects of temperature rise. J. Trop. Oceanogr. 2022, 43, 101–110, (In Chinese with English abstract). [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Henley, W.J. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J. Phycol. 1993, 29, 729–739. [Google Scholar] [CrossRef]
- Williams, P.J.L.; Rainea, R.C.T.; Bryan, J.R. Agreement between the 14C and oxygen methods of measuring phytoplankton production: Reassessment of the photosynthetic quotient. Oceanol. Acta 1979, 2, 411–416. [Google Scholar]
- Enríquez, S.; Agustí, S.; Duarte, C.M. Light absorption by marine macrophytes. Oecologia 1994, 98, 121–129. [Google Scholar] [CrossRef]
- Dudgeon, S.R.; Johnson, A.S. Thick vs. thin: Thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. J. Exp. Mar. Biol. Ecol. 1992, 165, 23–43. [Google Scholar] [CrossRef]
- Bischof, K.; Peralta, G.; Kräbs, G.; van de Poll, W.H.; Pérez-Lloréns, J.L.; Breeman, A.M. Effects of solar UV-B radiation on canopy structure of Ulva communities from Southern Spain. J. Exp. Bot. 2002, 53, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Vitlin Gruber, A.; Feiz, L. Rubisco assembly in the chloroplast. Front. Mol. Biosci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Chao-Rodríguez, Y.; Domínguez-Gómez, J.A.; Sánchez-Carnero, N.; Rodríguez-Pérez, D. A comparison of spectral macroalgae taxa separability methods using an extensive spectral library. Algal Res. 2017, 26, 463–473. [Google Scholar] [CrossRef]
- Engelman, T.W. Farbe und Assimilation. Bot. Zeit. 1883, 41, 1–13, 17–29. [Google Scholar]
- Stomp, M.; Huisman, J.; de Jongh, F.; Veraart, A.J.; Gerla, D.; Rijkeboer, M.; Ibelings, B.W.; Wollenzien, U.I.A.; Stal, L.J. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 2004, 432, 104–107. [Google Scholar] [CrossRef] [PubMed]
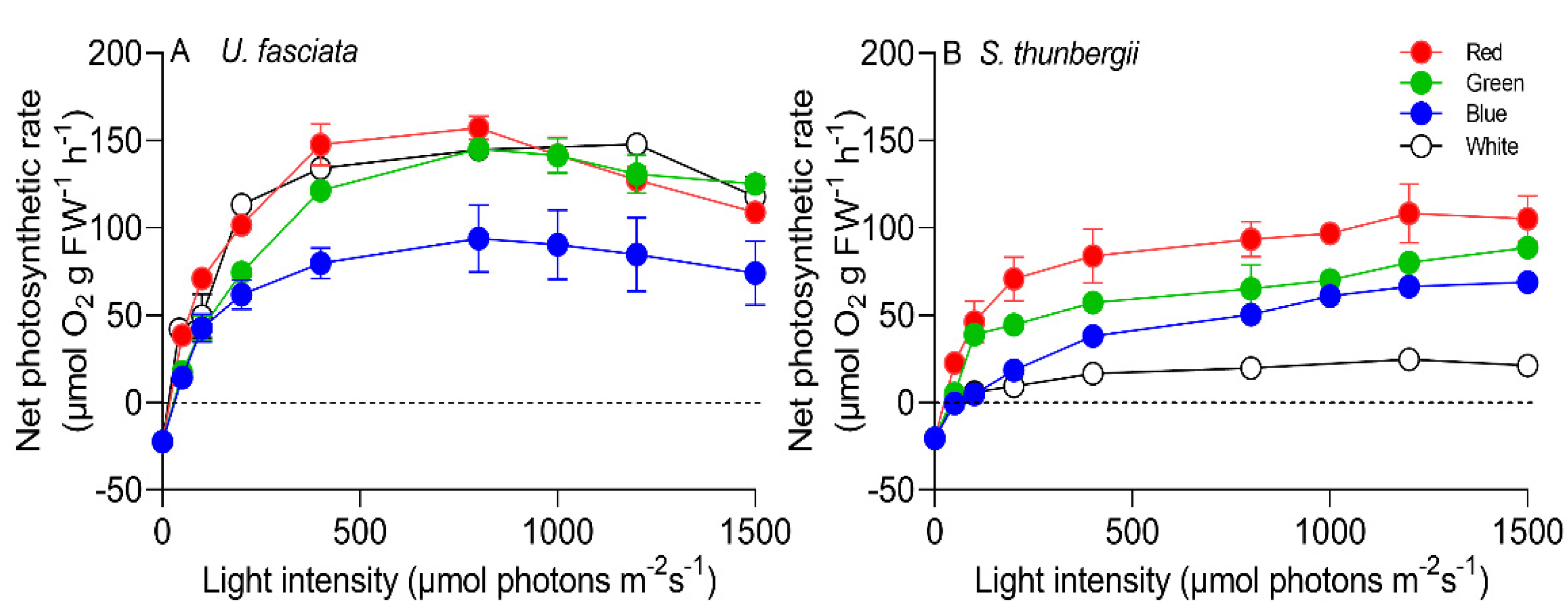
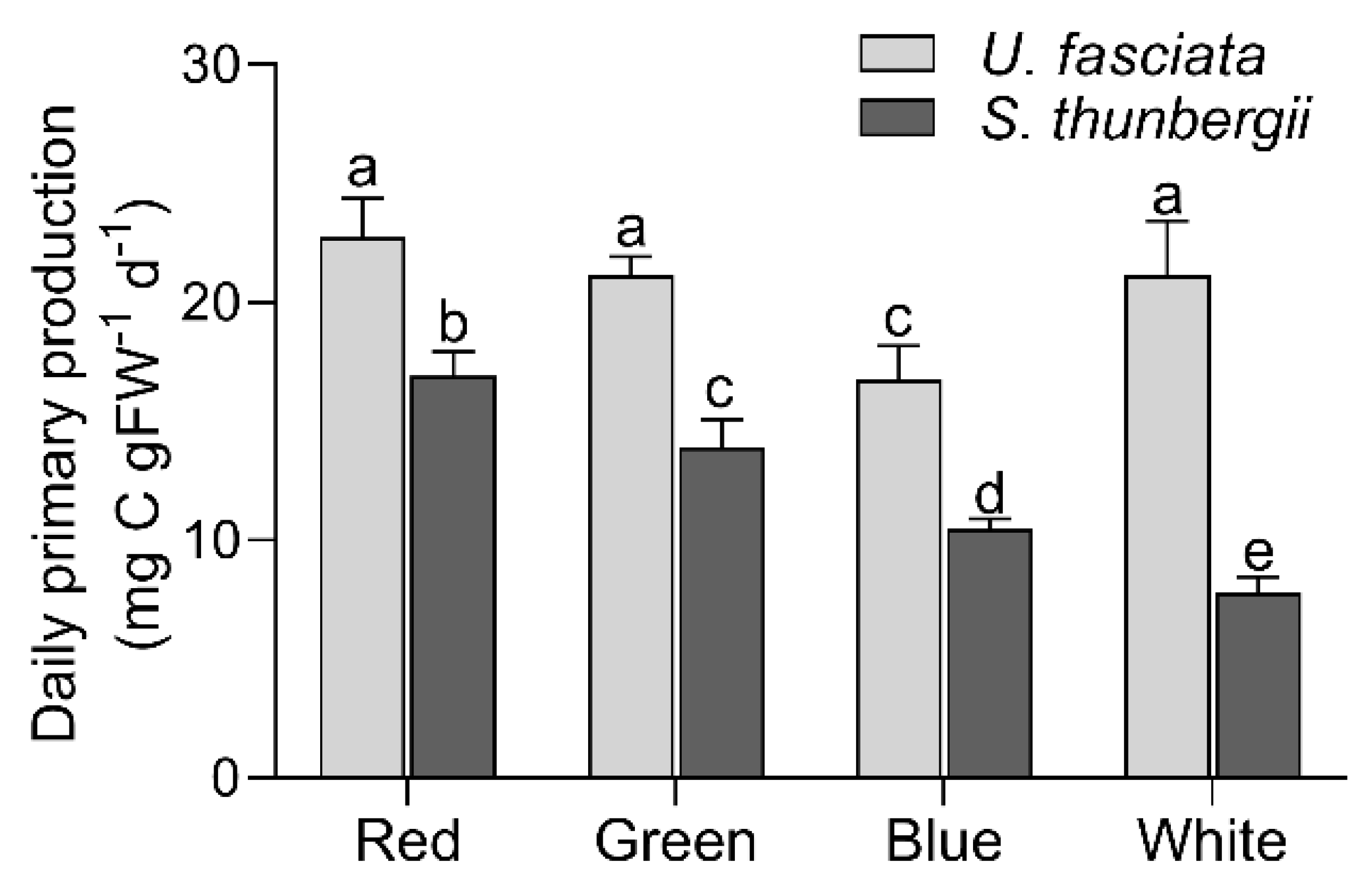


| Species | Parameters | Red | Green | Blue | White |
|---|---|---|---|---|---|
| U. fasciata | α | 0.98 ± 0.18 a | 0.58 ± 0.06 b | 0.66 ± 0.15 b | 0.79 ± 0.20 ab |
| EK | 73.1 ± 11.5 a | 123 ± 8.53 b | 243 ± 11.3 c | 91.9 ± 15.9 a | |
| Pmax | 153 ± 11.2 a | 153 ± 5.65 a | 104 ± 8.86 b | 148 ± 15.8 a | |
| S. thunbergii | α | 0.64 ± 0.10 a | 0.45 ± 0.10 a | 0.15 ± 0.02 b | 0.20 ± 0.04 b |
| EK | 82.0 ± 8.95 a | 91.9 ± 14.2 a | 244 ± 19.6 b | 91.9 ± 13.6 a | |
| Pmax | 113 ± 6.84 a | 89.3 ± 7.58 b | 78.3 ± 3.24 c | 39.2 ± 3.44 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Wang, Z.; Mai, G.; Ma, Z.; Xia, X.; Tan, Y.; Li, G. Photosynthetic Characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with Special Reference to the Effects of Light Quality. Sustainability 2022, 14, 8063. https://doi.org/10.3390/su14138063
Wan M, Wang Z, Mai G, Ma Z, Xia X, Tan Y, Li G. Photosynthetic Characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with Special Reference to the Effects of Light Quality. Sustainability. 2022; 14(13):8063. https://doi.org/10.3390/su14138063
Chicago/Turabian StyleWan, Mingyue, Zhiqin Wang, Guangming Mai, Zengling Ma, Xiaomin Xia, Yehui Tan, and Gang Li. 2022. "Photosynthetic Characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with Special Reference to the Effects of Light Quality" Sustainability 14, no. 13: 8063. https://doi.org/10.3390/su14138063
APA StyleWan, M., Wang, Z., Mai, G., Ma, Z., Xia, X., Tan, Y., & Li, G. (2022). Photosynthetic Characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with Special Reference to the Effects of Light Quality. Sustainability, 14(13), 8063. https://doi.org/10.3390/su14138063








