Abstract
Groundwater nitrate contamination is challenging and requires efficient solutions for nitrate removal. This study aims to investigate nitrate removal using a novel adsorbent, biochar-supported aluminum-substituted goethite (BAG). The results showed that an increase in the initial Al/(Al + Fe) atomic ratio for BAGs from 0 to 20% decreased the specific surface area from 115.2 to 75.7 m2/g, but enhanced the surface charge density from 0.0180 to 0.0843 C/m2. By comparison, 10% of Al/(Al + Fe) led to the optimal adsorbent for nitrate removal. The adsorbent’s adsorption capacity was effective with a wide pH range (4–8), and decreased with increasing ionic strength. The descending order of nitrate adsorption inhibition by co-existing anions was SO42−, HCO3−, PO43−, and Cl−. The adsorption kinetics and isotherms agreed well with the pseudo-first-order equation and Langmuir model, respectively. The theoretical maximum adsorption capacity was 96.1469 mg/g. Thermodynamic analysis showed that the nitrate adsorption was spontaneous and endothermic. After 10-cycle regeneration, the BAG still kept 92.6% of its original adsorption capacity for synthetic nitrate-contaminated groundwater. Moreover, the main adsorption mechanism was attributed to electrostatic attraction due to the enhancement of surface charge density by Al substitution. Accordingly, the BAG adsorbent is a potential solution to remove nitrate from groundwater.
1. Introduction
Nitrogen fertilizer has become necessary to support the sustainability of agriculture for the global human population [1]. The amount of agriculturally-used nitrogen fertilizers increased from around 12 megatons (Mt) in 1961 to 110 Mt in 2014 [2]. However, excess use of nitrogen has led to the widespread contamination of surficial fresh groundwater [3]. Field studies have suggested that 30% of applied N in harvested crops is lost through leaching to groundwater as nitrate nitrogen (NO3−-N) [3]. Owing to the mobility of NO3−, such leaching often causes the contamination of shallow unconfined aquifers underlying agricultural lands [4]. Today, groundwater is estimated to supply 36% of drinking water globally, and the elevated concentrations of nitrate in drinking water sources often present a potential risk to public health. Consuming too much nitrate could affect how blood carries oxygen and may cause infant methemoglobinemia [5]. The maximum level of nitrate in drinking water suggested by the World Health Organization is 10 mgNO3−-N/L. Therefore, it is necessary to find an efficient approach to remove nitrate from the contaminated groundwater.
Nitrate is a stable and highly mobile ion with a low possibility for co-precipitation or separation. These properties suggest that it is difficult to remove using conventional water treatment approaches such as lime softening and filtration [6,7]. By contrast, nitrate could be successfully removed by more sophisticated methods such as chemical reduction, catalytic reduction, photocatalytic reduction, ion exchange, reverse osmosis, biological denitrification, and adsorption [8,9,10]. Among these methods, adsorption technologies present an attractive feature with a simple design and convenient operation [9]. The interest in adsorption is based on the fact that the other methods have certain limitations. Specifically, chemical reduction often leads to great quantities of metal sludge and converts nitrate into ammonia which must be removed by air stripping [11]. Moreover, catalytic reduction generally requires an expensive catalyst of noble metal such as palladium [7]. Photocatalytic reduction has emerged as a highly promising transformative technology capable of reducing nitrate to innocuous nitrogen with noticeable selectivity; however, more efforts are still needed to solve the question of photocatalytic nitrate reduction in real water matrices [10]. In addition, ion exchange frequently generates large quantities of waste brine that requires further disposal [12]. Additionally, reverse osmosis generally requires a high energy input to develop pressure for the operation of RO units, and thus, it needs higher operating costs [13]. Moreover, biological denitrification often presents concerns for treated water such as possible microbial contamination, the present residual organics, and the potential rise of chlorine requirements [7,14]. For these reasons, adsorption could be a preferred alternative for nitrate removal in the water treatment for groundwater.
Recently, more interest regarding the adsorptive removal of nitrate from water has been paid to biochar-based adsorbents [15,16,17]. Biochar refers to a carbon-rich material generated from the pyrolysis process (heating in the absence or limited supply of oxygen) of biomass. Many studies have found that, when derived from different feedstocks and pyrolysis conditions, biochar presented with excellent adsorption properties for water remediation [18], sensing [19], and catalysis [20]. Nevertheless, there are still challenges for pristine biochar to remove nitrate from water through adsorption [21]. For example, Gai et al. found that there was no nitrate removal in water with 12 biochars derived from three feedstocks [22]. Similar results were also observed for biochars prepared from corn stover and oak wood pyrolyzed at 300–450 °C [23], cacao shell and corn cob pyrolyzed at 300–350 °C (Hale et al., 2013), and sugarcane bagasse, peanut hull, pepperwood, and bamboo pyrolyzed at 300–450 °C [24,25]. The intrinsic limitations in nitrate adsorption are mainly attributed to the electrostatic repulsion between the negatively charged surface of biochar and the nitrate anion. Surface modification of biochar may improve its adsorption capability for nitrate by weakening the electrostatic repulsion between biochar and nitrate anions. Popular modification approaches consist of the protonation of negatively charged functional groups or loading of metal oxide/hydroxide particles on the biochar surface [26]. In particular, the supported iron oxide/hydroxide on biochar have been frequently reported due to their excellent properties, such as improved binding sites and point of zero charge [27]. Such properties make biochar-supported iron oxide/hydroxide suitable for nitrate removal. Dewage et al. found that biochar-supported α-Fe2O3/Fe3O4 particles generated more adsorption sites and caused more nitrate adsorption [16]. Long et al. suggested that the increased nitrate adsorption resulted from the formation of iron oxides, which presented with more positive surface charge than that of pristine biochar [17]. Obviously, the formed iron oxide particles played a dominant role in the biochar-based adsorbents. Because the adsorbents were often prepared by iron salt-impregnated biochar, the iron oxide formed on biochar were mainly hematite (α-Fe2O3) and magnetite (Fe3O4) [28,29,30]. Yet, different from α-Fe2O3 and Fe3O4, there are many other iron oxide species [31]. Among them, goethite (α-FeOOH) is one of the most widely used iron oxides. It serves as a model system for a great variety of surveys on adsorption because its surface chemistry and surface structure have been well characterized, and partially because it is one of the most abundant iron oxides in natural environments. Indeed, like hematite, goethite could be used to modify biochar [32,33,34]. There is a large number of studies that have proved the feasibility of biochar-supported goethite to remove oxyanions, such as anionic As(III) [34,35], As(V) [32,33], phosphate [36,37,38,39], Cr(VI) [40], and nitrate [41]. Prior to the above studies, Hsi and Langmuir have found goethite presented with a point of zero charge (PZC) of 8.9, while hematite had a PZC of 7.5 [42]. This suggests that goethite would provide better nitrate adsorption than hematite, because the adsorption mechanisms of nitrate mainly depend on electrostatic interaction. In addition, the dispersion of goethite particles on the biochar surface resulted in more adsorption sites, and avoided the deterioration of adsorption due to the agglomeration of pristine goethite particles in solution [43]. You et al. prepared biochar-supported goethite to remove nitrate in alkaline rare-earth industry effluent and successfully removed nitrate, with a maximum adsorption amount of 10.75 mg/g [41]. Therefore, biochar-supported goethite could provide benefits for nitrate removal from groundwater.
Successful adsorption depends not only on the PZC of the adsorbent but also on the surface charge density of the adsorbent. A larger surface charge density means the accumulation of more charge at the direct surface of the adsorbent, and thus would lead to a stronger electric field. Specially, the stronger electric field would lead to the larger adsorption capacity of oppositely charged adsorbates on the adsorbent. For example, Smith observed that the adsorption of Cs+ increased with increasing charge density [44]. Li et al. investigated the impact of high, medium, and low charge density on the formation of methane hydrate and found that the high charge density led to more Na+ adsorbed [45]. Lee et al. studied phosphate removal by biochar coated with Mg/Al-calcined layered double hydroxide (LDH) and found that a lower Mg:Al molar ratio increased the surface charge density of the adsorbent, which then resulted in larger phosphate adsorption [46]. Seftel et al. provided insights into phosphate adsorption behavior on structurally modified ZnAl LDH and found that when the surface charge density changed from ~4.019 e/nm2 to ~4.980 e/nm2, the adsorption capacity of phosphate increased from 56 mg/g to 88 mg/g [47]. Obviously, the increase in surface charge density could result in the increase in adsorption capacity. Therefore, new strategies should be developed to improve the surface charge density of adsorbents for the removal of oppositely charged adsorbates.
Many studies reported that aluminum substitution in goethite was able to increase the surface charge density of goethite. The crystal structure of goethite determines its surface chemistry properties, especially its surface charge [31]. In the diaspore structure of goethite, each Fe ion is surrounded by three O2− and three OH− to give FeO3(OH)3 its octahedra. Because of the similarity of ionic radii and the valency of Fe(III) and Al(III) ions, isomorphous substitution for Fe(III) by Al(III) becomes possible [31]. In fact, goethite is by far the most investigated example of an isomorphous substituted iron oxide, and Al is the best-known example of the various possible substituents in both natural and synthetic samples [31,48,49,50,51,52,53,54]. Ni et al. found Al-substituted goethite presented with an increase in adsorption capacity of Cr(VI) compared with pristine goethite [55]. Hsu et al. showed the maximum adsorption capacity of phosphate rose from 135 to 584 mmol/kg when Al/(Al + Fe) mol% changed from 0 to 17.4% [56]. Tufo et al. suggested that a small incorporation of Al would enhance the adsorption properties of the goethite surface. The enhanced adsorption of anions is supported by the fact that Al substitution could enhance the surface charge density of goethite [57]. Zhu et al. suggested that the incorporation of Al increased the effective proton reactive sites on the (021) crystal face and caused a rougher surface by the rise of Stern layer capacitance, both of which enhanced the surface charge density of the goethite [58]. Therefore, Al substitution could increase the surface density of the positive charge of biochar-supported goethite.
Despite the efforts to improve the surface charge density of goethite by Al substitution, the Al-substituted goethite on biochar and its application for nitrate adsorption remains largely unexplored. In this study, we tested the hypothesis that a novel adsorbent, Al substitution of biochar-supported goethite, would enhance the adsorption of nitrate from groundwater. Here, biochar-supported Al-substituted goethite samples were prepared with different Al substitution amounts. Characterizations of the prepared samples were analyzed using X-ray diffraction, XPS, potentiometric titration and specific surface area. In addition, the adsorption behaviors were investigated under different operational conditions, including pH, initial nitrate concentration, adsorbent dosage, and the presence of co-existing anions. Additionally, further investigations were carried out for kinetic, isotherm and thermodynamic studies of nitrate adsorption on biochar-supported Al-substituted goethite. Meanwhile, the as-prepared adsorbents were also tested for synthetic nitrate-contaminated groundwater. This study provides an innovative alternative for nitrate removal from groundwater using biochar-supported Al-substituted goethite.
2. Materials and Methods
2.1. Materials
The raw wheat straw used in this study was obtained from Lintong, Shaanxi Province, China. It was washed with deionized (DI) water and dried in an oven at 80 °C. Nitrate stock solution was prepared by dissolving potassium nitrate in DI water. The experimental solution was prepared by diluting the stock solution to desired concentrations using DI water. All reagents used were of analytical reagent grade.
2.2. Synthesis of BC-AF
Biochar was produced through the slow pyrolysis of wheat straw in a tube furnace at 400 °C with a heating rate of 5 °C/min under limited oxygen for 1 h [59]. The produced biochar was ground to less than 0.15 mm.
A series of six biochar-supported Al-substituted goethite samples were synthesized and labeled BAG0, BAG5, BAG10, BAG15 BAG20, and BAG30 according to the initial Al/(Fe + Al) mole ratios in solution during preparation, which were 0%, 5%,10%, 15%, 20%, 30% mol/mol, respectively. The preparation method was proposed by Schwertmann and Cornell [60]. Briefly, 5 g of biochar was added into 200 mL of DI water with ultrasonic-associated dispersion for 30 min (ultrasound frequency 40 KHz) in a 2 L polypropylene bottle. Then, 89.5 mL of freshly prepared 1 M FeCl2 solution and a certain volume of 1 M AlCl3 solution were added to the bottle according to the aforementioned Al/(Fe + Al) mole ratios. Subsequently, the mixed liquid was diluted to 1 L, and the pH adjusted to 11 with 1 M KOH. Next, the liquid in the bottle was mixed thoroughly in an orbital incubator for 60 days. The suspension in the liquid was slowly oxidized through opening the bottle daily and swirling the contents. After that, the suspension was centrifuged and washed twice with 0.01 M KOH to remove the excess of Al. Finally, the suspension was washed with DI water and dried in a vacuum oven at 60 °C.
2.3. Characterizationi of BC-AF
The crystalline phases of the biochars were characterized using the PANalytical Empyren X-ray diffractometer(Malvern, Almelo, The Netherlands). The XRD patterns of the as-prepared samples were recorded from 10 to 80 degrees (2 theta) with a step size of 0.025 degrees and operated with Cu-Kα irradiation (λ = 0.15406 nm) in reflection mode at 40 kV and 30 mA. Rietveld structure refinement was conducted with the software TOPAS according to the goethite structure model (JCPDS 81-0464). The X-ray photoelectron spectroscopy (XPS) analysis was carried out using Thermo ESCALAB 250 Xi systems with a multi-detection analyzer and an Al Kα monochromatized source (1486.6 eV) at 10 kV and 5 mA under 10-8 Pa residual pressure. The specific surface area (SSA) was determined using nitrogen gas adsorption at 77 K under atmospheric pressure on a Micromeritics TriStar II 3020 instrument (USA). To investigate the particles on the biochar surface, transmission electron microscope (TEM) images were recorded using a JEM-1011 Transmission Electron Microscope (JEOL, Tokyo, Japan).
Surface charge density of as-prepared samples was determined with the potentiometric titration approach. Titrations were carried out in a thermostated vessel at 298 K under N2 atmosphere using Metrohm 877 Titrino plus (Metrohm Ltd., Herisau, Switzerland). The background electrolyte was prepared from potassium chloride (KCl), and its concentration was set at three different levels, 0.01, 0.05 and 0.1 M. As titrants, the volumetric standards, 0.1 M HCl and 0.1 M KOH, were used as received. The details about titration were stated in Małgorzata and Piotr [61]. In brief, 0.2 g solid powder of the as-prepared samples was mixed into a titration vessel containing 100 mL of background electrolyte solution. Before titrations, the suspensions were purged with nitrogen gas for at least 10 min at pH 4.0 to prevent the carbonation phenomena. Then, the titrations were carried out from pH 4.0 to pH 10.0 with 0.1 M NaOH and, subsequently, got back to pH 4.0 with 0.1 M HCl to check the titration reversibility at different levels of NaCl concentration. Meanwhile, blank titrations, i.e., background electrolyte titrations in the absence of the solid samples, were performed to compare with theoretical blanks to calculate the surface charge density. The surface charge density could be estimated as follows:
where F is the Farady constant; C is the concentration of titrant; vb and vd are the volumes of titrant consumed in titration to reach the same pH without and with a solid sample, respectively; m is the mass of the solid sample; and SSA is the specific surface area of the solid sample.
In addition, the titration curves at different electrolyte concentrations would have a common intersection point (CIP). The pH at the intersection point is often considered the point of zero charge (PZC), so PZC is established as the CIP of titration curves.
The suspension stability of the as-prepared adsorbents in water was explored with optical absorbance measurements. The measurement approach was modified according to Maamoun et al. [62]. The UV-visible absorption peak was found at 430 nm through the first derivative of UV-visible spectra (the scanning range is 200 nm–800nm). At the beginning of the measurement of suspension stability, 10 mg of the adsorbent was mixed with 10 mL of 2.5 mM NaHCO3 solution. Then the mixture was stirred for 30 min in a vortexing mixer. The prepared mixture was transferred into a 10 mL optical cuvette and was monitored at 430 nm wavelength for 20 min using UV-Vis Spectrophotometer (UV–2601, Rayleigh, China). The change in the absorbance intensity was recorded with respect to time. At last, the recorded absorbance intensity was used to evaluate the suspension stability of the as-prepared adsorbents.
The particle size distribution of the samples was determined in suspension using time-average laser light scattering (Malvern Mastersizer 3000, Malvern, UK) recording the particulate scattering volume adopting the Fraunhofer particle mode. The De Brouckere mean diameter was used as the mean diameter of the samples.
2.4. Batch Adsorption Experiments
The performance of adsorbents with different Al/(Al + Fe) mole ratios was investigated according to the following conditions: the dosage of 0.2 g, the solution volume of 50 mL, the initial nitrate concentration of 50 NO3−-N mg/L, the initial pH of 7, the contact time of 4 h, the shaking speed of 150 rpm/min, and the solution temperature of 25 °C.
The effect of initial pH on nitrate adsorption by BAG10 was investigated with different initial solution pH values (4, 5, 6, 7, 8, 9, 10, and 11). The other conditions were as follows: the dosage of 0.2 g, the solution volume of 50 mL, three initial nitrate concentrations (50, 70 and 100 NO3−-N mg/L), the contact time of 4 h, the shaking speed of 150 rpm/min, and the solution temperature of 25 °C.
The effect of ionic strength on nitrate adsorption by BAG10 was investigated with different sodium chloride concentrations (0, 0.01, 0.03, 0.15, 0.3, 0.75, and 1.5 M). The other conditions were as follows: the dosage of 0.2 g, the solution volume of 50 mL, the initial nitrate concentration of 50 NO3−-N mg/L, the initial pH of 7, the contact time of 4 h, the shaking speed of 150 rpm/min, and the solution temperature of 25 °C.
The effect of co-existing anions on nitrate adsorption by BAG10 was investigated with the following combinations: 50 mg/L NO3−-N (blank), 50 mg/L NO3−-N + 100 mg/L Cl−, 50 mg/L NO3−-N + 100 mg/L PO43−, 50 mg/L NO3−-N + 100 mg/L HCO3−, and 50 mg/L NO3−-N + 100 mg/L SO42−. The other conditions were as follows: the dosage of 0.2 g, the solution volume of 50 mL, the initial pH of 7, the contact time of 4 h, the shaking speed of 150 rpm/min, and the solution temperature of 25 °C.
The kinetic experiment was conducted with the following conditions: the dosage of 0.2 g, the solution volume of 50 mL, the initial nitrate concentration of 50 NO3−-N mg/L, the initial pH of 7, the contact time of 4 h, the shaking speed of 150 rpm/min, and the solution temperature of 25 °C. During the experiment, liquid samples were analyzed at 0, 5, 10, 15, 25, 35, 50, 65, 80, 100, 150, 200, 300, and 400 min, respectively.
Adsorption isotherm experiments were conducted with different initial nitrate concentrations (5, 20, 30, 60, 100, 150, and 250 mol/L). The other conditions were as follows: the dosage of 0.2 g, the solution volume of 50 mL, the initial nitrate concentration of 50 NO3−-N mg/L, the initial pH of 7, the contact time of 4 h, the shaking speed of 150 rpm/min, and three solution temperatures (15, 25 and 35 °C).
2.5. Reusability Potential of the Adsorbent
Based on our earlier groundwater investigations in Weinan City, Shaanxi Province, China, we prepared simulated groundwater to study the reusability of BC-AF for nitrate adsorption. The statistical data and the simulated data are listed in Table 1. In each adsorption cycle, 0.2 g adsorbent was added into 50 mL of simulated groundwater. Adsorption of each cycle was carried out under the condition of the solution temperature of 25 °C, the shaken speed of 150 rpm/min, and the contact time of 4 h. After each adsorption, the adsorbent was filtered and washed with DI water. Then, the washed adsorbent was added into 50 mL of a saturated solution of NaCl and was shaken with a speed of 120 rpm/min for 60 min. After regeneration, the desorbed nitrate was analyzed. Meanwhile, the regenerated adsorbent was washed with DI water and then used for the next adsorption-desorption experiment. Ten cycles were carried out in this study.

Table 1.
Water quality data of groundwater and simulated experimental value.
2.6. Analysis Method
2.6.1. Determination of NO3−-N Concentration in Solution
The phenoldisulfonic acid spectrophotometric method was used to measure the NO3−-N content in water [63,64]. Three parallel measurements were conducted and the average value was taken.
The NO3−-N adsorption amount qe was calculated by the following formula:
where, qe is NO3− equilibrium adsorption capacity, mg/g; c0 and ce are the initial concentraion and the equilibrium concentration NO3−-N in solution, respectively, mg/L; V is the volume of solution, L; and W is the adsorbent dosage, g.
2.6.2. Kinetic Studies
The adsorption kinetics of NO3− are described by the pseudo-first-order kinetic model [65] and the pseudo-second-order kinetic model [66] as follows:
where qe and qt (mg/g) are the adsorption capacity of BC-AF at the equilibrium time and the sampling time, respectively; k1 (1/h) and k2 (g/(mg·h)) are the rate constants of the two kinetic models.
2.6.3. Isothermal Studies
The adsorption isotherms of NO3−-N were analyzed by the Langmuir model and the Freundlich model as follows:
where ce (mg/L) is the equilibrium concentration; kL (L/mg) and kF (mg1−1/n·L1/n/g) are the model constants; qm (mg/g) is the theoretical maximum adsorption capacity; and 1/n is the heterogeneity factor.
The abovementioned isotherm models and kinetic models were fitted with experimental data by nonlinear regression. The nonlinear regression was carried out using the nlsLM() function in R software (Version 4.2.0) (the R Core Team, Toulouse, France). The Akaike information criterion (AICc) was used to evaluate how well the models fit the experimental data. In statistics, AICc is a mathematical method for comparing different possible models and determining which one is the best fit for the data that the models are generated from. According to AICc, the best-fit model is the one with the minimum AICc value. The formula for AICc is [67]:
where K is the number of parameters of the model; N is the number of experimental data; SSE is the sum of squared errors; yiexp is the experimental value of the ith point; and yical is the calculated value of the ith point of the model.
2.6.4. Thermodynamic Studies
The thermodynamic analysis focused on three thermodynamic parameters: standard enthalpy (ΔH), standard free energy (ΔG), and standard entropy (ΔS). The three parameters were calculated according to the van’t Hoff equation as follows:
where K is thermodynamic equilibrium constant; R is the gas constant (8.314 J/mol K); T is the absolute temperature (K).
3. Results
3.1. Characterization of BC-AF
The X-ray diffraction (XRD) results (Figure 1a) for all synthetic samples showed sharp peaks based on a straight baseline, which suggest the presence of well crystallized materials. Goethite was the only product in all materials except BAG30 which contained mainly hematite [68]. The diffraction peaks of BAG0 at 21.21°, 33.25°, 34.70°, 36.65°, 53.20°, and 59.03° were recognized corresponding to (110), (130), (021), (111), (221), and (151) planes, respectively. For the XRD patterns of BAG5, BAG10, BAG15, and BAG20, no new diffraction peaks were found. However, Figure 1b showed that the XRD peaks progressively shifted to larger angles as the Al content increased. The positive shift of diffraction peaks was due to the incorporation of Al [52,69,70]. Because the small-sized Al3+ (ionic radius ~0.53 Å) replaced Fe3+ (ionic radius ~0.65 Å) in the crystal lattices [71], this led to the shrinking of d-spacing between the crystal planes (Table 2), which caused the diffraction peaks shift to larger angles. On the other hand, Al substitution caused a decline of the average lattice parameters in goethite crystals (Table 2) [68]. Similar observations were made for the synthetic substitution of Al for Fe in goethite. Accordingly, such characteristics of XRD patterns suggest the successful synthesis of biochar-supported Al-substituted goethite.
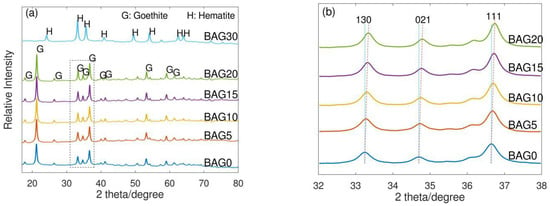
Figure 1.
(a) XRD patterns of biochar-supported Al-substituted iron oxide hydroxides with Al/(Al + Fe) mol% of 0 (BAG0), 5 (BAG5), 10 (BAG10), 15 (BAG15), 20 (BAG20), and 30 (BAG30); (b) the amplified XRD patterns at (130), (021), and (111) reflection planes of BAG0, BAG5, BAG10, BAG15, and BAG20, respectively.

Table 2.
Unit cell dimensions, point of zero charge, and specific surface area of prepared samples.
The diffraction peaks of BAG30 showed the presence of hematite, where eight XRD peaks at 24.17°, 33.19°, 35.66°, 39.32°, 49.51°, 54.12°, 62.49°, and 64.06° were identified corresponding to (012), (104), (110), (006), (024), (116), (214), and (300) planes, respectively. This indicates that a higher Al substitution favors the development of hematite over goethite on biochar. Similar phase changes have also been observed in other studies [58,68]. In fact, the addition of Al decreased the solubility of ferrihydrite, which was the intermediate product used in order to prepare iron oxide hydroxide. Such a decrease appeared to prefer to transform the ferrihydrite into hematite rather than goethite, which suggests excessive Al substitution could not bring about isomorphous Al substitution in goethite. Therefore, in order to produce biochar-supported Al-substituted goethite, the upper threshold of Al/(Al + Fe) atomic ratio was 20 mol%.
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that can recognize the elements that are within a material or covering its surface, as well as their chemical state [72,73]. In the high resolution XPS spectra of the as-prepared samples (Figure 2), the characteristic peaks of Al 2p electrons became more significant with the increasing Al substitution amount, which suggested that the successful substitution of Al atoms for Fe atoms in biochar-supported goethite. Meanwhile, such substitution caused a positive shift in the binding energy of the Al 2p shell. On the contrary, Al substitution led to the peak intensity attenuation of Fe 2p XPS spectra, and simultaneously made a negative shift in the binding energies of Fe 2p1/2 and Fe 2p3/2. As Al substitution happened, the structure of goethite on biochar was present with Fe-O-Al pairs. In Fe-O-Al pairs, the electron density around the Fe atom is larger than that in Fe-O-Fe pairs, because the electronegativity of Fe3+ (χ = 1.7053) is stronger than that of Al3+ (χ = 1.5149). This caused the negative shift of the binding energies of Fe 2p. In addition, the formation of Fe-O-Al pairs accompanies the stronger charge coupling because of the heteroatomic hybridization, which led to the positive shift of the Al 2p XPS peaks. Therefore, the XPS spectra of Al 2p and Fe 2p again confirmed the successful synthesis of biochar-supported Al-substituted goethite.
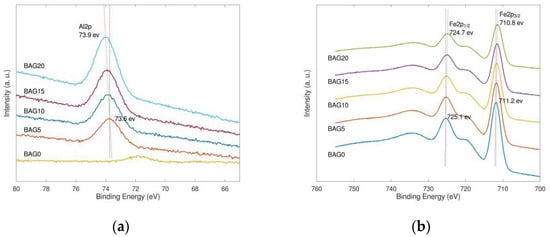
Figure 2.
High-resolution X-ray photoelectron spectroscopy (XPS) scans of (a) Fe 2p and (b) Al 2p for biochar-supported goethite and Al-substituted samples.
Potentiometric acid-base titration has been used for decades to investigate the surface charge density of metal oxides in aqueous solution. With such an approach, we determined the charge density of the as-prepared samples, as shown in Figure 3. For each sample, the lower the pH, the larger the positive charge density, and vice versa. On the other hand, there was a positive correlation between charge density and ionic strength. Increasing ionic strength led to an increase in surface charge density. In addition, the intersection of titration curves with different ionic strengths determined the PZC. For BAG0, BAG5, BAG10, BAG15, and BAG20, the PZCs were 8.7, 8.59, 8.57, 8.61, and 8.71, respectively. The distinction of PZCs for the different samples did not appear to be significant, which indicated that Al substitution did not notably change the point of zero charge of biochar-supported goethite. Despite this fact about PZC, Figure 3f showed that Al substitution increased the surface charge density significantly. At pH 6 and ionic strength 0.01 M, the charge densities of BAG0, BAG5, BAG10, BAG15, and BAG20 were 0.0180, 0.0590, 0.0686, 0.0781, and 0.0843 C/m2, respectively. This would certainly have a beneficial effect on nitrate adsorption in aqueous solution.
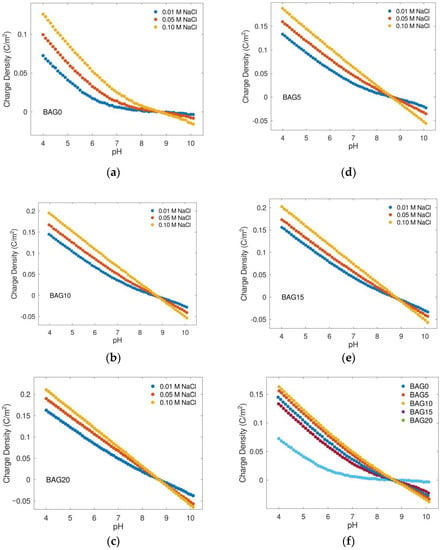
Figure 3.
Charge density (C/m2) as a function of pH and NaCl concentration for biochar-supported goethite and Al-substituted samples of (a) BAG0, (b) BAG10, (c) BAG20, (d) BAG5, and (e) BAG15; (f) the comparision of charge density of different Al-substituted samples at ionic strength 0.01 M.
The suspension stability of the adsorbents was explored with the change of absorbance intensity of the UV-visible spectrum at 430 nm for 20 min. Figure 4a shows that the absorbance intensity of BAG0 decreased more steeply than the others, and that of BAG20 descended more slowly than the others. In addition, the decreasing trend in absorbance intensity became slower when the adsorbent presented with more Al substitution. In fact, the substitution of Al atoms for Fe atoms in goethite would make the goethite lighter because the molar mass of Al (26.98 g/mol) is less than that of Fe (55.84 g/mol). Thus, more Al substitution could improve the suspension stability of the as-prepared adsorbents. Figure 4a also suggests that most of the as-prepared samples settled totally in 20 min. The faster settlement was mainly related to the adsorbent particle size (Figure 4b). The mean particle size of the BAG10 samples was 88.5 μm, which is far greater than the size of nanoscale composites. Although the size of the composites was beyond the nanoscale, the Al-substituted goethite particles were as well dispersed as nano particles on the biochar surface (Figure 4c). The well dispersed Al-substituted goethite particles indicate that BAG10 could act as an adsorbent. Because of the faster settlement of the adsorbent, an effective mixing is needed to ensure the sufficient suspension of the adsorbent in solution.
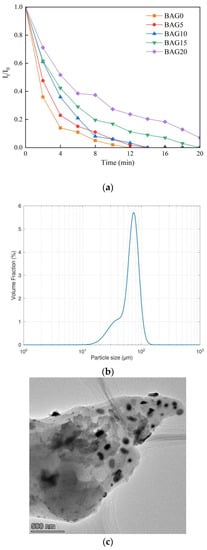
Figure 4.
(a) Normalized absorbance intensity of the as-prepared adsorbents; (b) the particle size distribution of the adsorbent BAG10; and (c) the TEM image of the adsorbent BAG10.
3.2. Effect of Al/(Al + Fe) mol%
Figure 5 shows the effect of the Al/(Al + Fe) mol% of adsorbent on nitrate adsorption. Obviously, the BAG10 with 10% of Al/(Al + Fe) mol% showed the largest removal efficiency, and removed 91.5% of initial nitrate in solution. By comparison, the BAG0 without Al substitution presented with the lowest removal efficiency, and only removed 57.8% of initial nitrate in solution. When the Al/(Al + Fe) mol% changed from 0 to 10%, the nitrate removal efficiency increased by 33.7%. However, the nitrate removal efficiency decreased significantly when the Al/(Al + Fe) mol% was beyond 10%. The removal efficiency of nitrate reduced by 23.3% after the Al/(Al + Fe) mol% increased from 10% to 20%. Obviously, 20% of Al/(Al + Fe) mol% led to the adsorbent BAG10 with the optimal nitrate removal efficiency, and the BAG10 was selected for the following studies.
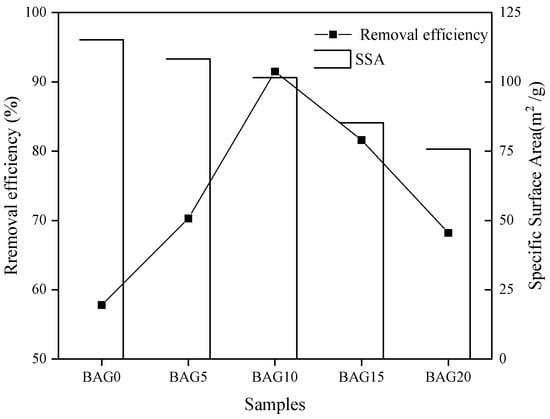
Figure 5.
Nitrate removal by BC-AFs prepared with different Al/Fe ratios (conditions: C0 = 50 mg/L, pH 7.0, contact time = 4 h, and T = 25 ± 1 °C).
3.3. Effect of Adsorbent Dose
Figure 6 shows the nitrate removal with different adsorbent dosages. The nitrate removal efficiency increased when the dosage increased from 0.05 to 0.20 g, and then reached a plateau with the dosage beyond 0.20 g. The dosage of 0.05 g presented with the lowest removal efficiency of 20.8%, while the dosage greater than 0.20 g resulted in removal efficiencies not less than 91.3%. On the other hand, the variation of adsorption capacity changed little when the dosage was less than 0.20 g, but it decreased significantly from 11.4125 mg/g to 4.5720 mg/g. Obviously, the dosage of 0.20 g presented with a more reasonable performance in both nitrate removal efficiency and adsorption capacity. Thus, the dosage of 0.20 g was selected for the following studies.
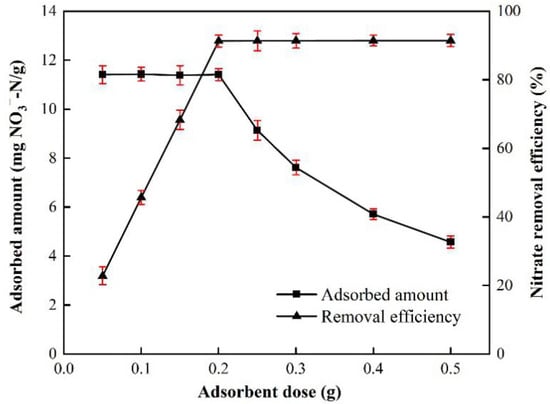
Figure 6.
Effect of adsorbent dose on NO3−-N removal onto BAG10 (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, contact time = 4 h, and T = 25 ± 1 °C).
3.4. Effect of Solution pH
Figure 7 shows the effect of the initial solution pH on nitrate adsorption with different initial nitrate concentrations. The nitrate adsorption was certainly preferable when the initial pH of solution was not greater than 8. For C0 = 50 mg/L, when the pH changed from 4 to 8, the adsorbed amount changed from 11.9450 to 10.8950 mg/L, which was just a slight decrease. However, when the initial pH changed from 8 to 9, the adsorbed amount decreased from 10.8950 to 1.100 mg/L, which was a great decrease. When the initial pH was greater than 9, there was almost no nitrate adsorption. Similar results were also found for C0 = 70 mg/L and 100 mg/L, respectively. Above all, a reasonable nitrate adsorption was observed at the initial pH of 7, which is common in groundwater. Therefore, the initial solution pH was chosen as 7 for the following research.
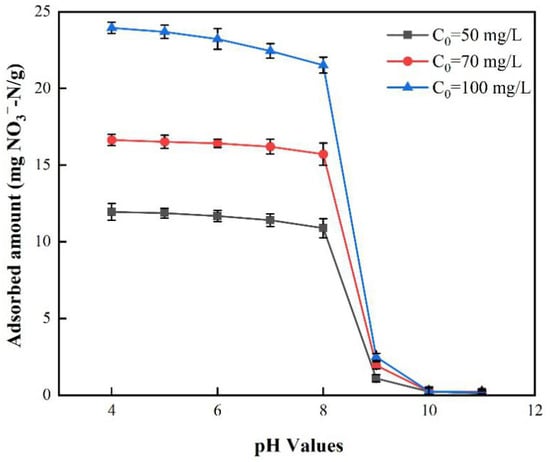
Figure 7.
Effect of solution pH on NO3−-N removal onto BAG10 (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, contact time = 4 h, and T = 25 ± 1 °C).
3.5. Effect of Ionic Strength
Figure 8 shows the effect of ionic strength on nitrate adsorption by BAG10. The ionic strength was investigated through seven different NaCl concentrations, including 0.00, 0.01, 0.03, 0.15, 0.30, 0.75, and 1.50 mol/L. The nitrate adsorption achieved 11.4125 mg/L without NaCl in solution, but it decreased to 5.7826 mg/L when the concentration of NaCl became 1.5 mol/L. The increase in ionic strength resulted in an almost 50% loss of the adsorbed amount of nitrate. The decrease was slow when the concentration of NaCl was less than 0.15 mol/L, but it became fast when the concentration of NaCl was beyond 0.15 mol/L. Obviously, a lower ionic strength had a low impact on the nitrate adsorption, while a stronger ionic strength resulted in a large negative impact on the nitrate adsorption by BAG10.
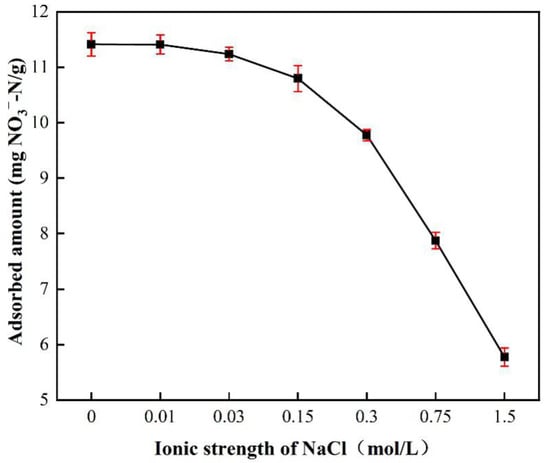
Figure 8.
Effect of ionic strength on NO3−-N removal onto BAG10 (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, pH 7.0, contact time = 4 h, and T = 25 ± 1 °C).
3.6. Effect of Co-Existing Anions
Figure 9 shows the effect of co-existing anions on nitrate adsorption by BAG10. The common anions (Cl−, PO43−, HCO3− and SO42−) in groundwater were investigated. The presence of SO42− in the nitrate solution caused only 47.3% of nitrate removed by BAG10, while the nitrate removal efficiency without SO42− was 91.3%. Compared with 91.3% of nitrate removal efficiency without any other anions in solution, the presence of Cl−, PO43−, and HCO3− also resulted in a decline to 91.0%, 88.4%, and 85.4%, respectively. Among the co-existing anions, SO42− led to the largest inhibition of nitrate adsorption on BAG10, while the inhibition by Cl−, PO43−, and HCO3− was not significant. The co-existing anions inhibited nitrate adsorption with a descending order of SO42−, HCO3−, PO43−, and Cl−.
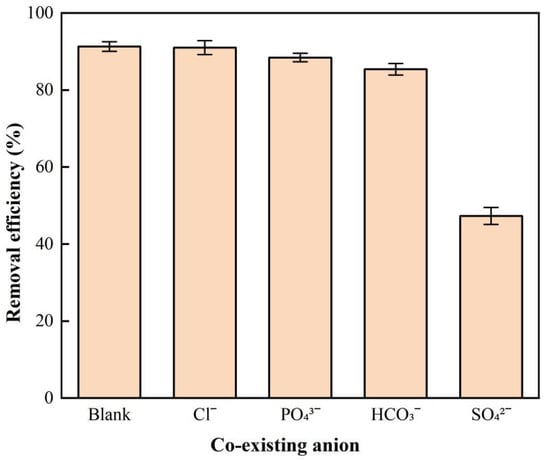
Figure 9.
Effect of co-existing anion on NO3−-N removal onto BAG10 (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, pH = 7.0, contact time = 4 h, and T = 25 ± 1 °C).
3.7. Adsorption Kinetic Modeling
Figure 10 shows the kinetics of nitrate adsorption on BAG10. The adsorbed amount of nitrate increased quickly in the first 50 min and achieved 80% of the maximum adsorption capacity with an average adsorption rate of 11.97 mg NO3−-N/g/h. The increase became slow from 50 min to 80 min, with an average adsorption rate of 2.14 mg NO3−-N/g/h. From 80 min, the adsorption reached a plateau after 100 min.
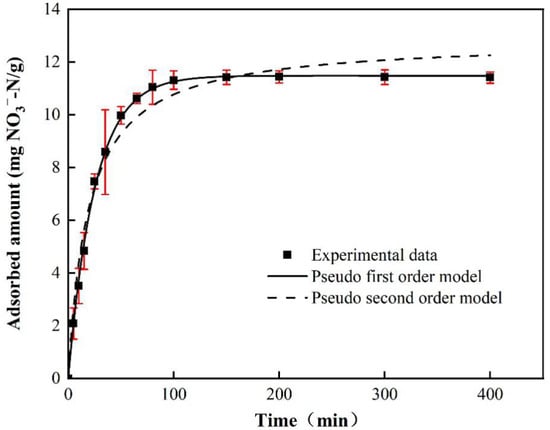
Figure 10.
Adsorption kinetics of BAG10 for NO3−-N (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, pH = 7.0 and T = 25 ± 1 °C).
The kinetic data were fitted using the pseudo-first-order and pseudo-second-order equations, as shown in Figure 10. The fitting curve in Figure 10 suggested that the pseudo-first-order equation fitted the data well. In addition, a statistics analysis also found that the pseudo-first-order equation described the kinetic data better than the pseudo-second-order equation. Table 3 shows that the determination coefficient R2 of the pseudo-first-order equation was 0.9989, and that of the pseudo-second-order equation was 0.9748. In addition, the AICc of the pseudo-first-order equation was 77.1, and that of the pseudo-second-order equation was 79.0. Both the greater R2 and the lower AICc suggested that the pseudo-first-order equation described the kinetic data better than the pseudo-second-order equation. Moreover, the pseudo-first-order equation also calculated the adsorption capacity close to that by experimental measurement (Table 3). Therefore, the pseudo-first-order equation was more suitable for the description of the kinetic data.

Table 3.
Parameter values of BAG10 adsorption NO3−-N kinetic equation.
3.8. Adsorption Equilibrium Modeling
Figure 11 shows the isotherm of nitrate adsorption by BAG10. Seven different initial concentrations were used to analyze the equilibrium isotherm. Obviously, the adsorbed amount of nitrate increased when the initial concentration increased from 5 to 250 mg/L. At the initial nitrate concentration of 5 mg/L, BAG10 removed 94.8% of nitrate from the water. At the initial nitrate concentration of 250 mg/L, BAG10 removed 84.4% of nitrate from the water. While the removal efficiency became lower, the equilibrium which adsorbed amount of nitrate still increased as shown in Figure 11.
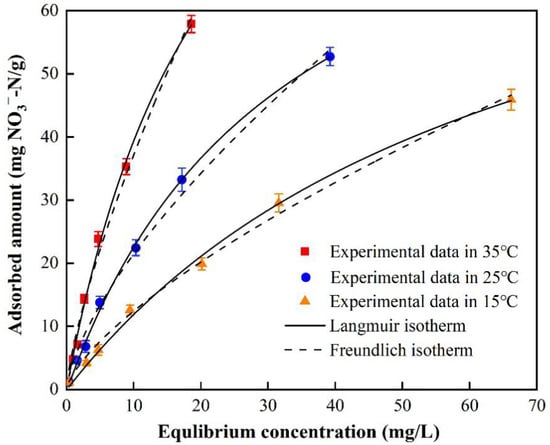
Figure 11.
BAG10 isothermal adsorption model for NO3−-N (conditions: adsorbent dose = 0.2 g, pH 7.0, contact time = 4 h and T = 25 ± 1 °C).
The equilibrium data were fitted with two isotherm models, the Langmuir model and Freundlich mode. The fitting parameters were listed in Table 4. The R2 and AICc of the Langmuir model were 0.9986 and 26.4, respectively. On the other hand, the R2 and AICc of the Freundlich model were 0.9928 and 39.6, respectively. Obviously, the R2 of the Langmuir model was greater than that of the Freundlich model. Meanwhile, the AICc of the Langmuir model was lower than that of the Freundlich model. Both the greater R2 and the lower AICc proved the superiority of the Langmuir model over the Freundlich model. Therefore, the Langmuir model was more suitable for describing the isotherm of nitrate adsorption.

Table 4.
Parameter values of BAG10 adsorption NO3−-N isothermal adsorption model equation.
3.9. Adsorption Thermodynamic Study
The thermodynamic equilibrium constant of the adsorption process, K, is the constant for nitrate distribution between the solid and liquid phases at equilibrium. K was computed using the method of plotting ln(qe/Ce) vs. qe and extrapolating qe to zero [74]. Table 5 presents the corresponding thermodynamic parameters. The positive value of ΔH suggested that the interaction of nitrate and BAG10 was endothermic in nature [75]. The adsorption process was spontaneous in nature when the temperature was at 298 K and 308 K, as indicated by the negative value of ΔG [64,76]. The positive value of ΔG at 288 K became more negative as the temperature increased, indicating that the higher the solution temperature was, the stronger the spontaneity of the adsorption process. On the contrary, a low temperature was not conducive to the adsorption of nitrate by BAG10. The positive values of ΔS indicated that the adsorption of nitrate was dependent on BAG10’s adsorbent and circumstantial interface in the solid/liquid phases [77], and the disorder of the adsorption process increased at the solid−liquid interface.

Table 5.
Thermodynamic parameters of NO3−-N adsorption by BAG10 adsorbent.
3.10. Reusability Potential of the Adsorbent
Desorption could be regarded as a substantial process because of the importance of economic issues. Figure 12 shows the changes of BAG10’s adsorption efficiency for NO3−-N and the desorption efficiency of NO3−-N in BAG10 by saturated NaCl with the increase in regeneration times. The results showed that the performance of BAG10 on the adsorption of nitrate from simulated groundwater was slightly worse than that under blank conditions. This was mainly due to the influence of anions such as SO42−, HCO3−, and Cl− in the simulated groundwater environment. Experimental data indicated that the desorption efficiency of nitrate using saturated NaCl was about 99.5%, and a slight decrease (from 88.7% to 82.1%) in the adsorption capacity of BAG10 was observed throughout the regeneration cycles. Considering the reusability of adsorbent, the results also showed that the novel adsorbent BAG10 had considerable adsorption properties, which made the adsorbent be reused nine times without any significant loss in adsorption efficiency. These results are positive for our need to reuse adsorbent to reduce nitrate levels in groundwater from 50 mg/L to below the standard 10 mg/L. Therefore, BAG10 showed an excellent regeneration ability for nitrate adsorption from simulated groundwater.
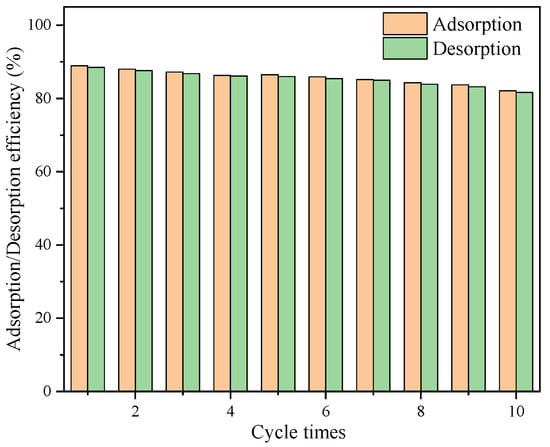
Figure 12.
Adsorption–desorption cycles for NO3−-N adsorption by BAG10 (conditions: C0 = 50 mg/L, adsorbent dose = 0.2 g, pH = 7.0, contact time = 4 h and T = 25 ± 1 °C).
4. Discussion
4.1. Effect of Al/(Al + Fe) Molar Ratio
The effect of the Al/(Al + Fe) molar ratio in Figure 5 is a compromise between the surface charge density and the specific surface area. The amount of Al substitution plays an important role in the nitrate removal efficiency of biochar-supported Al-substituted goethite. According to the characterization analysis, the Al substitution amount led to little change in the PZCs of the as-prepared adsorbents (Table 2), but caused a significant increase in the surface charge density (Figure 3f). This is consistent with those reported for goethite by Zhu et al. [46]. Meanwhile, the increase in surface charge density also indicates an increased proton uptake by Al substitution. The difference in proton uptake may be caused by the difference in the inner Stern layer capacitance and/or difference in the site density. In particular, Al substitution could increase the amount of single coordination groups of ≡AlOH−0.5 and ≡FeOH−0.5. Such an increase eventually enhanced the surface protonation, and thus led to the increase in surface charge density. On the other hand, the increase in Al substitution caused a decline of specific surface area of the as-prepared adsorbent (Table 2). The decline is consistent with the previous reported results [40,57]. The substitution of Fe3+ by Al3+ happened at the octahedral site in goethite on the biochar. Because the radius of Al3+ (~0.53 Å) was about 17% smaller than that of the Fe3+ (~0.65 Å), Al substitution caused crystal lattice distortion and decreased the interplanar spacing of the goethite. As a result, the average unit cell dimensions of the Al-substituted goethite on biochar gradually decreased after Al substitution (Table 2). Zhu et al. suggested that the decrease in crystal cell dimensions influences the microstructure of goethite [46]. The microstructure of the goethite after Al substitution presents with shorter and wider laths, and thus leads to a lower specific surface area [57]. According to the above, there must be a compromise between the increasing surface charge density and the decreasing specific surface area. Such a compromise gives the reason that the 10% of Al/(Al + Fe) molar ratio led to the adsorbent with the optimal nitrate removal efficiency.
4.2. Effect of Operational Conditions
Nitrate adsorption was greatly affected by the operational conditions [16,62,65]. In this study, the effects of adsorbent dosage, initial pH, ionic strength, and co-existing ions were investigated.
Generally, a higher adsorbent dosage leads to more removal of adsorbate from water [78,79]. However, a higher adsorbent dosage also caused less nitrate in the bulk solution, and this indicated a lower difference of nitrate concentration between the bulk solution and the adsorbent-water interface. The lower difference certainly caused less nitrate transfer from the bulk to the interface. Thus, when the dosage was greater than 0.2 g, there was little improvement for the adsorption (Figure 6).
The impact of the initial pH was consistent with the previous studies [52,58,66] but presented with a wider pH range for effective adsorption. The variation of adsorption capacity at the different initial pH was correlated with the point of zero charge of the adsorbent BAG10. According to Table 2, BAG10 showed a PZC of 8.57, which indicated that the surface charge would be positive when the solution pH was less than 8.57. According to Bhatnagar et al. [63], NO3− can be removed by electrostatic adsorption on the surface of metal oxide hydrate. Thus, the positively charged surface was beneficial to the nitrate adsorption onto BAG10. In fact, the positive surface charge was mainly derived from the protonation of the surface of BAG10, and this could facilitate electrostatic adsorption between NO3− and the adsorbent surface [80,81]. Meanwhile, BAG10 could be more protonated than the other biochar-supported Al-substituted goethites. BAG10 presented with a surface charge density of 0.0686 C/m2 and a specific surface area of 101.5 m2/g, both of which led to the largest charge amount per gram, 6.9629 C/g, among the as-prepared adsorbents. That is why the nitrate adsorption of BAG10 outperformed that of the others (Figure 6). On the other hand, when the initial pH was greater than 8.57, the surface of BAG10 was negatively charged, and this caused an electrostatic repulsion for the anion [58,82]. Thus, little nitrate was removed. Meanwhile, the negative charge also made little difference for nitrate adsorption even when the initial nitrate concentration increased. This means that the nitrate adsorption was dominated by the electrostatic interaction between nitrate and the adsorbent.
Ionic strength further confirmed the domination of the electrostatic interaction for nitrate adsorption. It is generally believed that a high ionic strength can compress the thickness of the double electric layer and weaken the electrostatic interaction between adsorbent and adsorbent [69,83]. At a high ionic strength, charged equilibrium ions surround the adsorption sites with an opposite charge, partially neutralize the charge at the adsorption sites, and weaken the electrostatic interaction between adsorption sites and adsorbent [59,84]. The effect of ionic strength suggests that outer-sphere adsorption is the main adsorption mechanism, which is consistent with the findings of previous reports [67].
The effect of co-existing anions suggested the presence of SO42− and showed the largest inhibition for nitrate removal. The inhibition effect of SO42− can be related to the fact that the bivalent anions have a higher adsorption tendency than monovalent ones [52,85,86,87]. Several previous studies have also reported that sulfate has the maximum inhibition effect on nitrate adsorption [50,58,71]. This is because that the outer-spherically sorbing anions, especially SO42−, can significantly interfere with the sorption of nitrate at elevated concentrations, where the sorption competition can occur for the limited amount of sorption sites on BAG10 [63].
4.3. Kinetics, Isotherms and Thermodynamics
The kinetics of nitrate adsorption complied with the pseudo-first-order equations. This further indicated that nitrate ions were physically adsorbed by BAG10. Meanwhile, the majority of nitrate was quickly removed in the first 50 min, which also implied a physical adsorption. Figure 9 shows that the adsorption went through three stages. The first stage was surface adsorption, which had the steepest slope and meant that film diffusion of nitrate ions from the bulk liquid phase to the adsorbent external surface occured [88]. This phenomenon could be explained by the fact that the positively charged surface of BAG10 was easy to generate electrostatic attraction with nitrate, and there were enough adsorption sites available for nitrate occupation at this stage [47]. Besides, the initial concentration of nitrate was relatively high, and thus, at the beginning of adsorption, the mass transfer driving force was large, and so NO3− was easily sent to the active site on the surface of the adsorbent. The second stage was pore adsorption. As the contact time between the adsorbent and the solution increased, the solution concentration decreased continuously, and the adsorption sites on the surface of BAG10 were occupied by NO3−. As a result, the electrostatic adsorption on the surface of the material was weakened, while the effect of electrostatic repulsion slowly appeared, and the driving force of adsorption mass transfer was reduced [75,89]. Then, the main adsorption process was changed to intra-particle adsorption, and the adsorption rate slowed down gradually. Stage 3 was the adsorption equilibrium stage. With a longer contact time, the availability of adsorbent active sites was reduced. After 80 min, the adsorption sites on the surface of the biochar tended to be saturated, and NO3− hardly entered the surface of biochar again.
An isotherm analysis suggested that the Langmuir model described the equilibrium data well. This indicated that NO3− was mainly monolayer adsorbed onto BAG10 and was limited by adsorption saturation. The maximum adsorption capacity of NO3− reached 96.1469 mg/g, which is comparable to previous studies [72,73]. In addition, a thermodynamic analysis suggested that the nitrate adsorption on BGA10 was endothermic and spontaneous. Therefore, both the calculated maximum adsorption capacity and the interaction nature of adsorption indicate that BAG10 is preferable to nitrate removal.
4.4. Adsorption Mechanism
The above discussion suggests that electrostatic attraction is the main adsorption mechanism for nitrate adsorption on biochar-supported Al-substituted goethite. The as-prepared adsorbent presented with higher point of zero charge, 8.57, which could make the adsorbent BAG10 positively charged at a wide pH range below 8.75. Meanwhile, more nitrate removal was attributed the larger surface charge density of BAG10, which was derived from the Al substitution for Fe in goethite on the biochar. Al substitution caused more effective adsorption sites, the single coordination groups ≡AlOH0.5− and ≡FeOH0.5−. The surface of the Al-substituted goethite also comprised triply-coordinated surface groups in the forms of ≡Fe3-O0.5− and ≡AlFe2-O0.5−. The single and triply coordination groups could produce protonation sites as follows:
≡AlOH0.5− + H+(aq) → ≡AlOH20.5+
≡FeOH0.5− + H+(aq) → ≡FeOH20.5+
≡Fe3-O0.5− + H+(aq) → ≡Fe3OH+
≡AlFe2-O0.5− + H+(aq) → ≡AlFe2OH+
Thus, the protonated sites attracted nitrate anions by an electrostatic force as follows:
≡AlOH20.5+ +NO3− → ≡AlOH20.5+···NO3−
≡FeOH20.5+ +NO3− → ≡FeOH20.5+···NO3−
≡Fe3OH+ + NO3− → ≡Fe3OH+···NO3−
≡AlFe2OH+ + NO3− → ≡AlFe2OH+···NO3−
Above all, the main mechanism of adsorption is attributed to the surface charge properties of BAG10, which are the higher point of zero charge and the larger surface charge density. The proposed mechanism is shown in Figure 13.
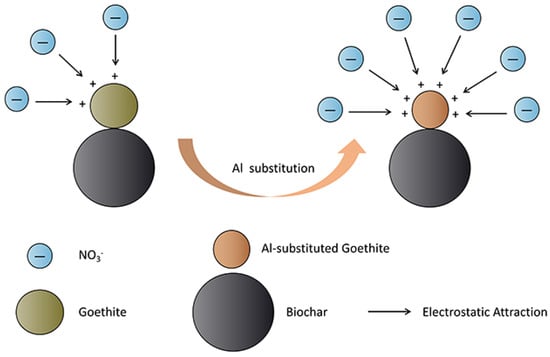
Figure 13.
The proposed adsorption mechanism of nitrate adsorption on biochar-supported Al-substituted goethite.
5. Conclusions
In this study, a novel adsorbent, biochar-supported Al-substituted goethite, was explored to remove nitrate from groundwater by adsorption. The novel adsorbent was prepared through the coprecipitation method and was confirmed XRD and XPS analysis. Potentiometric titrations revealed that the substitution caused an increase in surface charge density from 0.0180 to 0.0843 C/m2. However, the substitution also led to a decrease in specific surface area of the adsorbents from 115.2 to 75.7 m2/g. The compromise between specific surface area and surface charge density made the BAG10 present with the most charged sites, 6.9629 C/g. The performance of nitrate adsorption further suggested that the BAG10 with 10% of the initial Al/(Fe + Al) mole ratio outperformed other adsorbents derived from different Al/(Fe + Al) mole ratios. The investigations of initial solution pH demonstrated that the pH range of 4–8 was preferable to nitrate adsorption because the PZC of BAG10 was 8.57. In addition, the effect of ionic strength indicated that the adsorbed nitrate formed outer-sphere complexes at the external surface of BAG10. Meanwhile, the effect of co-existing anions indicated that the competing anions weakened nitrate adsorption in the order of SO42− > HCO3− > PO43− > Cl−. The kinetic data were well fitted by the pseudo-first-order equation, which implied a physical adsorption between nitrate and BAG10. Moreover, the isotherm data were well described by the Langmuir model, and the model determined a comparable maximum adsorption capacity of 96.1469 mg/g. A thermodynamic analysis indicated that the nitrate adsorption was spontaneous and endothermic. In addition, when regenerated with saturated NaCl solution, the BAG10 kept 92.6% of its original adsorption capacity for synthetic nitrate-contaminated groundwater. Furthermore, electrostatic attraction was supposed as the dominated mechanism of adsorption, and the enhanced electrostatic attraction was mainly attributed to the increased surface charge density by Al substitution. Above all, the prepared biochar-supported Al-substituted goethite could act as a novel adsorbent for nitrate removal from water, and this is of great significance for the control of groundwater nitrate pollution.
Author Contributions
Conceptualization, A.W.; methodology, S.L. (Siyuan Liu) and A.W.; software, S.L. (Siyuan Liu); validation, W.X. and S.L. (Shaopeng Li); formal analysis, S.L. (Siyuan Liu); investigation, W.X.; resources, L.W. and S.L. (Shaopeng Li); data curation, S.L. (Siyuan Liu); writing—original draft preparation, L.W. and S.L. (Siyuan Liu); writing—review and editing, A.W.; visualization, L.W.; supervision, A.W. and L.W.; project administration, A.W.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xianyang Vocational Technical College, grant number 2020KJA02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors declare no conflict of interest.
References
- Dong, N.-Q.; Lin, H.-X. Higher yield with less nitrogen fertilizer. Nat. Plants 2020, 6, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.Q.; Qiao, S.C.; Huang, G.R.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Puckett, L.J.; Tesoriero, A.J.; Dubrovsky, N.M. Nitrogen Contamination of Surficial Aquifers—A Growing Legacy. Environ. Sci. Technol. 2011, 45, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen Fertilization. A Review of the Risks Associated with the Inefficiency of Its Use and Policy Responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: A case study from the semi-urban region of South India. Ecotoxicol. Environ. Saf. 2021, 207, 111277. [Google Scholar] [CrossRef]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Nitrate Removal from Drinking Water—Review. J. Environ. Eng. 1997, 123, 371–380. [Google Scholar] [CrossRef]
- Younker, J.; Zamlynny, L.; Spearns, C.; Rand, J. Nitrate management in a rural drinking water supply. J. Water Process Eng. 2021, 43, 102301. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef]
- Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, J.; Yuan, M.; Wang, L.; Wang, X. A critical review of existing mechanisms and strategies to enhance N2 selectivity in groundwater nitrate reduction. Water Res. 2022, 209, 117889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Haddad, M.; Sauvé, S.; Barbeau, B. Alleviating the burden of ion exchange brine in water treatment: From operational strategies to brine management. Water Res. 2021, 205, 117728. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Zamyadi, A.; Wang, Q.; Li, M. Life-cycle cost analysis of a hybrid algae-based biological desalination—Low pressure reverse osmosis system. Water Res. 2021, 195, 116957. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, S.; Ma, X.; Yu, P.; Zuo, P.; Shi, B.; Wang, H.; Alvarez, P.J. Opportunistic pathogens and their health risk in four full-scale drinking water treatment and distribution systems. Ecol. Eng. 2021, 160, 106134. [Google Scholar] [CrossRef]
- Darajeh, N.; Alizadeh, H.; Leung, D.W.M.; Nodeh, H.R.; Rezania, S.; Farraji, H. Application of Modified Spent Mushroom Compost Biochar (SMCB/Fe) for Nitrate Removal from Aqueous Solution. Toxics 2021, 9, 277. [Google Scholar] [CrossRef]
- Dewage, N.B.; Liyanage, A.S.; Pittman, C.U.; Mohan, D.; Mlsna, T. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour. Technol. 2018, 263, 258–265. [Google Scholar] [CrossRef]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ. Sci. Pollut. Res. 2019, 26, 3065–3074. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Minh, T.D.; Song, J.; Deb, A.; Cha, L.; Srivastava, V.; Sillanpää, M. Biochar based catalysts for the abatement of emerging pollutants: A review. Chem. Eng. J. 2020, 394, 124856. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, C.; Zhu, Y.; Wei, W.; Qin, H. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water. J. Water Process Eng. 2018, 25, 96–104. [Google Scholar] [CrossRef]
- Anfar, Z.; Zbair, M.; Ahsiane, H.A.; Jada, A.; El Alem, N. Microwave assisted green synthesis of Fe2O3/biochar for ultrasonic removal of nonsteroidal anti-inflammatory pharmaceuticals. RSC Adv. 2020, 10, 11371–11380. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, S.; Laird, D.A.; Smith, R.G.; Brown, R.C. Capture and Release of Orthophosphate by Fe-Modified Biochars: Mechanisms and Environmental Applications. ACS Sustain. Chem. Eng. 2021, 9, 658–668. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, And Uses; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Abdelrhman, F.; Gao, J.; Ali, U.; Wan, N.; Sharaf, A.; Hu, H. Assessment of goethite modified biochar on the immobilization of cadmium and arsenic and uptake by Chinese cabbage in paddy soil. Arch. Agron. Soil Sci. 2022, 1–16. [Google Scholar] [CrossRef]
- Irshad, M.K.; Ibrahim, M.; Noman, A.; Shang, J.; Mahmood, A.; Mubashir, M.; Khoo, K.S.; Ng, H.S.; Show, P.L. Elucidating the impact of goethite-modified biochar on arsenic mobility, bioaccumulation in paddy rice (Oryza sativa L.) along with soil enzyme activities. Process Saf. Environ. Prot. 2022, 160, 958–967. [Google Scholar] [CrossRef]
- Zhu, S.; Qu, T.; Irshad, M.K.; Shang, J. Simultaneous removal of Cd(II) and As(III) from co-contaminated aqueous solution by α-FeOOH modified biochar. Biochar 2020, 2, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2020, 247, 119579. [Google Scholar] [CrossRef]
- Schneider, F.; Haderlein, S.B. Potential effects of biochar on the availability of phosphorus—Mechanistic insights. Geoderma 2016, 277, 83–90. [Google Scholar] [CrossRef]
- Kamran, M.A.; Bibi, S.; Chen, B.; Jiang, J.; Xu, R.-K. Elucidating the mechanisms determining the availability of phosphate by application of biochars from different parent materials. Environ. Geochem. Health 2022, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Zhu, R.; Zhang, X.; Yan, L. Phosphate adsorption performance and mechanisms by nanoporous biochar–iron oxides from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 28132–28145. [Google Scholar] [CrossRef]
- Zhang, X.; Gang, D.D.; Sun, P.; Lian, Q.; Yao, H. Goethite dispersed corn straw-derived biochar for phosphate recovery from synthetic urine and its potential as a slow-release fertilizer. Chemosphere 2021, 262, 127861. [Google Scholar] [CrossRef]
- Liu, L.; Liu, G.; Zhou, J.; Jin, R. Interaction between hexavalent chromium and biologically formed iron mineral-biochar composites: Kinetics, products and mechanisms. J. Hazard. Mater. 2021, 405, 124246. [Google Scholar] [CrossRef]
- You, H.; Zhang, Y.; Li, W.; Li, Y.; Ma, Y.; Feng, X. Removal of NO3-N in alkaline rare earth industry effluent using modified coconut shell biochar. Water Sci. Technol. 2019, 80, 784–793. [Google Scholar] [CrossRef]
- Hsi, C.-K.D.; Langmuir, D. Adsorption of uranyl onto ferric oxyhydroxides: Application of the surface complexation site-binding model. Geochim. Cosmochim. Acta 1985, 49, 1931–1941. [Google Scholar] [CrossRef]
- Guo, X.; Dong, H.; Yang, C.; Zhang, Q.; Liao, C.; Zha, F.; Gao, L. Application of goethite modified biochar for tylosin removal from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 502, 81–88. [Google Scholar] [CrossRef]
- Smith, D.E. Molecular Computer Simulations of the Swelling Properties and Interlayer Structure of Cesium Montmorillonite. Langmuir 1998, 14, 5959–5967. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, C.; Song, H.; Yuan, P.; Zhang, B.; Liu, D.; Du, P. Effects of Layer-Charge Distribution of 2:1 Clay Minerals on Methane Hydrate Formation: A Molecular Dynamics Simulation Study. Langmuir 2020, 36, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, J.-W.; Song, K.G.; Choi, K.; Lee, Y.J.; Jung, K.-W. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis. Compos. Part B Eng. 2019, 176, 107209. [Google Scholar] [CrossRef]
- Seftel, E.M.; Ciocarlan, R.G.; Michielsen, B.; Meynen, V.; Mullens, S.; Cool, P. Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides. Appl. Clay Sci. 2018, 165, 234–246. [Google Scholar] [CrossRef]
- Latta, D.E.; Bachman, J.E.; Scherer, M.M. Fe Electron Transfer and Atom Exchange in Goethite: Influence of Al-Substitution and Anion Sorption. Environ. Sci. Technol. 2012, 46, 10614–10623. [Google Scholar] [CrossRef]
- Xu, J.; Koopal, L.K.; Wang, M.; Xiong, J.; Hou, J.; Li, Y.; Tan, W. Phosphate speciation on Al-substituted goethite: ATR-FTIR/2D-COS and CD-MUSIC modeling. Environ. Sci. Nano 2019, 6, 3625–3637. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Liu, F.; Liang, X.; Feng, X.; Tan, W.; Zheng, L.; Yin, H. Effects of Al3+ doping on the structure and properties of goethite and its adsorption behavior towards phosphate. J. Environ. Sci. 2016, 45, 18–27. [Google Scholar] [CrossRef]
- Belelli, P.G.; Fuente, S.A.; Castellani, N.J. Phosphate adsorption on goethite and Al-rich goethite. Comput. Mater. Sci. 2014, 85, 59–66. [Google Scholar] [CrossRef]
- Wang, H.; Cao, S.; Kang, F.; Chen, R.; Liu, H.; Wei, Y. Effects of Al substitution on the microstructure and adsorption performance of α-FeOOH. J. Alloy. Compd. 2014, 606, 117–123. [Google Scholar] [CrossRef]
- Souza, T.G.; Freitas, E.T.; Mohallem, N.D.; Ciminelli, V.S. Defects induced by Al substitution enhance As(V) adsorption on ferrihydrites. J. Hazard. Mater. 2021, 420, 126544. [Google Scholar] [CrossRef] [PubMed]
- Bahashi, J.; Bi, E. Effects of Al substitution on sorption of diclofenac to Fe(III) (hydr)oxides: Roles of phase transition and sorption mechanisms. Environ. Sci. Pollut. Res. 2022, 29, 21314–21327. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Liu, S.; Wang, H.; Liu, H.; Chen, R. Studies on Adsorption Characteristics of Al-Free and Al-Substituted Goethite for Heavy Metal Ion Cr(VI). Water Air Soil Pollut. 2017, 228, 40. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Tzou, Y.-M.; Ho, M.-S.; Sivakumar, C.; Cho, Y.-L.; Li, W.-H.; Chiang, P.-N.; Teah, H.Y.; Liu, Y.-T. Preferential phosphate sorption and Al substitution on goethite. Environ. Sci. Nano 2020, 7, 3497–3508. [Google Scholar] [CrossRef]
- Tufo, A.E.; Afonso, M.D.S.; Sileo, E.E. Arsenic adsorption onto aluminium-substituted goethite. Environ. Chem. 2016, 13, 838. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, P.; Liang, Y.; Wang, M.; Xiong, J.; Tan, W. Effects of aluminum substitution on the surface charge of colloidal goethite particles: Experiments and MUSIC modeling. Environ. Sci. Pollut. Res. 2020, 27, 38397–38406. [Google Scholar] [CrossRef]
- Wei, A.; Ma, J.; Chen, J.; Zhang, Y.; Song, J.; Yu, X. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Paterson, E.J.C.M. Iron Oxides in the Laboratory. Prep. Charact. 2000, 27, 393. [Google Scholar]
- Wawrzkiewicz, M.; Polska-Adach, E.; Wiśniewska, M.; Fijałkowska, G.; Goncharuk, O. Adsorptive removal of C.I. Direct Yellow 142 from textile baths using nanosized silica-titanium oxide. Eur. Phys. J. Plus 2019, 134, 108. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, O.; Eljamal, R.; Falyouna, O.; Sugihara, Y. Promoting aqueous and transport characteristics of highly reactive nanoscale zero valent iron via different layered hydroxide coatings. Appl. Surf. Sci. 2020, 506, 145018. [Google Scholar] [CrossRef]
- Mehdinejadiani, B.; Amininasab, S.M.; Manhooei, L. Enhanced adsorption of nitrate from water by modified wheat straw: Equilibrium, kinetic and thermodynamic studies. Water Sci. Technol. 2019, 79, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Gao, B.; Xu, X.; Wang, F.; Xue, N.; Sun, S.; Song, W.; Jia, R. Adsorption of nitrate from aqueous solution by magnetic amine-crosslinked biopolymer based corn stalk and its chemical regeneration property. J. Hazard. Mater. 2016, 304, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S.J. About the theory of so-called adsorption of solution substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Maamoun, I.; Bensaida, K.; Eljamal, R.; Falyouna, O.; Tanaka, K.; Tosco, T.; Sugihara, Y.; Eljamal, O. Rapid and efficient chromium (VI) removal from aqueous solutions using nickel hydroxide nanoplates (nNiHs). J. Mol. Liq. 2022, 358, 119216. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Effect of Al content on the structure of Al-substituted goethite: A micro-Raman spectroscopic study. J. Raman Spectrosc. 2013, 44, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.G.; Schwertmann, U.J.C. The Influence of Aluminum on the Formation of Iron Oxides. IV. The Influence of [Al], [OH], and Temperature. Clays Clay Miner. 1979, 27, 195–200. [Google Scholar] [CrossRef]
- Bowles, J.F.W. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses. Mineral. Mag. 1997, 61, 740–741. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Rakovan, J.; Becker, U.; Hochella, M.F. Aspects of goethite surface microtopography, structure, chemistry, and reactivity. Am. Miner. 1999, 84, 884–894. [Google Scholar] [CrossRef]
- Gemici, B.T.; Ozel, H.U.; Ozel, H.B. Removal of methylene blue onto forest wastes: Adsorption isotherms, kinetics and thermodynamic analysis. Environ. Technol. Innov. 2021, 22, 101501. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Dehghanifard, E.; Mohseni-Bandpi, A.; Rezaei, L.; Esrafili, A.; Kakavandi, B.; Azari, A. Nitrate adsorption by synthetic activated carbon magnetic nanoparticles: Kinetics, isotherms and thermodynamic studies. DESALINATION Water Treat. 2015, 57, 16445–16455. [Google Scholar] [CrossRef]
- You, H.; Lin, H.; Li, Y.; Yang, Y.; Ma, Y.; Shang, Z.; Niu, X. Iron-aluminum and aluminum-single impregnated biochar composite for nitrate adsorption in rare earth wastewater: Behavior and mechanism. Biomass-Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Nitrate removal from water by nano-alumina: Characterization and sorption studies. Chem. Eng. J. 2010, 163, 317–323. [Google Scholar] [CrossRef]
- Zare, L.; Ghasemi-Fasaei, R. Investigation of Equilibrium Isotherm and Kinetic Modeling to Asses Sorption Characteristics of Nitrate onto Palm Leaf Biochar. Iran J. Chem. Chem. Eng. 2019, 38, 143–153. [Google Scholar]
- Herath, A.; Reid, C.; Perez, F.; Pittman, C.U.; Mlsna, T.E. Biochar-supported polyaniline hybrid for aqueous chromium and nitrate adsorption. J. Environ. Manag. 2021, 296, 113186. [Google Scholar] [CrossRef]
- Antelo, J.; Avena, M.; Fiol, S.; López, R.; Arce, F. Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite–water interface. J. Colloid Interface Sci. 2005, 285, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Exerowa, D. Effect of adsorption, ionic strength and pH on the potential of the diffuse electric layer. Kolloid-Z. Z. Polym. 1969, 232, 703–710. [Google Scholar] [CrossRef]
- Naidu, R.; Bolan, N.; Kookana, R.S.; Tiller, K. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur. J. Soil Sci. 1994, 45, 419–429. [Google Scholar] [CrossRef]
- Ilay, R. Short-lived Effects of Olive Pomace Biochar Produced at Different Temperatures on Nitrate (NO3−), Bromide (Br−), Sulfate (SO42−) and Phosphate (PO43−) Leaching from Sandy Loam Soils. Commun. Soil Sci. Plant Anal. 2020, 51, 2223–2243. [Google Scholar] [CrossRef]
- Mazarji, M.; Aminzadeh, B.; Baghdadi, M.; Bhatnagar, A. Removal of nitrate from aqueous solution using modified granular activated carbon. J. Mol. Liq. 2017, 233, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Yang, Z.; Xu, P.; Li, Y.; Niu, X. Nitrate adsorption from aqueous solutions by calcined ternary Mg-Al-Fe hydrotalcite. Water Sci. Technol. 2017, 75, 2194–2203. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Xu, Z.; Cao, X.; Song, J.; Huang, W.; Ge, X.; Wang, H. Adsorption of Nitrate and Ammonium from Water Simultaneously Using Composite Adsorbents Constructed with Functionalized Biochar and Modified Zeolite. Water Air Soil Pollut. 2021, 232, 198. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Application of pinewood waste-derived biochar for the removal of nitrate and phosphate from single and binary solutions. Chemosphere 2021, 278, 130361. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).