Comparison of Adsorption Capacity and Removal Efficiency of Strontium by Six Typical Adsorption Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Instruments
2.2. Adsorption Experiments
2.3. Adsorption Theory
2.3.1. Isothermal Adsorption Model
2.3.2. Kinetic Model
2.4. Characterizations and Measurements
3. Results and Discussions
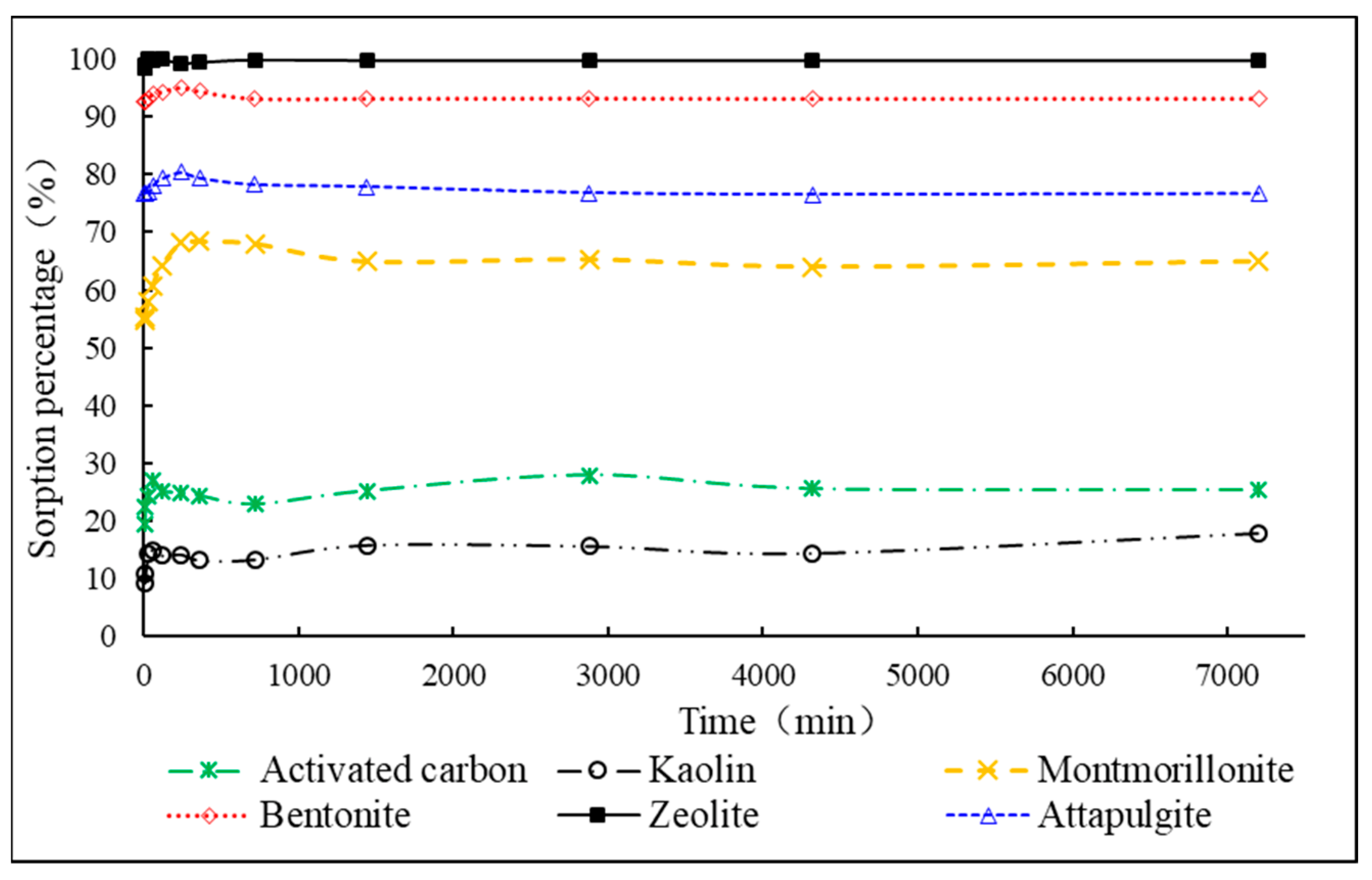
3.1. Comparison of Adsorption Efficiency of Different Adsorbents
3.2. Adsorption Isotherms and Kinetics
3.2.1. Adsorption Isotherms
3.2.2. Adsorption Kinetics
3.3. Characterization
3.3.1. SEM Image Analysis
3.3.2. EDS Analysis
3.3.3. Specific Surface Area Analysis
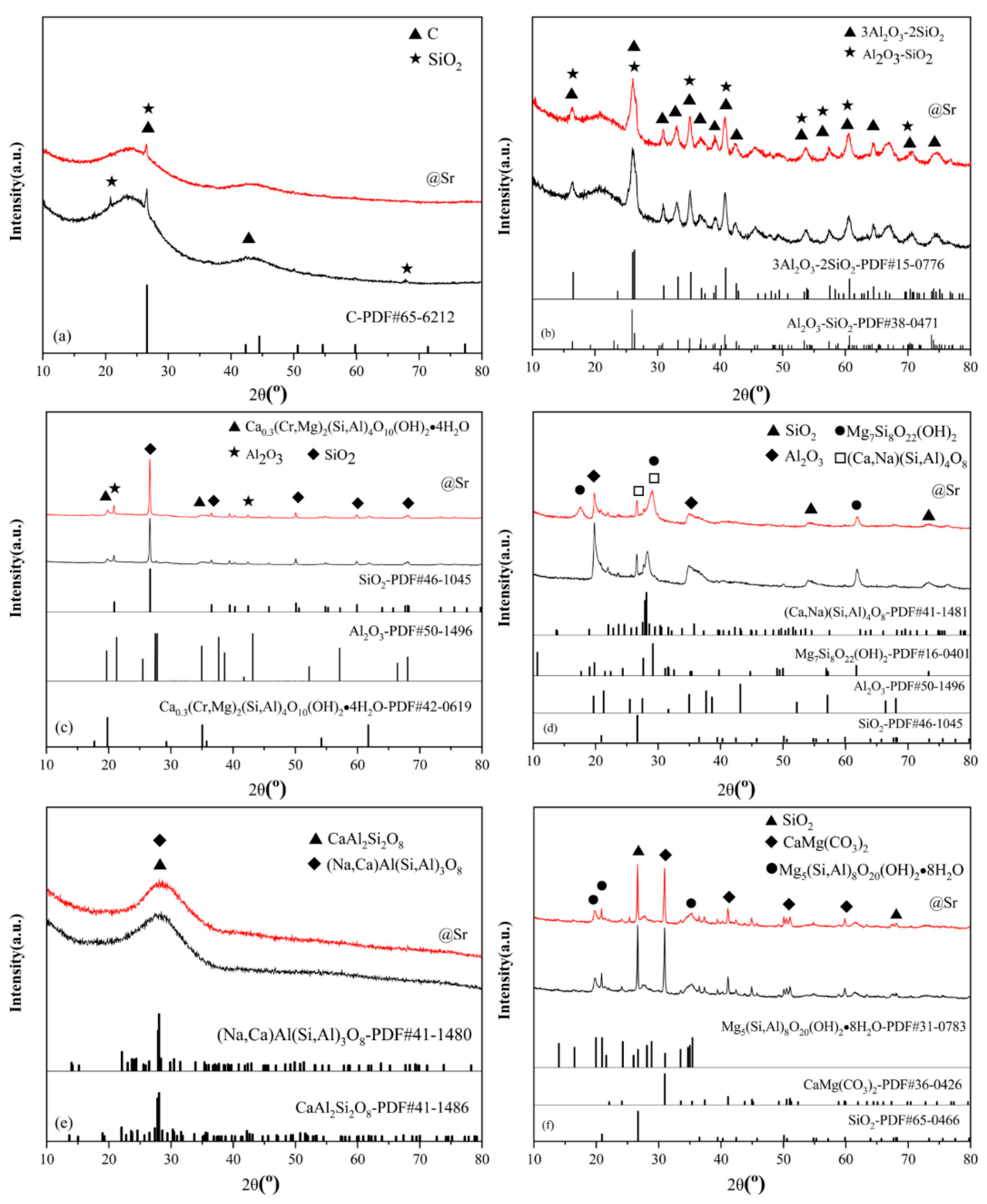
3.3.4. XRD Analysis
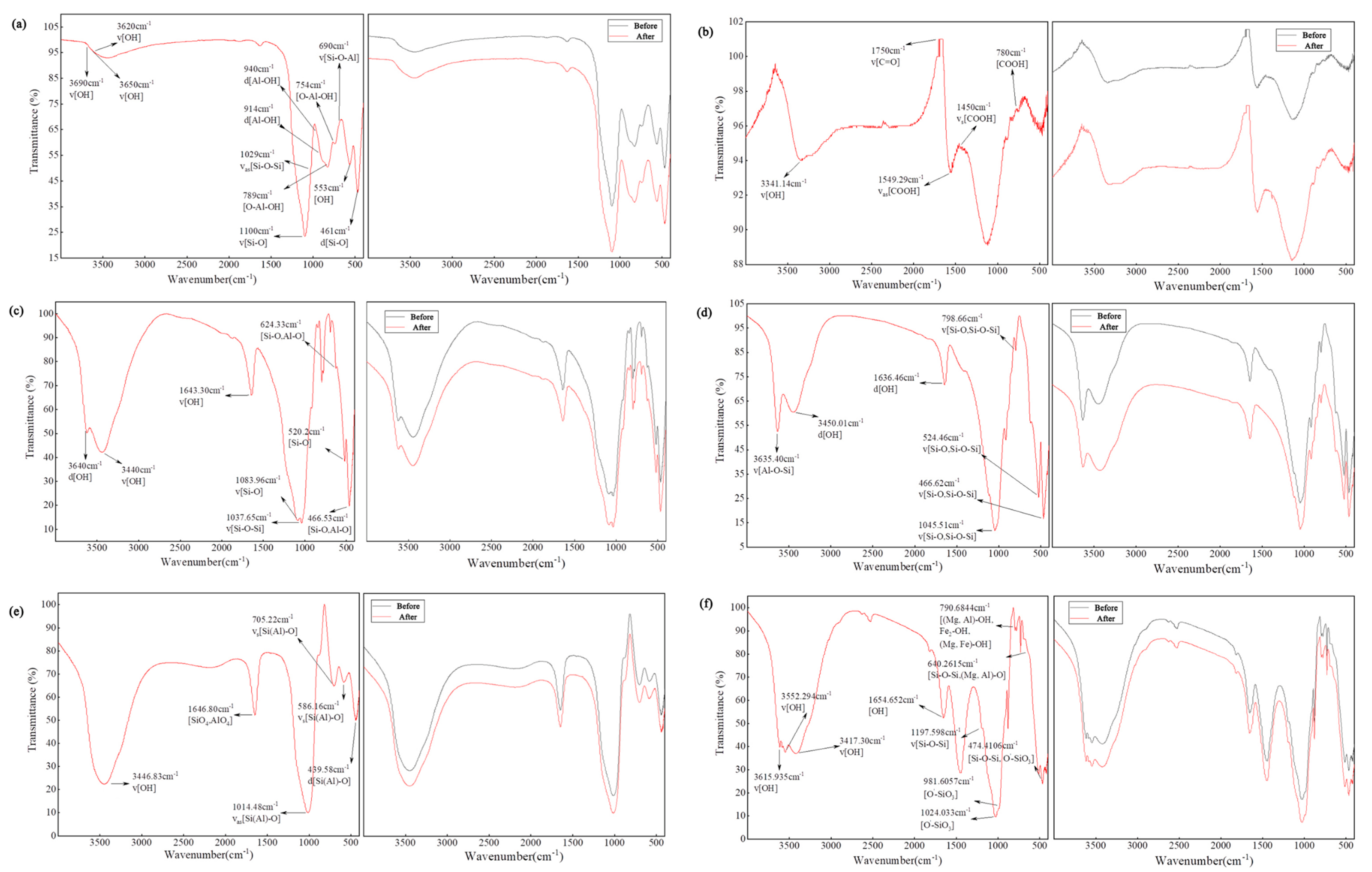
3.3.5. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calabrese, E. Improving the scientific foundations for estimating health risks from the Fukushima incident. Proc. Natl. Acad. Sci. USA 2011, 108, 19447–19448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.L.; Zhang, A.Y.; Chai, Z.F. Synthesis and characterization of novel macroporous silica-polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium. J. Hazard. Mater. 2014, 267, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Finck, N.; Heberling, F.; Pruessmann, T.; Liu, C.L.; Lutzenkirchen, J. Adsorption of Selenium and Strontium on Goethite: EXAFS Study and Surface Complexation Modeling of the Ternary Systems. Environ. Sci. Technol. 2017, 51, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Liu, Y. Ultrafast removal of radioactive strontium ions from contaminated water by nanostructured layered sodium vanadosilicate with high adsorption capacity and selectivity. J. Hazard. Mater. 2020, 398, 122907. [Google Scholar] [CrossRef]
- Lee, S.D.; Snyder, E.G.; Willis, R.; Fischer, R.; Gates-Anderson, D.; Sutton, M.; Viani, B.; Drake, J.; MacKinney, J. Radiological dispersal device outdoor simulation test: Cesium chloride particle characteristics. J. Hazard. Mater. 2010, 176, 56–63. [Google Scholar] [CrossRef]
- Pongener, C.; Bhomick, P.C.; Supong, A.; Baruah, M.; Sinha, U.B.; Sinha, D. Adsorption of fluoride onto activated carbon synthesized from Manihot esculenta biomass-Equilibrium, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 2382–2389. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption behavior of inorganic- and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.H.; He, Y.J.; Chai, L.Y.; Yang, W.C.; Deng, H.Y.; Wang, H.Y.; Chen, Y.S.; Crittenden, J. Adsorption mechanism for removing different species of fluoride by designing of core-shell boehmite. J. Hazard. Mater. 2020, 394, 122555. [Google Scholar] [CrossRef]
- Jan, Y.L.; Tsai, S.C.; Wei, Y.Y.; Tung, N.C.; Wei, C.C.; Hsu, C.N. Coupled mechanics, hydraulics and sorption properties of mixtures to evaluate buffer/backfill materials. Phys. Chem. Earth 2007, 32, 789–794. [Google Scholar] [CrossRef]
- Li, Z.T.; Wang, L.; Meng, J.; Liu, X.M.; Xu, J.M.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H.; Hummadi, E.H. Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Iasir, A.R.M.; Ghosh, T.K.; Sen Gupta, B.; Prelas, M.A. Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maranescu, B.; Popa, A.; Lupa, L.; Maranescu, V.; Visa, A. Use of chitosan complex with aminophosphonic groups and cobalt for the removal of Sr2+ ions. Sep. Sci. Technol. 2018, 53, 1058–1064. [Google Scholar] [CrossRef]
- Du, C.; Zuo, R.; Chen, M.; Wang, J.; Liu, X.; Liu, L.; Lin, Y. Influence of colloidal Fe(OH)(3) on the adsorption characteristics of strontium in porous media from a candidate high-level radioactive waste geological disposal site. Environ. Pollut. 2020, 260, 113997. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.H.; Sinnathamby, G.; Russell, M.I.; Anderson, C.; Paksy, A. Mineralogy of selected geological deposits from the United Kingdom and the Republic of Ireland as possible capping material for low-level radioactive waste disposal facilities. Appl. Clay Sci. 2011, 53, 395–401. [Google Scholar] [CrossRef]
- Aytas, S.; Yurtlu, M.; Donat, R. Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J. Hazard. Mater. 2009, 172, 667–674. [Google Scholar] [CrossRef]
- Krishna, B.S.; Murty, D.S.R.; Prakash, B.S.J. Thermodynamics of chromium(VI) anionic species sorption onto surfactant-modified montmorillonite clay. J. Colloid Interface Sci. 2000, 229, 230–236. [Google Scholar] [CrossRef]
- Xue, A.L.; Zhou, S.Y.; Zhao, Y.J.; Lu, X.P.; Han, P.F. Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes. J. Hazard. Mater. 2011, 194, 7–14. [Google Scholar] [CrossRef]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J.P. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211, 216–223. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Wang, Q.P.; Jin, X.Y.; Chen, Z.L. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, S.; Wu, H.; Deligeer, W.; Asuha, S. Direct acid activation of kaolinite and its effects on the adsorption of methylene blue. Appl. Clay Sci. 2016, 126, 98–106. [Google Scholar] [CrossRef]
- Tournassat, C.; Tinnacher, R.M.; Grangeon, S.; Davis, J.A. Modeling uranium(VI) adsorption onto montmorillonite under varying carbonate concentrations: A surface complexation model accounting for the spillover effect on surface potential. Geochim. Cosmochim. Acta 2018, 220, 291–308. [Google Scholar] [CrossRef]
- Wu, P.X.; Wu, W.M.; Li, S.Z.; Xing, N.; Zhu, N.W.; Li, P.; Wu, J.H.; Yang, C.; Dang, Z. Removal of Cd2+ from aqueous solution by adsorption using Fe-montmorillonite. J. Hazard. Mater. 2009, 169, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sanchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Arulkumar, M.; Thirumalai, K.; Sathishkumar, P.; Palvannan, T. Rapid removal of chromium from aqueous solution using novel prawn shell activated carbon. Chem. Eng. J. 2012, 185, 178–186. [Google Scholar] [CrossRef]
- Tang, N.; Niu, C.G.; Li, X.T.; Liang, C.; Guo, H.; Lin, L.S.; Zheng, C.W.; Zeng, G.M. Efficient removal of Cd(2+) and Pb(2+) from aqueous solution with amino- and thiol-functionalized activated carbon: Isotherm and kinetics modeling. Sci. Total Environ. 2018, 635, 1331–1344. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Supriyanto, G.; Kusuma, H.S. Adsorption performance of Cr(VI)-imprinted poly(4-VP-co-MMA) supported on activated Indonesia (Ende-Flores) natural zeolite structure for Cr(VI) removal from aqueous solution. J. Environ. Chem. Eng. 2018, 6, 3436–3443. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.N.; Riwu, A.A.P.; Darmokoesoemo, H.; Supriyanto, G.; Holdsworth, C.I.; Amenaghawon, A.N.; Kusuma, H.S. A Cr(VI)-imprinted-poly(4-VP-co-EGDMA) sorbent prepared using precipitation polymerization and its application for selective adsorptive removal and solid phase extraction of Cr(VI) ions from electroplating industrial wastewater. React. Funct. Polym. 2020, 147, 104451. [Google Scholar] [CrossRef]
- Janusz, W.; Skwarek, E. Study of sorption processes of strontium on the synthetic hydroxyapatite. Adsorpt.-J. Int. Adsorpt. Soc. 2016, 22, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Donat, R.; Akdogan, A.; Erdem, E.; Cetisli, H. Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J. Colloid Interface Sci. 2005, 286, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.H.; Yu, L.; Wang, C.Z.; Yin, X.Q.; Mosa, A.; Lv, J.L.; Sun, H.M. Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 2017, 169, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Biosorption of reactive dyes by dried activated sludge: Equilibrium and kinetic modelling. Biochem. Eng. J. 2001, 7, 79–84. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Wu, W.S.; Fan, Q.H.; Xu, J.Z.; Niu, Z.W.; Lu, S.S. Sorption-desorption of Th(IV) on attapulgite: Effects of pH, ionic strength and temperature. Appl. Radiat. Isot. 2007, 65, 1108–1114. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, A. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Shaban, M.; Hassouna, M.E.M.; Nasief, F.M.; AbuKhadra, M.R. Adsorption properties of kaolinite-based nanocomposites for Fe and Mn pollutants from aqueous solutions and raw ground water: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. 2017, 24, 22954–22966. [Google Scholar] [CrossRef]
- Nguyen-Thanh, D.; Block, K.; Bandosz, T.J. Adsorption of hydrogen sulfide on montmorillonites modified with iron. Chemosphere 2005, 59, 343–353. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.J.; Ma, J.X.; Xia, P.; Zhao, J.F. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef]
- Kacan, E.; Kutahyali, C. Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J. Anal. Appl. Pyrolysis 2012, 97, 149–157. [Google Scholar] [CrossRef]
- Wu, P.X.; Dai, Y.P.; Long, H.; Zhu, N.W.; Jai, P.; Wu, J.H.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr(II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Peng, Y.R.; Azeem, M.; Li, R.H.; Xing, L.B.; Li, Y.M.; Zhang, Y.C.; Guo, Z.Q.; Wang, Q.; Ngo, H.H.; Qu, G.Z.; et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J. Hazard. Mater. 2022, 423, 127081. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Wang, A.Q. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91. [Google Scholar] [CrossRef]

| Disposal Methods | Advantages | Disadvantages |
|---|---|---|
| Chemical precipitation | Low cost, simple method and proven technology | Low selectivity and poor purification |
| Ion exchange | High selectivity and wide range of applications | Higher cost and large amount of waste generated |
| Evaporation and concentration | High efficiency of removal | High thermal energy consumption and high operating costs |
| Electrolysis | Vulnerability to other factors | Incomplete technical system |
| Redox methods | High selectivity | Costly |
| No. | Equipment Name | Source and Description |
|---|---|---|
| 1 | Centrifuge | LXJ-IIB, Feige |
| 2 | Electronic balance | BSA224S-CW, Sartorius, Göttingen, Germany |
| 3 | Thermostat oscillator | WS20, Wiggens |
| 4 | pH meter | PHS-3C, Leici |
| 5 | Specific surface analyzer | Quadrasorb SI, Quantachrome |
| 6 | Scanning electron microscope | S-4800, Hitachi High Technologies Corporation, Tokyo, Japan |
| 7 | Electronic dispersive spectrometer | S-4800, Hitachi High Technologies Corporation, Tokyo, Japan |
| 8 | X-ray diffractometer | PANalytical, X’Pert PRO MPD |
| 9 | Fourier transform infrared spectrometer | IRAffinity−1, SHIMADZU |
| Adsorption Materials | Adsorption Capacities (mg g−1) | Contrast Value (mg g−1) | References |
|---|---|---|---|
| Activated carbon | 1.03 | 2.5 | [20] |
| Kaolin | 0.63 | 4.2 | [16] |
| Montmorillonite | 2.78 | 15 | [19] |
| Bentonite | 3.85 | 4.5 | [12] |
| Zeolite | 4.07 | 11.52 | [10] |
| Attapulgite | 3.16 | 3.25 | [36] |
| Samples | Freundlich Isotherm | Langmuir Isotherm | Temkin Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KF | 1/n | R2 | KL | Qm | R2 | A | B | R2 | |
| Activated carbon | 0.05 | 0.64 | 0.8966 | 0.006 | 2.59 | 0.8511 | 0.39 | 1070.3 | 0.7625 |
| Kaolin | 0.007 | 0.61 | 0.9504 | 0.007 | 1.49 | 0.9511 | 0.19 | 1072.9 | 0.8053 |
| Montmorillonite | 1.08 | 0.38 | 0.9632 | 0.103 | 5.86 | 0.9378 | 1.55 | 2262.3 | 0.9597 |
| Bentonite | 4.83 | 0.42 | 0.6371 | 0.268 | 19.62 | 0.7639 | 2.03 | 532.3 | 0.7810 |
| Zeolite | 48.4 | 0.70 | 0.7745 | 4.002 | 35.67 | 0.8576 | 76.4 | 468.1 | 0.7211 |
| Attapulgite | 1.96 | 0.32 | 0.8311 | 0.167 | 7.53 | 0.8387 | 16.92 | 2672.2 | 0.7295 |
| Models | Parameters | Activated Carbon | Kaolin | Montmorillonite | Bentonite | Zeolite | Attapulgite |
|---|---|---|---|---|---|---|---|
| Elovich model | a | 45.68 | 26.88 | 22.13 | 29.51 | 28.31 | 35.77 |
| b | 6.19 | 248.53 | 1.9 | 5.92 | 1.17 | 3.77 | |
| R2 | 0.9545 | 0.8750 | 0.9828 | 0.9951 | 0.9957 | 0.9956 | |
| Two-Constant model | A | 0.02 | 0.07 | 0.02 | 0.008 | 0.008 | 0.008 |
| B | −0.12 | −1.06 | 0.86 | 1.28 | 1.35 | 1.09 | |
| R2 | 0.9538 | 0.8887 | 0.9822 | 0.9999 | 0.9999 | 0.9999 | |
| Pseudo-first-order model | K1 | 0.30 | 0.20 | 0.41 | 0.91 | 0.88 | 0.05 |
| q1 | 1.02 | 0.55 | 2.63 | 3.80 | 4.06 | 3.19 | |
| R2 | 0.9735 | 0.7312 | 0.9765 | 0.9997 | 0.9999 | 0.1538 | |
| Pseudo-second-order model | K2 | 0.81 | 0.63 | 0.43 | 4.12 | 3.77 | 0.18 |
| q2 | 1.03 | 0.57 | 2.67 | 3.81 | 4.07 | 3.19 | |
| R2 | 0.9681 | 0.7526 | 0.9876 | 0.9997 | 0.9999 | 0.6739 | |
| Intra-particle diffusion model | k | 0.004 | 0.005 | 0.009 | 0.011 | 0.012 | 0.009 |
| R2 | 0.0883 | 0.4854 | 0.0449 | -0.0014 | 0.0008 | 0.0003 |
| Element | Activated Carbon | Kaolin | Montmorillonite | Bentonite | Zeolite | Attapulgite |
|---|---|---|---|---|---|---|
| C | 88.03 | 16.89 | 12.92 | 4.45 | 12.12 | 22.97 |
| O | 11.60 | 62.81 | 66.06 | 67.93 | 58.01 | 58.15 |
| Mg | 0.12 | - | 0.79 | 1.09 | - | 2.24 |
| Al | 0.03 | 10.08 | 2.57 | 7.00 | 7.86 | 3.06 |
| Si | 0.14 | 9.76 | 17.15 | 16.69 | 13.19 | 9.91 |
| Ca | - | - | 0.17 | 0.06 | - | 0.96 |
| Fe | - | 0.06 | 0.34 | - | - | - |
| K | - | - | - | 0.16 | 0.15 | 0.39 |
| Na | - | - | - | 1.62 | 8.67 | - |
| Sample | Activated Carbon | Kaolin | Montmorillonite | Bentonite | Zeolite | Attapulgite |
|---|---|---|---|---|---|---|
| Specific surface area (m2/g) | 1407.754 | 10.227 | 183.492 | 28.546 | 110.213 | 205.630 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Han, K.; Shang, J.; Cai, W.; Pan, M.; Xu, D.; Du, C.; Zuo, R. Comparison of Adsorption Capacity and Removal Efficiency of Strontium by Six Typical Adsorption Materials. Sustainability 2022, 14, 7723. https://doi.org/10.3390/su14137723
Li H, Han K, Shang J, Cai W, Pan M, Xu D, Du C, Zuo R. Comparison of Adsorption Capacity and Removal Efficiency of Strontium by Six Typical Adsorption Materials. Sustainability. 2022; 14(13):7723. https://doi.org/10.3390/su14137723
Chicago/Turabian StyleLi, Hu, Kexue Han, Jinhua Shang, Weihai Cai, Minghao Pan, Donghui Xu, Can Du, and Rui Zuo. 2022. "Comparison of Adsorption Capacity and Removal Efficiency of Strontium by Six Typical Adsorption Materials" Sustainability 14, no. 13: 7723. https://doi.org/10.3390/su14137723
APA StyleLi, H., Han, K., Shang, J., Cai, W., Pan, M., Xu, D., Du, C., & Zuo, R. (2022). Comparison of Adsorption Capacity and Removal Efficiency of Strontium by Six Typical Adsorption Materials. Sustainability, 14(13), 7723. https://doi.org/10.3390/su14137723





