Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management
Abstract
:1. Introduction
2. Biogeochemical Processes of Fe in Groundwater
2.1. Fe Distribution in Groundwater
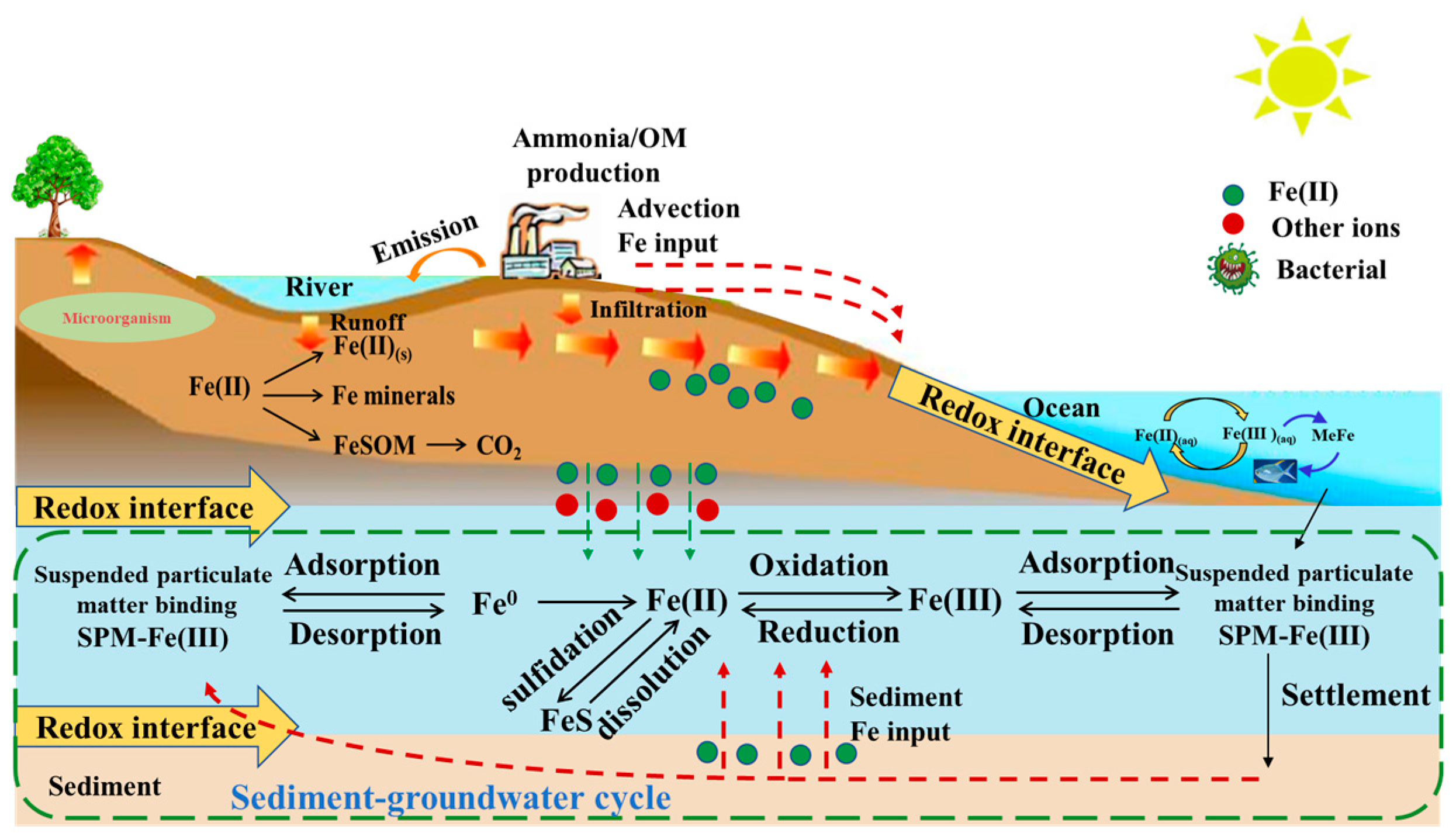
2.2. Origin of Fe in Groundwater
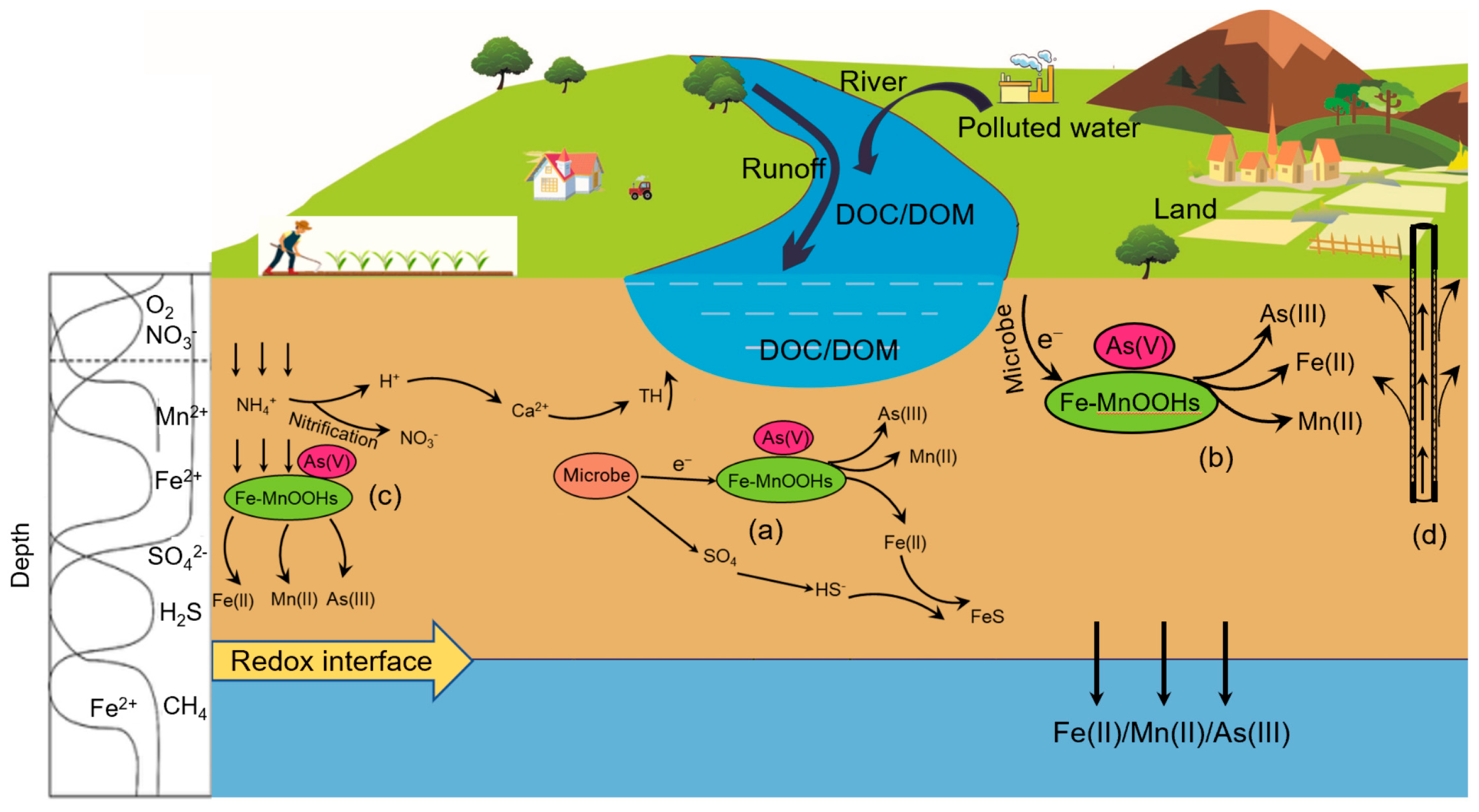
2.3. Biogeochemical Reaction of Fe in Groundwater
3. Factors Affecting Fe Biogeochemistry
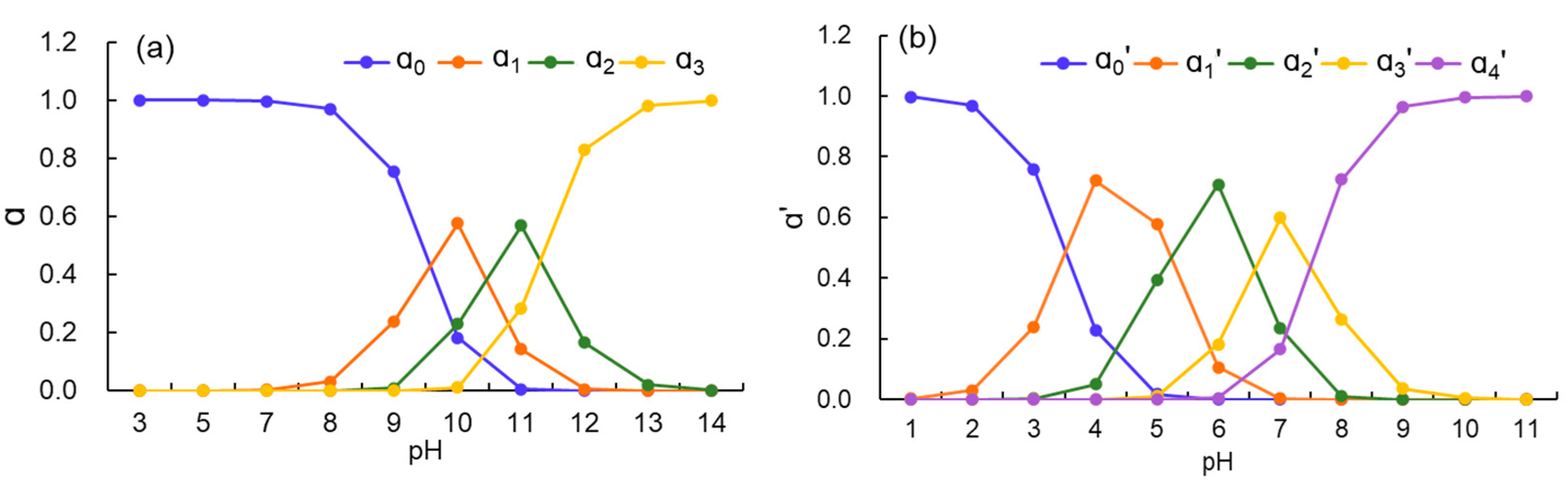
3.1. pH
3.2. Redox Potential
3.3. Organic Matter
3.4. Microorganisms
4. Environmental Management Based on Fe in Groundwater
4.1. Groundwater Monitoring and Assessment
4.2. Early Warning of Groundwater Pollution
5. Management and Prospects of Fe Pollution
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Ding, S.; Ling, L.; Di, X.; Gong, M.; Hao, T. Kinetics of phosphorus release from sediments and its relationship with iron speciation influenced by the mussel (corbicula fluminea) bioturbation. Sci. Total Environ. 2016, 542, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Guo, B.; Tsang, D.; Mechtcherine, V. Red mud-enhanced magnesium phosphate cement for remediation of pb and as contaminated soil. J. Hazard. Mater. 2020, 400, 123317. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.; Berg, G.; Breukelen, B.; Juhasz-Holterman, M.; Stuyfzand, P.J. Iron-hydroxide clogging of public supply wells receiving artificial recharge: Near-well and in-well hydrological and hydrochemical observations. Hydrogeol. J. 2013, 21, 1393–1412. [Google Scholar] [CrossRef]
- Hou, K.; Ma, X.; Li, Y.; Liu, F.; Han, D. Genesis of Huoqiu banded iron formation (BIF), southeastern North China Craton, constraints from geochemical and Hf-O-S isotopic characteristics. J. Geochem. Explor. 2019, 197, 60–69. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.; Fredrickson, J. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Manamsa, K.; Crane, E.; Stuart, M.; Talbot, J.; Lapworth, D.; Hart, A. A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci. Total Environ. 2016, 568, 712–726. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, J.; Wang, L.; Chen, J.; Hou, H.; Yang, J. Long-term stability of FeSO4 and H2SO4 treated chromite ore processing residue (COPR): Importance of H+ and SO42−. J. Hazard. Mater. 2017, 321, 720–727. [Google Scholar] [CrossRef]
- Teutsong, T.; Temga, J.; Enyegue, A.; Feuwo, N.; Bitom, D. Petrographic and geochemical characterization of weathered materials developed on BIF from the Mamelles iron ore deposit in the Nyong unit, South-West cameroon. Acta Geochim. 2021, 40, 163–175. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination 2010, 263, 240–248. [Google Scholar] [CrossRef]
- Heikkinen, K.; Saari, M.; Heino, J.; Ronkanen, A.; Kortelainen, P.; Joensuu, S. Iron in boreal river catchments: Biogeochemical, ecological and management implications. Sci. Total Environ. 2022, 805, 150256. [Google Scholar] [CrossRef]
- Hutchins, D.; Boyd, P. Marine phytoplankton and the changing ocean iron cycle. Nat. Clim. Chang. 2016, 6, 1072–1079. [Google Scholar] [CrossRef]
- Ye, X.; Cui, R.; Wang, L.; Du, X. The influence of riverbank filtration on regional water resources: A case study in the second Songhua River Catchment, China. Water Sci. Tech. Water Supply 2020, 20, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Xi, B.; Jiang, Y.; Guo, H.; Yang, Y.; Lian, X.; Han, S. Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: A review. Sci. Total Environ. 2018, 643, 967–993. [Google Scholar] [CrossRef]
- Peter, B.; Kenneth, B.; James, E.; Tyler, D. Elevated manganese concentrations in united states groundwater, role of land surface-soil-aquifer connections. Environ. Sci. Technol. 2019, 53, 29–38. [Google Scholar]
- Li, J.; Wan, Y.; Li, Y.; Yao, G.; Lai, B. Surface Fe(III)/Fe(II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl. Catal. B Environ. 2019, 256, 117782. [Google Scholar] [CrossRef]
- Burgos, W.; Borch, T.; Troyer, L.; Luan, F.; Larson, L. Schwertmannite and Fe oxides formed by b.;low-pH Fe(II) oxidation versus abiotic neutralization: Impact on trace metal sequestration. Geochim. Cosmochim. Acta 2012, 76, 29–44. [Google Scholar] [CrossRef]
- Chen, L.; Ma, T.; Wang, Y.; Zheng, J. Health risks associated with multiple metal(loid)s in groundwater: A case study at Hetao Plain, northern China. Environ. Pollut. 2020, 263, 114562. [Google Scholar] [CrossRef]
- Johnson, C.D.; Nandi, A.; Joyner, T.A.; Luffman, I. Iron and Manganese in Groundwater: Using Kriging and GIS to Locate High Concentrations in Buncombe County, North Carolina. Ground Water 2018, 56, 87–95. [Google Scholar] [CrossRef]
- DeSimone, L.; McMahon, P.; Rosen, M. The Quality of Our Nation’s Waters—Water Quality in Principal Aquifers of the United States, 1991–2010; US Geological Survey: Reston, WA, USA, 2014; Volume 1360, p. 151.
- Homoncik, S.; Macdonald, A.; Heal, K. Manganese concentrations in Scottish groundwater. Sci. Total Environ. 2010, 408, 2467–2473. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Teng, Y.; Zhai, Y.; Zheng, F.; Cao, X. Influencing factors and mechanism by which DOM in groundwater releases Fe from sediment. Chemosphere 2022, 300, 134524. [Google Scholar] [CrossRef]
- Markic, D.; Bjelic, D.; Drakulic, N.; Carapina, H.; Sobot Pesic, Z. Assessment of the impact of banjaluka landfill on groundwater quality. Carpath. J. Earth Env. 2015, 10, 271–280. [Google Scholar]
- Grisey, E.; Aleya, L. Prolonged aerobic degradation of shredded and pre-composted municipal solid waste: Report from a 21-year study of leachate quality characteristics. Environ. Sci. Pollut. Res. 2016, 23, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sohi, S.; Liu, S.; Guan, J.; Zhou, J.; Chen, J. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 2019, 235, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Catalytic degradation of 2,4-dichloro-phenoxyacetic acid (2,4-D) by nano-Fe2O3 activated peroxymonosulfate: Influential factors and mechanism determination. Chemosphere 2017, 169, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guo, H.; Xi, B.; Jiang, Y.; Zhang, Z.; Yuan, R. Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao basin, Inner Mongolia. Sci. Total Environ. 2017, 601, 691–702. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.; Huang, D. Use of iron oxide nanomaterials in wastewater treatment: A review-Sciencedirect. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Weng, H.; Qin, C.; Chen, X. Elevated iron and manganese concentrations in groundwater derived from the Holocene transgression in the Hang-Jia-Hu Plain, China. Hydrogeol. J. 2007, 15, 715–726. [Google Scholar] [CrossRef]
- Sparrow, L.; Uren, N. The role of manganese toxicity in crop yellowing on seasonally waterlogged and strongly acidic soils in northeastern Victoria. Aust. J. Exp. Agric. 1987, 27, 303–307. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, M.; Singh, J. Studies on the mobility of heavy metals in soil. Indian J. Environ. Health 1996, 38, 1–6. [Google Scholar]
- Buschmann, J.; Berg, M.; Stengel, C.; Sampson, M. Arsenic and manganese contamination of drinking water resources in Cam-bodia: Coincidence of risk areas with low relief topography. Environ. Sci. Technol. 2007, 41, 2146–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, C.; Santos, I.; Barcellos, R. Elevated concentrations of dissolve Ba, Fe and Mn in a mangrove subterranean estuary: Consequence of seal level rise? Cont. Shelf Res. 2012, 43, 86–94. [Google Scholar] [CrossRef]
- Kshetrimayum, K.; Hegeu, H. The state of toxicity and cause of elevated iron and manganese concentrations in surface water and groundwater around Naga Thrust of Assam-Arakan basin, Northeastern India. Environ. Earth Sci. 2016, 75, 1–14. [Google Scholar] [CrossRef]
- Szczucinska, A.; Siepak, M.; Ziota-Frankowska, A.; Marciniak, M. Seasonal and spatial changes of metal concentrations in groundwater outflows from porous sediments in the gryzyna-grabin tunnel valley in western poland. Environ. Earth Sci. 2010, 61, 921–930. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L. Groundwater Resources of China, Liaoning Province Volume; China Cartographic Publishing House: Beijing, China, 2005; (In Chinese with English Abstract). [Google Scholar]
- Jiao, J.; Wang, Y.; Cherry, J.; Wang, X.; Zhi, B.; Du, H. Abnormally high am-monium of natural origin in a coastal aquifer-aquitard system in the Pearl River Delta, China. Environ. Sci. Technol. 2010, 44, 7470–7475. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Giambastiani, B.; Colombani, N. Ammonium occurrence in a salinized lowland coastal aquifer (Ferrara, Italy). Hydrol. Process. 2013, 27, 3495–3501. [Google Scholar] [CrossRef]
- Lingle, D.; Kehew, A.; Krishnamurthy, R. Use of nitrogen isotopes and other geochemical tools to evaluate the source of ammonium in a confined glacial drift aquifer, Ottawa County, Michigan, USA. Appl. Geochem. 2017, 78, 334–342. [Google Scholar] [CrossRef]
- Postma, D.; Larsen, F.; Thai, N.; Trang, P.; Jakobsen, R.; Nhan, P.; Long, T.; Viet, P.; Murray, A. Groundwater arsenic concentrations in Vietnam controlled by sediment age. Nat. Geosci. 2012, 5, 656–661. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, H.; Jiang, Y.; Wu, Y.; Zhou, Y. Hydrogeochemical zonation and its im-plication for arsenic mobilization in deep groundwaters near alluvial fans in the Hetao Basin, Inner Mongolia. J. Hydrol. 2014, 518, 410–420. [Google Scholar] [CrossRef]
- Fei, Y.; Zhang, Z.; Song, H.; Qian, Y.; Chen, J.; Meng, S. Discussion of vertical variations of saline groundwater and mechanism in North China Plain. Water Resour. Prot. 2009, 26, 21–23, (In Chinese with English abstract). [Google Scholar]
- Han, D.; Currell, M.; Cao, G. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, R.; Guo, H.; Jia, Y.; Jiang, Y.; Cao, Y.; Zhao, W.; Wang, Z. Distribution character-istics and genesis of fluoride groundwater in the Hetao basin, Inner Mongolia. Earth Sci. Front. 2016, 23, 260–268, (In Chinese with English abstract). [Google Scholar]
- Lovley, D.; Holmes, D.; Nevin, K. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [PubMed] [Green Version]
- Ahmed, I.; Maher, B. Identification and Paleoclimatic Significance of Magnetite Nanoparticles in Soils. P. Natl. Acad. Sci. USA 2018, 115, 1736–1741. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.; Silver, W.; Timokhin, V.; Hammel, K. Lignin Decomposition Is Sustained under Fluctuating Redox Conditions in Humid Tropical Forest Soils. Global Change Biol. 2015, 21, 2818–2828. [Google Scholar] [CrossRef] [Green Version]
- Rozan, T.; Taillefert, M.; Trouwborst, R.; Glazer, B.; Ma, S.; Herszage, J.; Valdes, L.; Price, K.; Luther, G. Iron-Sulfur-Phosphorus Cycling in the Sediments of a Shallow Coastal Bay:Implications for Sediment Nutrient Release and Benthic Macroalgal Blooms. Limnol. Oceanog. 2002, 47, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Burdige, D.; Komada, T. Iron redox cycling, sediment resuspension and the role of sediments in low oxygen environments as sources of iron to the water column. Mar. Chem. 2020, 223, 103793. [Google Scholar] [CrossRef]
- Sheng, A.; Li, X.; Arai, Y.; Ding, Y.; Rosso, K.M.; Liu, J. Citrate controls Fe(II)-catalyzed transformation of ferrihydrite by complexation of the labile Fe(III) intermediate. Environ. Sci. Technol. 2020, 54, 7309–7319. [Google Scholar] [CrossRef]
- Scholz, F.; Löscher, C.; Fiskal, A.; Sommer, S.; Hensen, C.; Lomnitz, U.; Wuttig, K.; Göttlicher, J.; Kossel, E.; Steininger, R. Nitrate-dependent iron oxidation limits iron transport in anoxic ocean regions. Earth Planet. Sci. Lett. 2016, 454, 272–281. [Google Scholar] [CrossRef]
- Trusiak, A.; Treibergs, L.; Kling, G.; Cory, R. The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim. Cosmochim. Ac. 2018, 224, 80–95. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Liu, C.-C.; Zhang, H. Effects of Second Metal Oxides on Surface-Mediated Reduction of Contaminants by Fe(II) with Iron Oxide. ACS Earth Space Chem. 2019, 3, 680–687. [Google Scholar] [CrossRef]
- Miller, C.; Rose, A.; Waite, T. Hydroxyl radical production by H2O2− mediated oxidation of Fe(Ⅱ) complexed by suwannee river fulvic acid under circumneutral freshwater conditions. Environ. Sci. Technol. 2013, 47, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeng, X.; Wang, J.; Deng, Y.; Ma, T.; Guo, J.; Mu, Y.; Yang, Y.; Li, H.; Wang, Y. Microbial communities involved in arsenic mobilization and release from the deep sediments into groundwater in Jianghan plain, Central China. Sci. Total Environ. 2017, 579, 989–999. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Alam, I.; Chowdhury, I. Aggregation and stability of nanoscale plastics in aquatic environment. Water Res. 2019, 171, 115401. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yuan, S.; Tong, M.; Ma, S.; Chen, C. Contaminant degradation by oh during sediment oxygenation: Dependence on Fe(II) species. Environ. Sci. Technol. 2020, 54, 2975–2984. [Google Scholar] [CrossRef]
- Harvey, J.; Fuller, C. Effect of enhanced manganese oxidation in the hyporheic zone on basin-scale geochemical mass balance. Water Res. Res. 1998, 34, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Fuller, C.; Harvey, J. Reactive uptake of trace metals in the hyporheic zone of a mining-contaminated stream, pinal creek, arizona. Environ. Sci. Technol. 2000, 34, 1150–1155. [Google Scholar] [CrossRef]
- Rubasinghege, G. Simulated atmospheric processing of iron oxyhydroxide minerals at low pH: Roles of particle size and acid anion in iron dissolution. Proc. Natl. Acad. Sci. USA 2010, 107, 6628–6633. [Google Scholar] [CrossRef] [Green Version]
- Bryant, L.; Hsu-Kim, H.; Gantzer, P.; Little, J. Solving the problem at the source: Controlling Mn release at the sediment-water interface via hypolimnetic oxygenation. Water Res. 2011, 45, 6381–6392. [Google Scholar] [CrossRef]
- Ardo, S.; Nelieu, S.; Ona-Nguema, G.; Delarue, G.; Brest, J.; Pironin, E. Oxidative degradation of nalidixic acid by nano-magnetite via Fe2+/O2− mediated reactions. Environ. Sci. Technol. 2015, 49, 4506–4514. [Google Scholar] [CrossRef] [PubMed]
- Gypser, S.; Hirsch, F.; Schleicher, A.; Freese, D. Impact of crystalline and amorphous iron-and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Stosser, R.; Zehl, A. Nature and abundance of organic radicals in natural organic matter: Effect of pH and irradiation. Environ. Sci. Technol. 2006, 40, 5897–5903. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Neumann, A.; Yuan, S.; Liao, W.; Qian, A. Oxidative Degradation of Organic Contaminants by FeS in the Presence of O2. Environ. Sci. Technol. 2020, 54, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Cory, R.; Mcknight, D. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, T.; Yu, Y.; Wu, Y.; Yang, X. A novel peroxymonosulfate (pms)-enhanced iron coagulation process for simultaneous removal of trace organic pollutants in water. Water Res. 2020, 185, 116136. [Google Scholar] [CrossRef]
- Godowska, M.; Stopelli, E.; Pham, T.; Duyen, V.; Kleindienst, S. Arsenic behavior in groundwater in Hanoi (Vietnam) influenced by a complex biogeochemical network of iron, methane, and sulfur cycling. J. Hazard. Mater. 2020, 407, 124398. [Google Scholar] [CrossRef]
- Melton, E.; Swanner, E.; Behrens, S.; Schmidt, C.; Kappler, A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Wielinga, B.; Lucy, J.; Moore, J.; Seastone, O.; Gannon, J. Microbiological and geochemical characterization of fluvially deposited sulfidic mine tailings. Appl. Environ. Microb. 1999, 65, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yuan, S.; Peng, L. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Cosmochim. Ac. 2016, 172, 444–457. [Google Scholar]
- Huan, H.; Li, X.; Zhou, J.; Liu, W.; Jiang, Y. Groundwater pollution early warning based on qtr model for regional risk management: A case study in luoyang city, China. Environ. Pollut. 2020, 259, 113900. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gan, Y.; Wang, Y.; Deng, Y.; Guo, X.; Dong, C. Temporal variation of groundwater level and arsenic concentration at Jianghan Plain, central China. J. Geochem. Explor. 2015, 149, 106–119. [Google Scholar] [CrossRef]
- Huang, G.; Han, D.; Song, J.; Li, L.; Pei, L. A sharp contrasting occurrence of iron-rich groundwater in the Pearl River Delta during the past dozen years (2006–2018): The genesis and mitigation effect. Sci. Total Environ. 2022, 829, 154676. [Google Scholar] [CrossRef] [PubMed]
- Broclawik, O.; Lukawska-Matuszewska, K.; Brodecka-Goluch, A.; Bolalek, J. Impact of methane occurrence on iron speciation in the sediments of the Gdansk Basin (Southern Baltic Sea). Sci. Total. Environ. 2020, 721, 137718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Wang, H.; Cheng, Y.; Wang, Y. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J. Hazard. Mater. 2021, 411, 125146. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, Z.; Ding, Z.; Liu, J. Synthesis of different crystallographic FeOOH catalysts for peroxymonosulfate activation towards organic matter degradation. RSC Adv. 2018, 8, 7269–7279. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, X.; Zhang, W.; Huan, Y.; Yu, J.; Zhang, Y. Risk Assessment of Groundwater Organic Pollution Using Hazard, Intrinsic Vulnerability, and Groundwater Value, Suzhou City in China. Expo. Health 2017, 10, 99–115. [Google Scholar] [CrossRef]
- Jylhä-Ollila, M.; Laine-Kaulio, H.; Niinikoski-Fusswinkel, P.; Leveinen, J.; Koivusalo, H. Water quality changes and organic matter removal using natural bank infiltration at a boreal lake in Finland. Hydrogeol. J. 2020, 28, 1343–1357. [Google Scholar] [CrossRef] [Green Version]
- Dean, W.E.; Neff, B.P.; Rosenberry, D.O.; Winter, T.C.; Parkhurst, R. The Significance of Ground Water to the Accumulation of Iron and Manganese in the Sediments of Two Hydrologically Distinct Lakes in North-Central Minnesota: A Geological Perspective. Ground Water 2003, 41, 951–963. [Google Scholar] [CrossRef]
- Chen, R.; Teng, Y.; Chen, H.; Hu, B.; Yue, W. Groundwater pollution and risk assessment based on source apportionment in a typical cold agricultural region in northeastern China. Sci. Total Environ. 2019, 696, 133972. [Google Scholar] [CrossRef]
- Bhutiani, R.; Kulkarni, D.; Khanna, D.; Gautam, A. Water quality, pollution source apportionment and health risk assessment of heavy metals in groundwater of an industrial area in North India. Expos. Health 2015, 8, 3–18. [Google Scholar] [CrossRef]
- Boateng, T.; Opoku, F.; Acquaah, S.; Akoto, O. Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality, Ghana. Environ. Syst. Res. 2015, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Adeyeye, O.; Xiao, C.; Zhang, Z.; Liang, X. State, source and triggering mechanism of iron and manganese pollution in groundwater of Changchun, Northeastern China. Environ. Monit. Assess. 2020, 192, 619. [Google Scholar] [CrossRef] [PubMed]

| India (City) | China (the Middle and Lower Reaches of the River Basin) | ||||
|---|---|---|---|---|---|
| Name of location | Type of water | Fe (mg/L) | Region | Basin/Plain | Fe (mg/L) |
| Khoponala | Shallow groundwater | 16.861 | Northeastern China | Sanjiang Plain | 0.30–38.5 |
| Khusiabill | Shallow groundwater | 8.659 | Songnen Plain | 0.05–4.98 | |
| Burma camp | Shallow groundwater | 6.461 | Lower Liaohe Plain | 0.18–0.35 | |
| Eralibill | Shallow groundwater | 26.859 | Northern China | North China plain | 0.10–65.0 |
| Sovima | Shallow groundwater | 19.355 | Taiyuan Basin | 0.01–5.05 | |
| Naharbhari | Shallow groundwater | 13.305 | Hetao Basin | 0.01–3.03 | |
| Dancan | Shallow groundwater | 0.966 | Yinchuan Basin | 0.03–6.80 | |
| East police station | Deep groundwater | 0.491 | Southern China | Huai River Plain | 0.08–27.8 |
| Sovima | Deep groundwater | 19.355 | Jianghan Plain | 0.01-39.8 | |
| Chumukedima Gate | Deep groundwater | 24.136 | Dongting Lake Plain | 0.15–1.02 | |
| Purana bazar | Deep groundwater | 5.121 | Pearl River Delta | 0.01−94.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Teng, Y.; Zhai, Y. Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management. Sustainability 2022, 14, 7059. https://doi.org/10.3390/su14127059
Xia X, Teng Y, Zhai Y. Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management. Sustainability. 2022; 14(12):7059. https://doi.org/10.3390/su14127059
Chicago/Turabian StyleXia, Xuelian, Yanguo Teng, and Yuanzheng Zhai. 2022. "Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management" Sustainability 14, no. 12: 7059. https://doi.org/10.3390/su14127059
APA StyleXia, X., Teng, Y., & Zhai, Y. (2022). Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management. Sustainability, 14(12), 7059. https://doi.org/10.3390/su14127059








