Topography and Soil Properties Determine Biomass and Productivity Indirectly via Community Structural and Species Diversity in Karst Forest, Southwest China
Abstract
1. Introduction
2. Materials and Methods
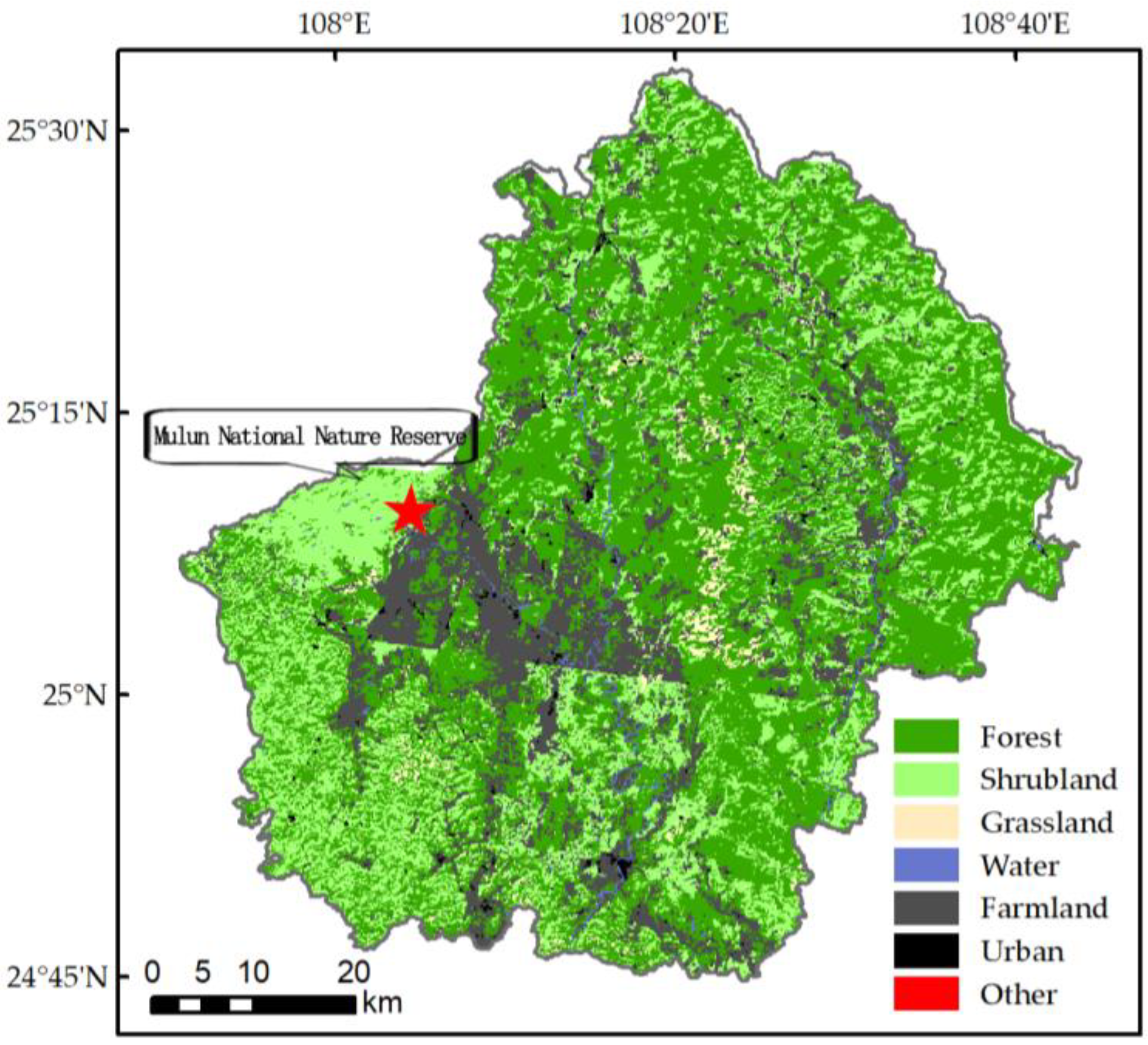
2.1. Study Description
2.2. Data Collection
2.2.1. Field Survey
2.2.2. Topographic Variables and Soil Variables
2.2.3. Species Diversity and Community Structure Diversity
2.2.4. Biomass and Productivity
2.3. Statistical Analysis
3. Results
3.1. Changes in Community Composition, Structure and Biomass
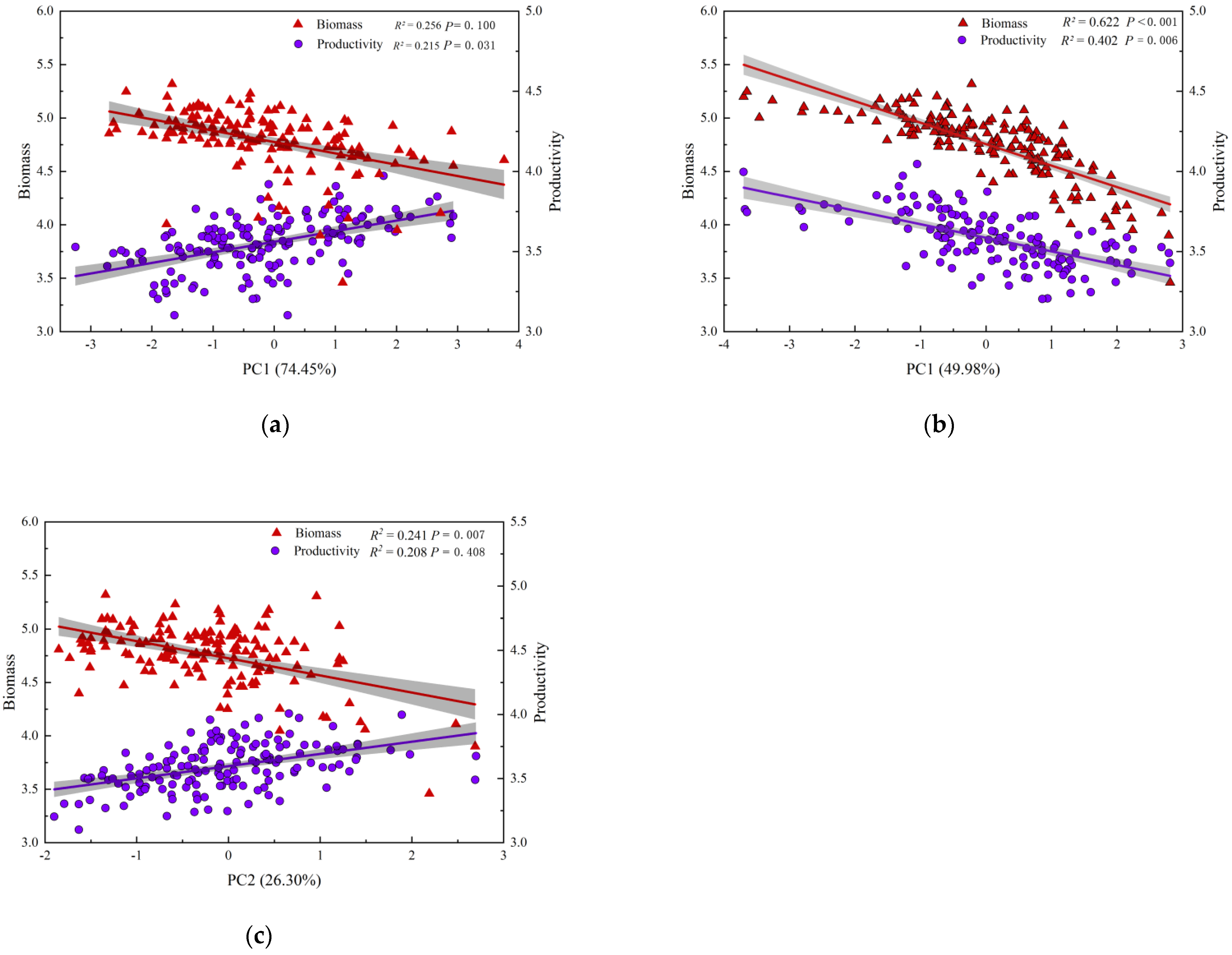
3.2. The Relationship between Biodiversity and Biomass, Productivity
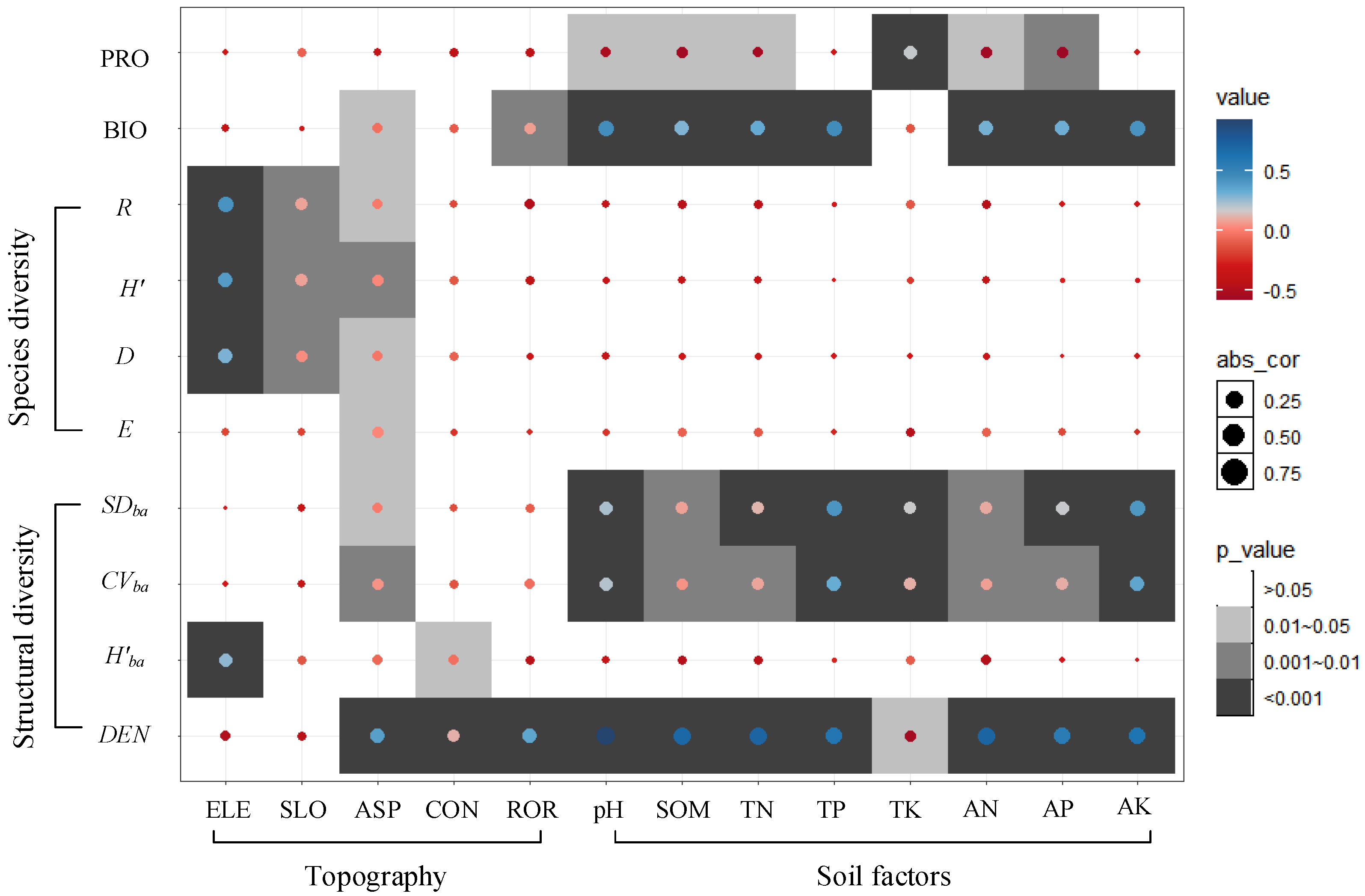
3.3. Correlation of Topography and Soil Factors with Biodiversity, Biomass, and Productivity
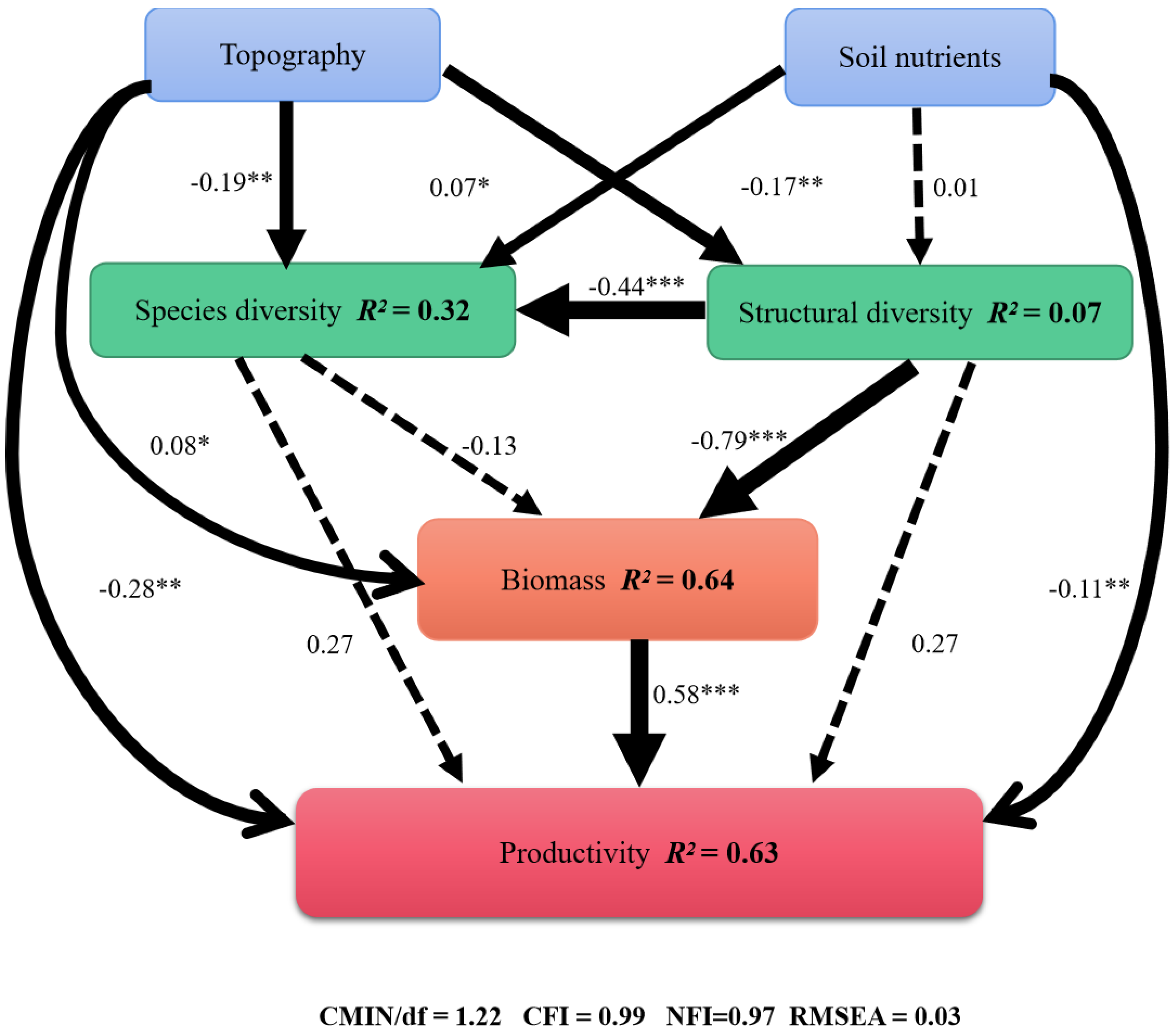
3.4. Relative Importance of Stand Conditions in Determining Productivity
4. Discussion
4.1. Dynamic Changes of Forest Biomass and Productivity
4.2. The Influence of Species Diversity and Structural Diversity on Community Dynamic
4.3. The Relative Influences of Biotic and Abiotic Factors on Community Dynamics
5. Conclusions
6. Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houghton, R.A.; Hall, F.; Goetz, S.J. Importance of biomass in the global carbon cycle. J. Geophys. Res.-Biogeosci. 2009, 114, G00E03. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Benito, P.; Ratcliffe, S.; Jump, A.S.; Gomez-Aparicio, L.; Madrigal-Gonzalez, J.; Wirth, C.; Kaendler, G.; Lehtonen, A.; Dahlgren, J.; Kattge, J.; et al. Functional diversity underlies demographic responses to environmental variation in European forests. Glob. Ecol. Biogeogr. 2017, 26, 128–141. [Google Scholar] [CrossRef]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Bluethgen, N.; Boehm, S.; Grassein, F.; Hoelzel, N.; Klaus, V.H.; et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Thom, D.; Seidl, R. Trade-offs between temporal stability and level of forest ecosystem services provisioning under climate change. Ecol. Appl. 2018, 28, 1884–1896. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A Review of Processes behind Diversity-Productivity Relationships in Forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Ecology-Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.; Wang, X.; Zeng, Y.; Lei, P.; Deng, X.; Peng, C. Significant effects of biodiversity on forest biomass during the succession of subtropical forest in south China. For. Ecol. Manag. 2016, 372, 291–302. [Google Scholar] [CrossRef]
- Wu, C.; Han, W.; Jiang, B.; Liu, B.; Yuan, W.; Shen, A.; Huang, Y.; Zhu, J. Relationships between species richness and biomass/productivity depend on environmental factors in secondary forests of Dinghai, Zhejiang Province. Biodivers. Sci. 2018, 26, 545–553. [Google Scholar] [CrossRef]
- Liang, J.; Buongiorno, J.; Monserud, R.A.; Kruger, E.L.; Zhou, M. Effects of diversity of tree species and size on forest basal area growth, recruitment, and mortality. For. Ecol. Manag. 2007, 243, 116–127. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.; Michael, S.; Eric, W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Qin, Y.; Jiang, M.; Qiao, X. The Effects of Biotic and Abiotic Factors on the Community Dynamics in a Mountain Subtropical Forest. Forests 2021, 12, 427. [Google Scholar] [CrossRef]
- Long, J.N.; Shaw, J.D. The influence of compositional and structural diversity on forest productivity. Forestry 2010, 83, 121–128. [Google Scholar] [CrossRef]
- Danescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Clark, J.S. Individuals and the Variation Needed for High Species Diversity in Forest Trees. Science 2010, 327, 1129–1132. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R. The forest strata-dependent relationship between biodiversity and aboveground biomass within a subtropical forest. For. Ecol. Manag. 2017, 401, 125–134. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef]
- Vila, M.; Carrillo-Gavilan, A.; Vayreda, J.; Bugmann, H.; Fridman, J.; Grodzki, W.; Haase, J.; Kunstler, G.; Schelhaas, M.; Trasobares, A. Disentangling Biodiversity and Climatic Determinants of Wood Production. PLoS ONE 2013, 8, e53530. [Google Scholar] [CrossRef]
- Diao, J.; Liu, J.; Zhu, Z.; Wei, X.; Li, M. Active forest management accelerates carbon storage in plantation forests in Lishui, southern China. For. Ecosyst. 2022, 9, 100004. [Google Scholar] [CrossRef]
- Coomes, D.A.; Holdaway, R.J.; Kobe, R.K.; Lines, E.R.; Allen, R.B. A general integrative framework for modelling woody biomass production and carbon sequestration rates in forests. J. Ecol. 2012, 100, 42–64. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Wang, S.; Gazol, A.; Freckleton, R.; Wang, X.; Lin, F.; Ye, J.; Zhou, L.; Hao, Z.; et al. Abiotic and biotic determinants of coarse woody productivity in temperate mixed forests. Sci. Total Environ. 2018, 630, 422–431. [Google Scholar] [CrossRef]
- Pretzsch, H.; Bielak, K.; Block, J.; Bruchwald, A.; Dieler, J.; Ehrhart, H.-P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zasada, M.; et al. Productivity of mixed versus pure stands of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) and European beech (Fagus sylvatica L.) along an ecological gradient. Eur. J. For. Res. 2013, 132, 263–280. [Google Scholar] [CrossRef]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Arantes, C.S.; van der Sande, M.T.; Lohbeck, M.; Poorter, L. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 2016, 104, 817–827. [Google Scholar] [CrossRef]
- Murphy, S.J.; Audino, L.D.; Whitacre, J.; Eck, J.L.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Species associations structured by environment and land-use history promote beta-diversity in a temperate forest. Ecology 2015, 96, 705–715. [Google Scholar] [CrossRef]
- Valencia, R.; Condit, R.; Muller-Landau, H.C.; Hernandez, C.; Navarrete, H. Dissecting biomass dynamics in a large Amazonian forest plot. J. Trop. Ecol. 2009, 25, 473–482. [Google Scholar] [CrossRef]
- Du, H.; Liu, L.; Su, L.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Song, T. Seasonal Changes and Vertical Distribution of Fine Root Biomass during Vegetation Restoration in a Karst Area, Southwest China. Front. Plant Sci. 2019, 9, 2001. [Google Scholar] [CrossRef]
- Du, H.; Hu, F.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Zeng, Z.; Zhang, F.; Song, T. Spatial distribution of tree species in evergreen-deciduous broadleaf karst forests in southwest China. Sci. Rep. 2017, 7, 15564. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.-Y.; Hu, G.; Zhang, Z.-H.; Cheng, A.-Y.; Wang, S.-J.; Ni, J. Biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China: A comprehensive inventory of a 2 ha plot. Silva Fenn. 2016, 50, 1492. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Theng, B.K.G.; Zhang, L.; Fang, B.; Li, F. Biomass accumulation and carbon sequestration in an age-sequence of Zanthoxylum bungeanum plantations under the Grain for Green Program in karst regions, Guizhou province. Agric. For. Meteorol. 2015, 203, 88–95. [Google Scholar] [CrossRef]
- Du, H.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Song, M.; Zhang, H. Spatial pattern of woody plants and their environmental interpretation in the karst forest of southwest China. Plant Biosyst. 2015, 149, 121–130. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Zhang, F. Pattern of forest vegetation and its environmental interpretation in Zhuweigou, Lishan Mountain Nature Reserve. Acta Ecol. Sin. 2003, 23, 421–427. [Google Scholar]
- Wang, Z.; Du, H.; Song, T.; Peng, W.; Zeng, F.; Zeng, Z.; Zhang, H. Allometric models of major tree species and forest biomass in Guangxi. Acta Ecol. Sin. 2015, 35, 4462–4472. [Google Scholar]
- Lasky, J.R.; Uriarte, M.; Boukili, V.K.; Erickson, D.L.; Kress, W.J.; Chazdon, R.L. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 2014, 17, 1158–1167. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.-L.; He, J.-K.; Kong, F.-M.; Yu, J.-H.; Jiang, H.-S. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Shen, W.; He, Q.; Peng, Y.; Huang, X.; Pang, S.; Li, Y. Species composition and diversity of different shrub communities in West Guangxi. Guihaia 2016, 36, 1165–1171. [Google Scholar]
- Cadotte, M.W. Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 2017, 20, 989–996. [Google Scholar] [CrossRef]
- Yu, W.; Dong, D.; Ni, J. Comparison of biomass and productivity between karst and non-karst forests in mountainous areas of southwest China. J. Subtrop. Resour. Environ. 2010, 5, 25–30. [Google Scholar]
- Lin, D.; Lai, J.; Mi, X.; Ren, H.; Ma, K. Spatial variation in community structure of a subtropical evergreen broad-leaved forest: Implications for sampling design. Chin. Sci. Bull. 2013, 58, 1181–1186. [Google Scholar] [CrossRef][Green Version]
- Lohbeck, M.; Poorter, L.; Martinez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef]
- Xu, Y.; Franklin, S.B.; Wang, Q.; Shi, Z.; Luo, Y.; Lu, Z.; Zhang, J.; Qiao, X.; Jiang, M. Topographic and biotic factors determine forest biomass spatial distribution in a subtropical mountain moist forest. For. Ecol. Manag. 2015, 357, 95–103. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.Y.H.; Yang, J. Linking understory species diversity, community-level traits and productivity in a Chinese boreal forest. J. Veg. Sci. 2019, 30, 247–256. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, A.; Zou, S.; Xiong, X.; Liu, S.; Chu, G.; Zhang, Q.; Liu, J.; Tang, X.; Yan, J.; et al. Relationships between tree diversity and biomass/productivity and their influence factors in a lower subtropical evergreen broad-leaved forest. Biodivers. Sci. 2021, 29, 1435–1446. [Google Scholar] [CrossRef]
- Fahey, R.T.; Fotis, A.T.; Woods, K.D. Quantifying canopy complexity and effects on productivity and resilience in late-successional hemlock-hardwood forests. Ecol. Appl. 2015, 25, 834–847. [Google Scholar] [CrossRef]
- Tan, L.; Fan, C.; Fan, X. Relationships between species diversity or community structure and productivity of woody-plants in a broad-leaved Korean pine forest in Jiaohe, Jilin, China. Chin. J. Plant Ecol. 2017, 41, 1149–1156. [Google Scholar]
- Fortunel, C.; Lasky, J.R.; Uriarte, M.; Valencia, R.; Joseph Wright, S.; Garwood, N.C.; Kraft, N.J.B. Topography and neighborhood crowding can interact to shape species growth and distribution in a diverse Amazonian forest. Ecology 2018, 99, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ali, A.; Jucker, T.; Ruiz-Benito, P.; Wang, S.; Jiang, L.; Wang, X.; Lin, F.; Ye, J.; Hao, Z.; et al. Multiple abiotic and biotic pathways shape biomass demographic processes in temperate forests. Ecology 2019, 100, e02650. [Google Scholar] [CrossRef] [PubMed]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Xiang, W.; Wang, X.; Xiao, W.; Chen, L.; Li, S.; Sun, H.; Deng, X.; Forrester, D.I.; Zeng, L.; et al. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Haerdtle, W.; von Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
| Variable | Range | Mean | Standard Deviation |
|---|---|---|---|
| Elevation (ELE) (m) | 438.11–522.07 | 72.11 | 20.77 |
| Slope (SLO) (°) | 2.34–60.15 | 31.58 | 13.47 |
| Cos (Aspect) (ASP) | −0.99–0.99 | −0.19 | 0.65 |
| Convexity (CON) | −5.99–5.27 | −0.26 | 2.01 |
| Rock outcrop ratio (ROR) (%) | 8–98 | 76.19 | 20.29 |
| Soil total nitrogen (TN) (g·kg−1) | 2.38–19.57 | 8.36 | 5.11 |
| Soil total phosphorus (TP) (g·kg−1) | 0.31–3.37 | 1.31 | 0.71 |
| Soil total potassium (TK) (g·kg−1) | 1.46–11.09 | 5.63 | 2.42 |
| Soil available nitrogen (AN) (mg·kg−1) | 266.51–2075.69 | 855.63 | 504.77 |
| Soil available phosphorus (AP) (mg·kg−1) | 0.20–25.28 | 8.88 | 6.69 |
| Soil available potassium (AK) (mg·kg−1) | 33.79–336.18 | 139.34 | 73.88 |
| Soil organic matter (SOM) (g·kg−1) | 46.08–372.17 | 156.28 | 95.27 |
| Soil pH value (pH) | 5.64–7.64 | 6.77 | 0.58 |
| Index | Formula | Range | Standard Deviation | |
|---|---|---|---|---|
| Species diversity | Species richness (R) | R = Ns | 1–30 | 4.54 |
| Shannon-Wiener diversity index (H’) | 0.64–3.16 | 0.45 | ||
| Simpson diversity index (D) | 0.44–0.95 | 0.09 | ||
| Pielou evenness index (E) | 0.60–0.98 | 0.08 | ||
| Structural diversity | Basal area standard deviation (SDba) | — | 0.56–117.65 | 20.19 |
| Basal area coefficient of variation (CVba) | 18.09–1526.15 | 220.35 | ||
| DBH Shannon-Wiener diversity index (H′ba) | 0.69–4.01 | 0.51 | ||
| Stand density (DEN) | — | 0.08–2.33 | 0.58 |
| Years | 2007 | 2017 | 2007–2017 |
|---|---|---|---|
| Number of genera | 100 | 95 | — |
| Number of species | 130 | 123 | — |
| Total number of individuals | 8073 | 5393 | — |
| Total number of recruitments | — | 1978↑ | — |
| Total number of recruitments | — | 4658↓ | — |
| The average of AGB (Mg ha−1) | 53.19 | 70.74 | — |
| Productivity (Mg ha−1 yr−1) | — | — | 1.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Du, H.; Yang, Z.; Song, T.; Zeng, F.; Peng, W.; Huang, G. Topography and Soil Properties Determine Biomass and Productivity Indirectly via Community Structural and Species Diversity in Karst Forest, Southwest China. Sustainability 2022, 14, 7644. https://doi.org/10.3390/su14137644
Zhang L, Du H, Yang Z, Song T, Zeng F, Peng W, Huang G. Topography and Soil Properties Determine Biomass and Productivity Indirectly via Community Structural and Species Diversity in Karst Forest, Southwest China. Sustainability. 2022; 14(13):7644. https://doi.org/10.3390/su14137644
Chicago/Turabian StyleZhang, Lijin, Hu Du, Zhiqi Yang, Tongqing Song, Fuping Zeng, Wanxia Peng, and Guoqin Huang. 2022. "Topography and Soil Properties Determine Biomass and Productivity Indirectly via Community Structural and Species Diversity in Karst Forest, Southwest China" Sustainability 14, no. 13: 7644. https://doi.org/10.3390/su14137644
APA StyleZhang, L., Du, H., Yang, Z., Song, T., Zeng, F., Peng, W., & Huang, G. (2022). Topography and Soil Properties Determine Biomass and Productivity Indirectly via Community Structural and Species Diversity in Karst Forest, Southwest China. Sustainability, 14(13), 7644. https://doi.org/10.3390/su14137644







