Leaf Carbon, Nitrogen and Phosphorus Stoichiometry in a Pinus yunnanensis Forest in Southwest China
Abstract
1. Introduction
2. Material and Methods
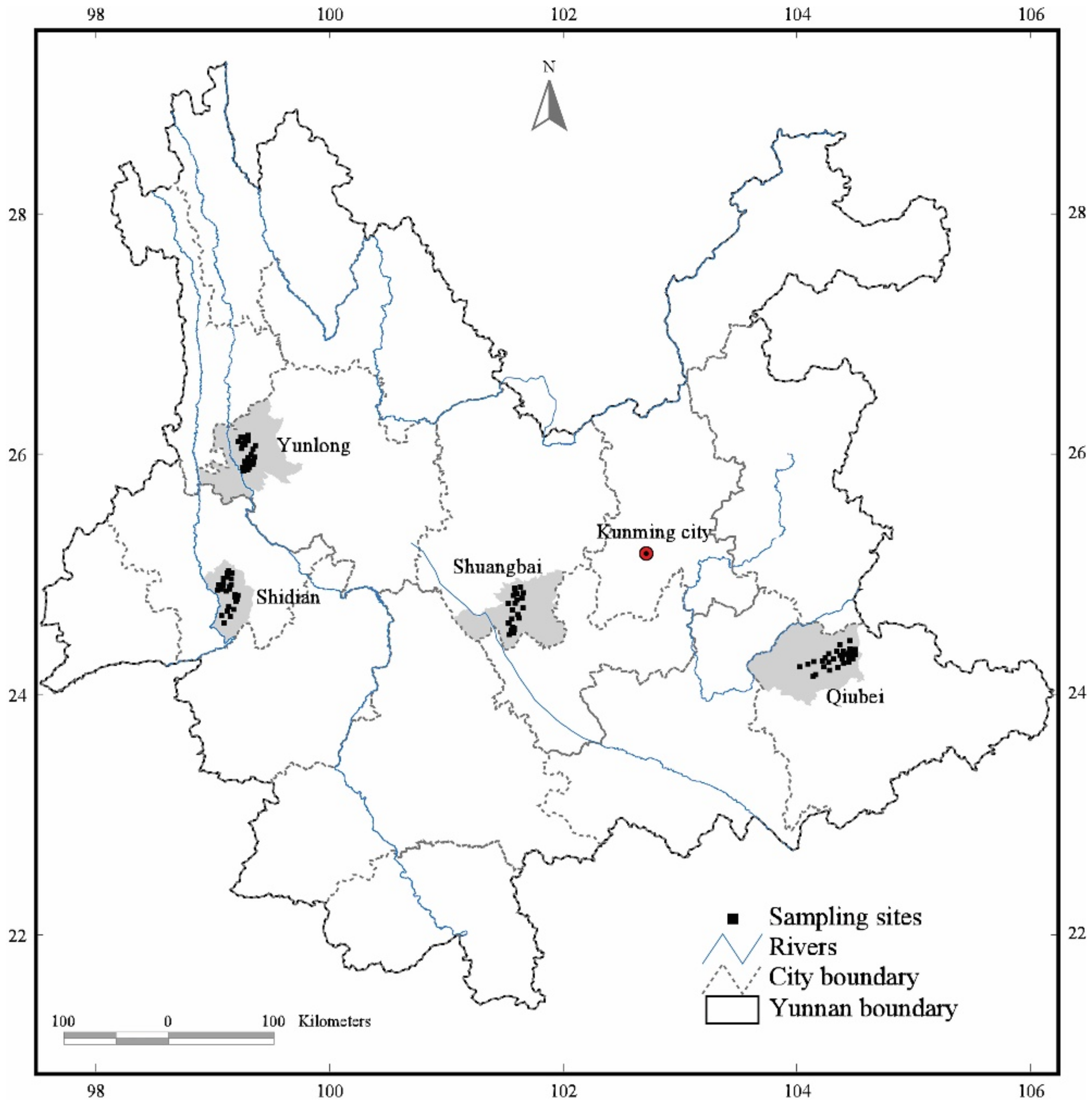
2.1. Study Area
2.2. Sampling and Measurements
2.3. Data Analysis
3. Results
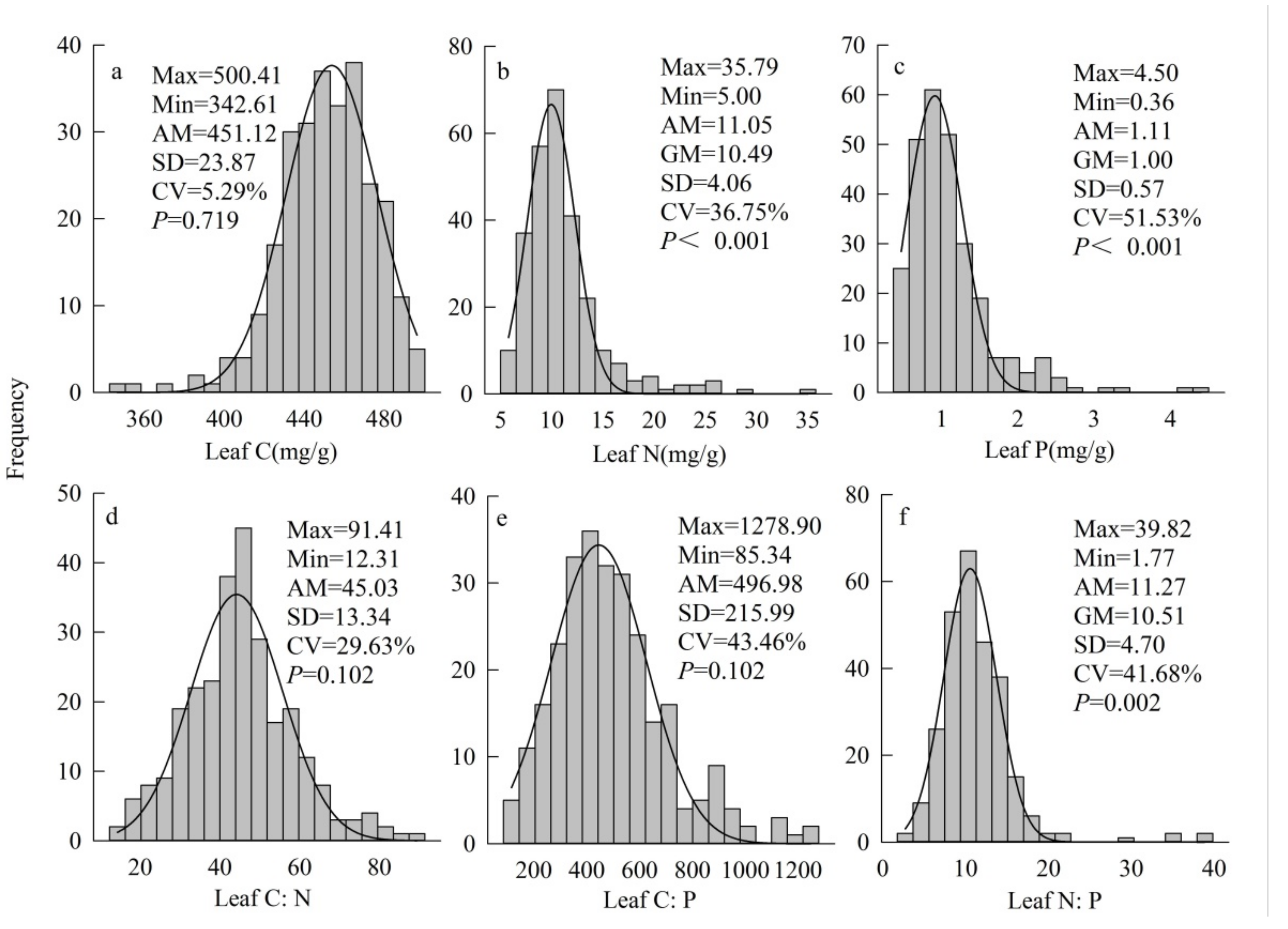
3.1. Patterns of Leaf C, N and P and Stoichiometric Ratios across All Species
3.2. Relationships among Leaf C, Leaf N and Leaf P for All Species
3.3. Comparison of Leaf C, N and P and Stoichiometric Ratios across Functional Groups
4. Discussion
4.1. Overall Leaf C, N and P in Natural P. yunnanensis Forests
4.2. Leaf C:N, C:P and N:P and Nutrient Limitation
4.3. Variations in Leaf C, N and P and Stoichiometry Ratios across Plant Types
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; He, M.; Li, X.; Tan, H.; Gao, Y.; Li, G.; Han, G.; Wu, Y. Foliar carbon, nitrogen and phosphorus stoichiometry of typical desert plants across the Alashan Desert. Acta Ecol. Sin. 2014, 34, 6538–6547. [Google Scholar] [CrossRef][Green Version]
- Ågren, G.I. The C: N: P stoichiometry of autotrophs–theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Knecht, M.F.; Göransson, A. Terrestrial plants require nutrients in similar proportions. Tree physiol. 2004, 24, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate–stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T. Biomass N: P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Makino, W.; Cotner, J.; Sterner, R.; Elser, J. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct. Ecol. 2003, 17, 121–130. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. Am. J. Bot. 2005, 92, 1256–1263. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen. J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Blanes, M.C.; Viñegla, B.; Merino, J.; Carreira, J.A. Nutritional status of Abies pinsapo forests along a nitrogen deposition gradient: Do C/N/P stoichiometric shifts modify photosynthetic nutrient use efficiency? Oecologia 2013, 171, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Xu, L.; Zhu, J.; Dai, G.; He, N. C: N: P stoichiometry in terrestrial ecosystems in China. Sci. Total Environ. 2021, 795, 148849. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yu, G.; Tao, B.; Wang, S. Leaf nitrogen and phosphorus stoichiometry across 654 terrestrial plant species in NSTEC. Environ. Sci. 2007, 28, 2665–2673. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Li, R.; Yan, J.; Sha, L.; Han, S. C: N: P stoichiometric characteristics of four forest types’ dominant tree species in China. Chin. J. Plant Ecol. 2011, 35, 587–595. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Zhang, Z.; Huang, X. Leaf carbon, nitrogen and phosphorus stoichiometry at different growth stages in dominant tree species of a monsoon broad-leaved evergreen forest in Pu’er, Yunnan Province, China. Chin. J. Plant Ecol. 2015, 39, 52–62. [Google Scholar] [CrossRef]
- Yang, K.; Huang, J.; Dong, D.; Ma, W.; He, J. Canopy leaf N and P stoichiometry in grassland communities of Qinghai-Tibetan Plateau, China. Chin. J. Plant Ecol. 2010, 34, 17–22. [Google Scholar] [CrossRef]
- Ding, X.; Luo, S.; Liu, J.; Li, K.; Liu, G. Longitude gradient changes on plant community and soil stoichiometry characteristics of grassland in Hulunbeir. Acta Ecol. Sin. 2012, 32, 3467–3476. [Google Scholar] [CrossRef]
- Xiong, X.; Cai, H.; Li, Y.; Ma, W.; Niu, K.; Chen, D.; Liu, N.; Su, X.; Jing, H.; Feng, X.; et al. Seasonal dynamics of leaf C, N and P stoichiometry in plants of typical steppe in Nei Mongol, China. Chin. J. Plant Ecol. 2020, 44, 1138–1153. [Google Scholar] [CrossRef]
- Li, Z.; Han, L.; Liu, Y.; An, S.; Leng, X. C, N and P stoichiometric characteristics in leaves of Suaeda salsa during different growth phase in coastal wetlands of China. Chin. J. Plant Ecol. 2012, 36, 1054–1061. [Google Scholar] [CrossRef]
- Liu, B.; Liao, B.; Fang, Z. Different tide status and salinity alter stoichiometry characteristics of mangrove Kandelia candel seedlings. Acta Ecol. Sin. 2012, 32, 7818–7827. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Wang, C.; Zeng, C.; Tong, C. Soil carbon, nitrogen and phosphorus ecological stoichiometry of Phragmites australis wetlands in different reaches in Minjiang River estuary. Acta Ecol. Sin. 2012, 32, 4087–4093. [Google Scholar] [CrossRef]
- An, Z.; Niu, D.; Wen, H.; Yang, Y.; Zhang, H.; Fu, H. Effects of N addition on nutrient resorption efficiency and C: N: P stoichiometric characteristics in Stipa bungeana of steppe grasslands in the Loess Plateau, China. Chin. J. Plant Ecol. 2011, 35, 801–807. [Google Scholar] [CrossRef]
- Yan, Z.; Kim, N.Y.; Han, T.; Fang, J.; Han, W. Effects of nitrogen and phosphorus fertilization on leaf carbon, nitrogen and phosphorus stoichiometry of Arabidopsis thaliana. Chin. J. Plant Ecol. 2013, 37, 551–557. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Song, Z.; Yang, X.; Li, Z.; Hao, Q.; Liu, L. Ecological stoichiometry of nitrogen, phosphorous, and sulfur in China’s forests. Acta Geochim. 2017, 36, 525–530. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N. C: N: P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2018, 32, 50–60. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Su, J. Selective logging enhances ecosystem multifunctionality via increase of functional diversity in a Pinus yunnanensis forest in Southwest China. For. Ecosyst. 2020, 7, 55. [Google Scholar] [CrossRef]
- Li, G.; Shi, H.; Meng, G.; Fang, X.; Chai, Y.; He, L.; Zhang, Z.; Yang, Y. Community structural properties and species diversity in primary Pinus yunnanensis forest. J. Zhejiang AF Univ. 2007, 24, 396–400. [Google Scholar] [CrossRef]
- John, M.K. Colorimetric determination in soil and plant material with ascorbic acid. Soil Sci. 1970, 109, 214–220. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, B.; Xiao, Y.; Pan, Y.; Chen, Y.; Xiao, J. Leaf stoichiometry of trees in three forest types in Pearl River Delta, South China. Chin. J. Plant Ecol. 2010, 34, 58–63. [Google Scholar] [CrossRef]
- Yu, Y.F.; Peng, W.; Song, T.; Zeng, F.; Wang, K.; Weng, L.; Fan, F. Stoichiometric characteristics of plant and soil C, N and P in different forest types in depressions between karst hills, southwest China. Chin. J. Appl. Ecol. 2014, 25, 947–954. [Google Scholar]
- Yan, K.; Fu, D.; He, F.; Duan, C. Leaf nutrient stoichiometry of plants in the phosphorus-enriched soils of the Lake Dianchi watershed, southwestern China. Chin. J. Plant Ecol. 2011, 35, 353–361. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X. Leaf Nutrient and Structural Characteristics of 32 Evergreen Broad-leaved Species. J. Eastchina Norm. Univ. 2003, 1, 92–97. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef]
- Han, W.; Wu, Y.; Tang, L.; Chen, Y.; Li, L.; He, J.; Fang, J. Leaf Carbon, Nitrogen and Phosphorus Stoichiometry across Plant Species in Beijing and Its Periphery. Acta Sci. Nat. Univ. Pekin. 2009, 45, 855–860. [Google Scholar]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Sterck, F.J.; Poorter, L.; Schieving, F. Leaf traits determine the growth-survival trade-off across rain forest tree species. Am. Nat. 2006, 167, 758–765. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar] [CrossRef]

| Number | Species | Family | Plant Type |
|---|---|---|---|
| 1 | Albizia kalkora | Leguminosae | Tree/Deciduous/Broadleaf |
| 2 | Alnus nepalensis | Betulaceae | Tree/Deciduous/Broadleaf |
| 3 | Anneslea fragrans | Theaceae | Tree/Evergreen/Broadleaf |
| 4 | Castanopsis delavayi | Fagaceae | Tree/Evergreen/Broadleaf |
| 5 | Cerasus dielsiana | Rosaceae | Tree/Evergreen/Broadleaf |
| 6 | Coriaria nepalensis | Coriariaceae | Shrub/Deciduous/Broadleaf |
| 7 | Craibiodendron stellatum | Ericaceae | Tree/Evergreen/Broadleaf |
| 8 | Cyclobalanopsis delavayi | Fagaceae | Tree/Evergreen/Broadleaf |
| 9 | Cyclobalanopsis glaucoides | Fagaceae | Tree/Evergreen/Broadleaf |
| 10 | Cyclobalanopsis kerrii | Fagaceae | Tree/Evergreen/Broadleaf |
| 11 | Dichotomanthus tristaniaecarpa | Rosaceae | Shrub/Evergreen/Broadleaf |
| 12 | Dodonaea viscosa | Sapindaceae | Shrub/Evergreen/Broadleaf |
| 13 | Elaeagnus conferta | Elaeagnaceae | Shrub/Evergreen/Broadleaf |
| 14 | Engelhardtia spicata | Juglandaceae | Tree/Evergreen/Broadleaf |
| 15 | Eurya groffii | Theaceae | Tree/Evergreen/Broadleaf |
| 16 | Ficus semicordata | Moraceae | Tree/Evergreen/Broadleaf |
| 17 | Glochidion hirsutum | Euphorbiaceae | Shrub/Evergreen/Broadleaf |
| 18 | Keteleeria evelyniana | Pinaceae | Tree/Evergreen/Conifer |
| 19 | Lithocarpus variolosus | Fagaceae | Tree/Evergreen/Broadleaf |
| 20 | Lyonia doyonensis | Ericaceae | Shrub/Evergreen/Broadleaf |
| 21 | Lyonia ovalifolia | Ericaceae | Shrub/Evergreen/Broadleaf |
| 22 | Myrica esculenta | Myricaceae | Tree/Evergreen/Broadleaf |
| 23 | Myrica rubra | Myricaceae | Tree/Evergreen/Broadleaf |
| 24 | Osyris wightiana | Santalaceae | Shrub/Evergreen/Broadleaf |
| 25 | Photinia glomerata | Rosaceae | Shrub/Evergreen/Broadleaf |
| 26 | Phyllanthus emblica | Euphorbiaceae | Tree/Evergreen/Broadleaf |
| 27 | Pinus armandii | Pinaceae | Tree/Evergreen/Conifer |
| 28 | Pinus yunnanensis | Pinaceae | Tree/Evergreen/Conifer |
| 29 | Pyrus pashia | Rosaceae | Tree/Evergreen/Broadleaf |
| 30 | Quercus aliena | Fagaceae | Tree/Deciduous/Broadleaf |
| 31 | Quercus semicarpifolia | Fagaceae | Tree/Evergreen/Broadleaf |
| 32 | Quercus variabilis | Fagaceae | Tree/Deciduous/Broadleaf |
| 33 | Rapanea neriifolia | Myrsinaceae | Shrub/Evergreen/Broadleaf |
| 34 | Rhododendron decorum | Ericaceae | Tree/Evergreen/Broadleaf |
| 35 | Rhododendron delavayi | Ericaceae | Shrub/Evergreen/Broadleaf |
| 36 | Rhododendron lapponicum | Ericaceae | Shrub/Evergreen/Broadleaf |
| 37 | Rhododendron moulmainense | Ericaceae | Shrub/Evergreen/Broadleaf |
| 38 | Rhododendron spinuliferum | Bignoniaceae | Shrub/Evergreen/Broadleaf |
| 39 | Schima argentea | Theaceae | Tree/Evergreen/Broadleaf |
| 40 | Schima noronhae | Theaceae | Tree/Evergreen/Broadleaf |
| 41 | Symplocos chinensis | Symplocaceae | Shrub/Deciduous/Broadleaf |
| 42 | Ternstroemia gymnanthera | Theaceae | Shrub/Evergreen/Broadleaf |
| 43 | Toona ciliata | Meliaceae | Tree/Deciduous/Broadleaf |
| 44 | Ulmus tonkinensis | Ulmaceae | Tree/Evergreen/Broadleaf |
| 45 | Vaccinium vitis-idaea | Ericaceae | Shrub/Evergreen/Broadleaf |
| 46 | Viburnum cylindricum | Caprifoliaceae | Shrub/Evergreen/Broadleaf |
| 47 | Wendlandia uvariifolia | Rubiaceae | Tree/Evergreen/Broadleaf |
| 48 | Zanthoxylum bungeanum | Rutaceae | Tree/Deciduous/Broadleaf |
| Growth Form | C mg/g | N mg/g | P mg/g | C:N | C:P | N:P |
|---|---|---|---|---|---|---|
| Tree | 449.93 ± 23.75 a | 11.38 ± 4.24 a | 1.12 ± 0.56 a | 43.62 ± 12.71 b | 483.99 ± 204.58 a | 11.35 ± 4.88 a |
| Shrub | 454.25 ± 24.04 a | 10.17 ± 3.41 b | 1.06 ± 0.59 a | 48.73 ± 14.31 a | 530.91 ± 241.50 a | 11.08 ± 4.21 a |
| Evergreen | 453.36 ± 22.64 a | 10.38 ± 2.99 b | 1.04 ± 0.50 b | 46.86 ± 12.43 a | 518.92 ± 212.29 a | 11.37 ± 4.88 a |
| Deciduous | 431.69 ± 25.76 b | 16.86 ± 6.75 a | 1.71 ± 0.77 a | 29.20 ± 10.28 b | 306.52 ± 143.66 b | 10.40 ± 2.49 a |
| Conifer | 468.32 ± 12.15 a | 9.29 ± 1.87 b | 0.97 ± 0.23 b | 52.51 ± 11.13 a | 509.86 ± 119.73 a | 9.80 ± 1.65 b |
| Broadleaf | 446.74 ± 24.15 b | 11.50 ± 4.34 a | 1.14 ± 0.62 a | 43.13 ± 13.21 b | 493.70 ± 234.38 a | 11.65 ± 5.13 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lang, X.; Li, S.; Liu, W.; Su, J. Leaf Carbon, Nitrogen and Phosphorus Stoichiometry in a Pinus yunnanensis Forest in Southwest China. Sustainability 2022, 14, 6365. https://doi.org/10.3390/su14106365
Huang X, Lang X, Li S, Liu W, Su J. Leaf Carbon, Nitrogen and Phosphorus Stoichiometry in a Pinus yunnanensis Forest in Southwest China. Sustainability. 2022; 14(10):6365. https://doi.org/10.3390/su14106365
Chicago/Turabian StyleHuang, Xiaobo, Xuedong Lang, Shuaifeng Li, Wande Liu, and Jianrong Su. 2022. "Leaf Carbon, Nitrogen and Phosphorus Stoichiometry in a Pinus yunnanensis Forest in Southwest China" Sustainability 14, no. 10: 6365. https://doi.org/10.3390/su14106365
APA StyleHuang, X., Lang, X., Li, S., Liu, W., & Su, J. (2022). Leaf Carbon, Nitrogen and Phosphorus Stoichiometry in a Pinus yunnanensis Forest in Southwest China. Sustainability, 14(10), 6365. https://doi.org/10.3390/su14106365








