Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions
Abstract
:1. Introduction
1.1. The Need to Develop H2 Production
1.2. Hydrogen Production by Water Splitting in Thermal Redox Systems
1.3. Literature Findings on MnOx/Na2CO3 Cycle
1.4. Objectives of the Research
2. Materials and Methods
2.1. Precursor Materials and Characteristics
2.2. Synthesis of the Redox Reactants
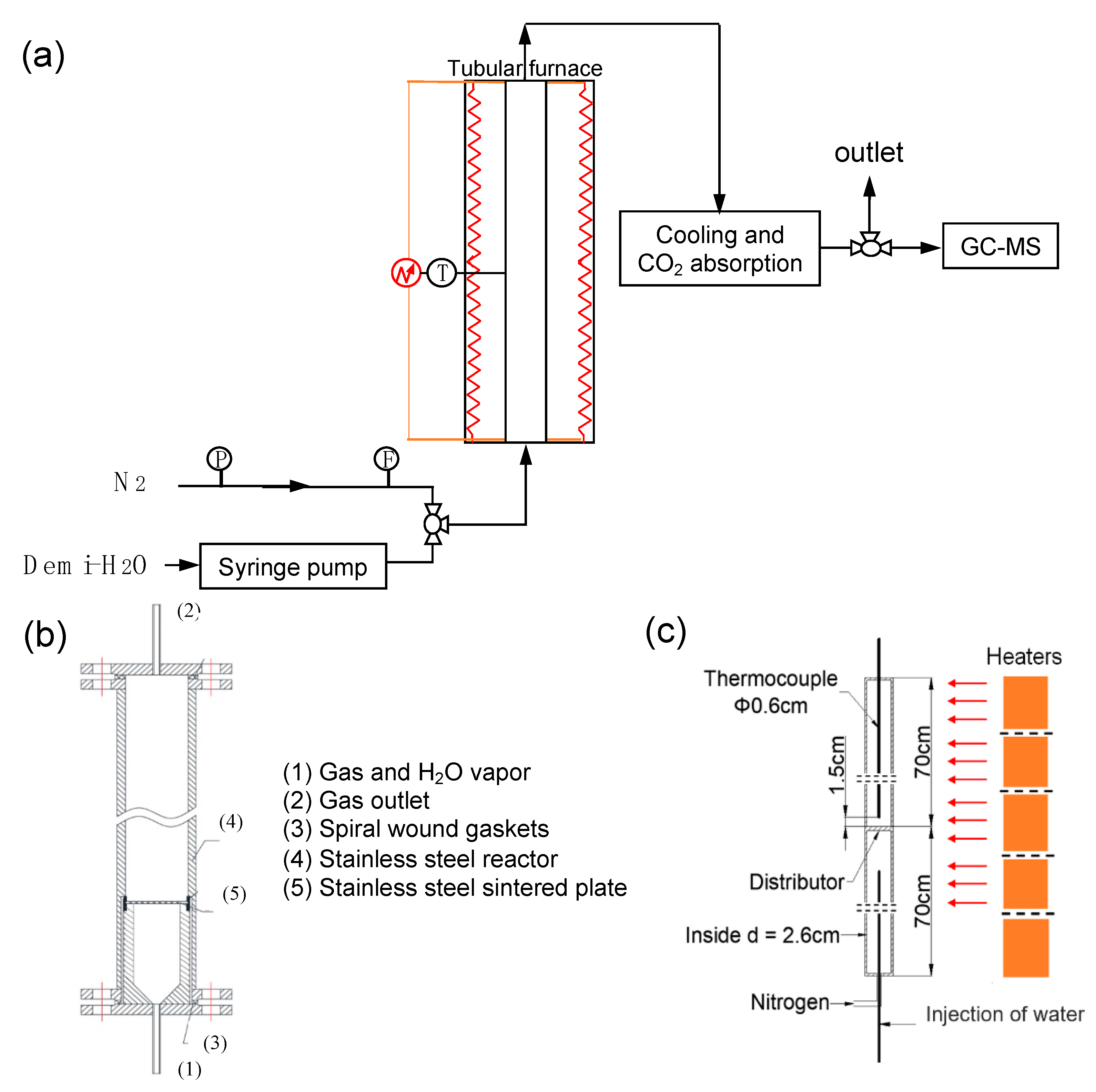
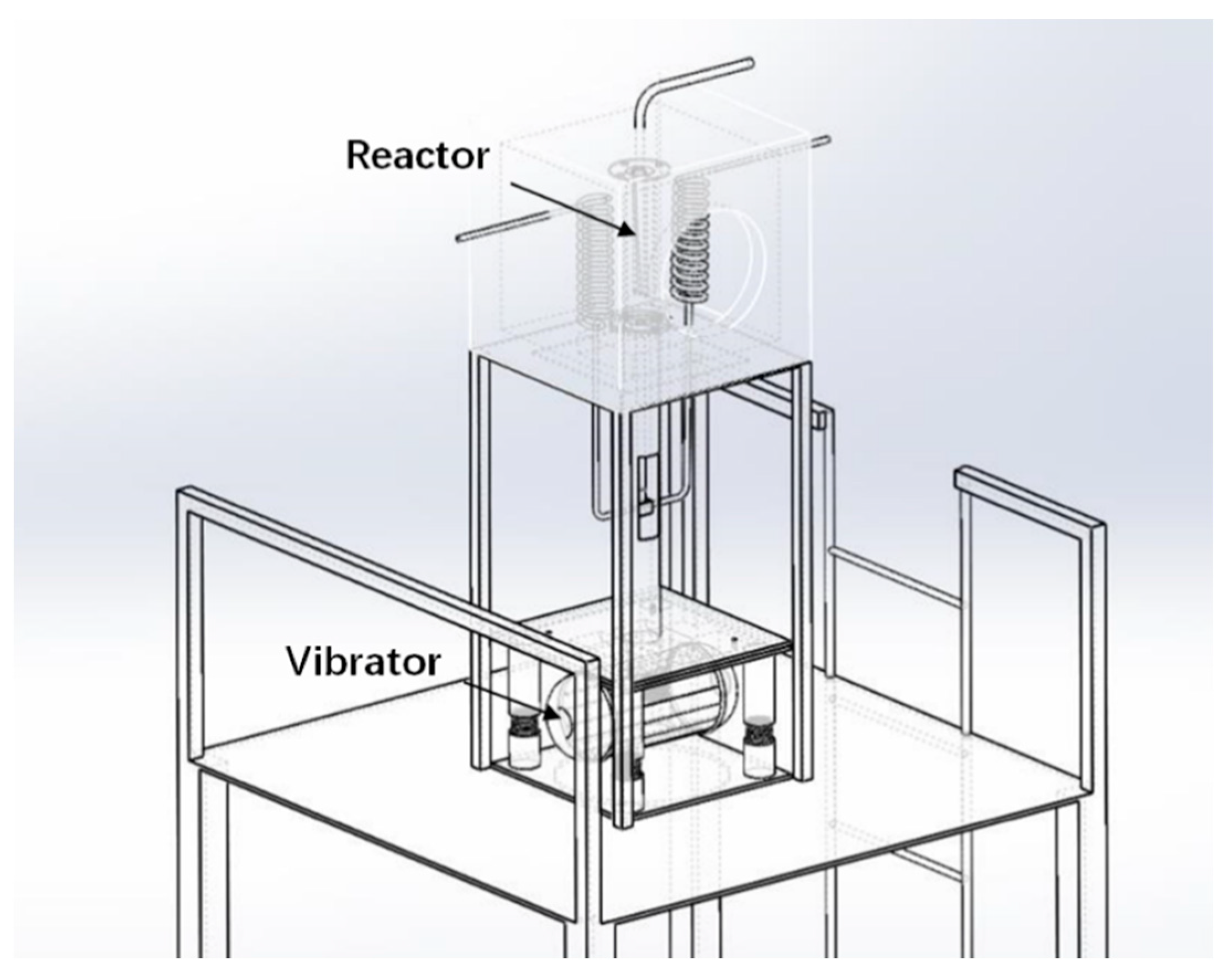
2.3. Experimental Set-Up
2.4. Mn3O4/Na2CO3 Cycle
- In a first step, the furnace was slowly heated to the required testing temperature (775 or 825 °C) under N2 flow. The outlet gas passed through saturated clarified lime water to capture evolved CO2. When a constant temperature was achieved in the testing section of the reactor, and when no CO2 exhaust was detected, water was injected by syringe pump and heated until reaching the steam temperature in the preheating section of the furnace and reactor. The high temperature steam reacted with the reactants, and the produced H2 was measured at the outlet by GC-MS. The column used was the TDX-01 packed column produced in Lanzhou Zhongke Antai Analysis Technology Co., Ltd. (Shenzhen, China). The outlet gas flow rate was measured by an electric flowmeter, and the real-time record was transmitted to the computer. A hydrogen alarm was placed at the outlet. The step was assumed to be nearly completed when the hydrogen concentration was lower than 0.1%.
- The reduction step consisted of 2 subsequent reactions: firstly, a cooling to 140 °C (for about 5 h), followed by the reduction step using pure CO2 at 825 °C (step 4 of the reaction scheme). It was observed that steps 1 and 2 of the reaction scheme were started simultaneously, although the quantity of produced H2 remained very low (<0.1%).
3. Results and Discussion
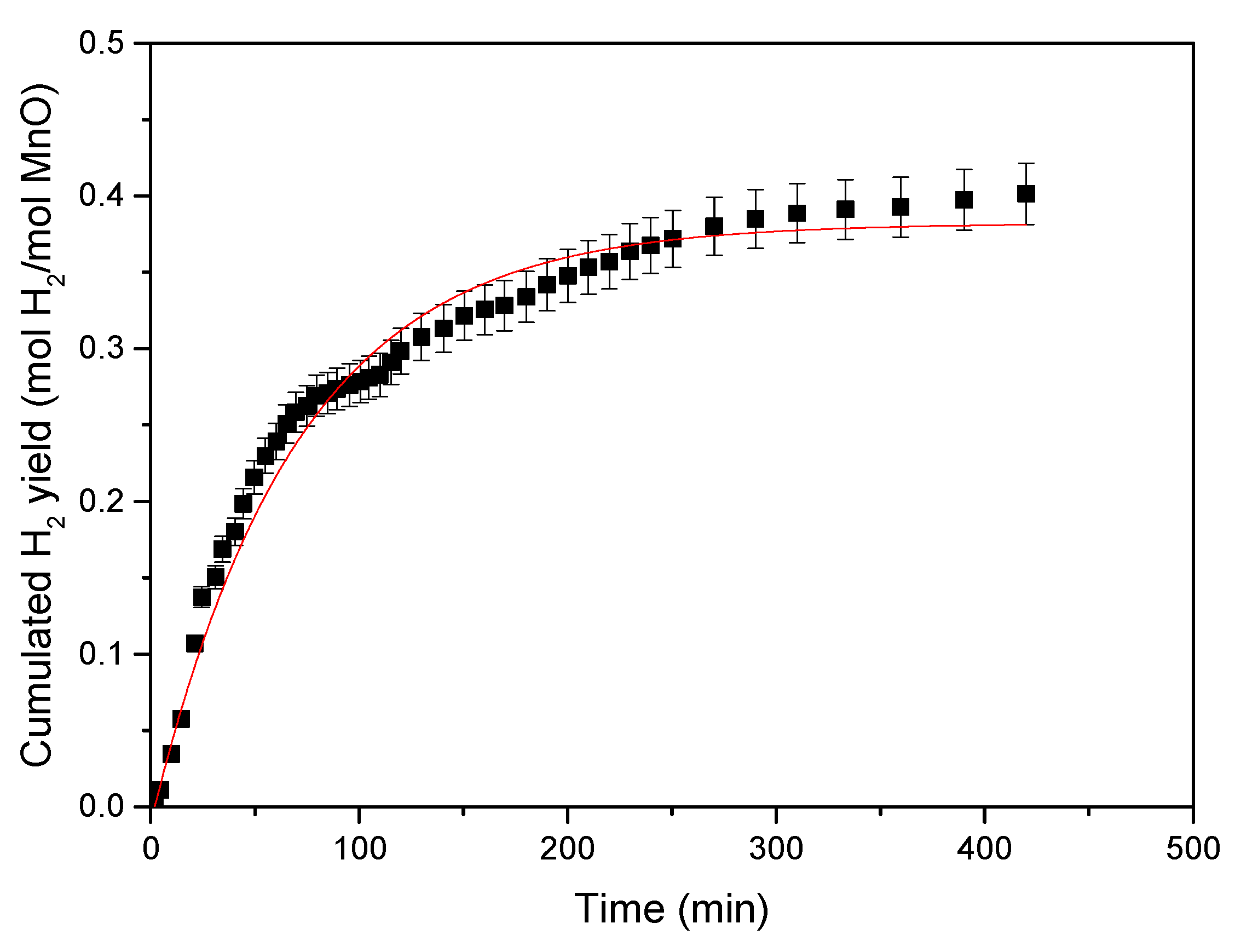
3.1. Hydrogen Yield in the Electric Furnace
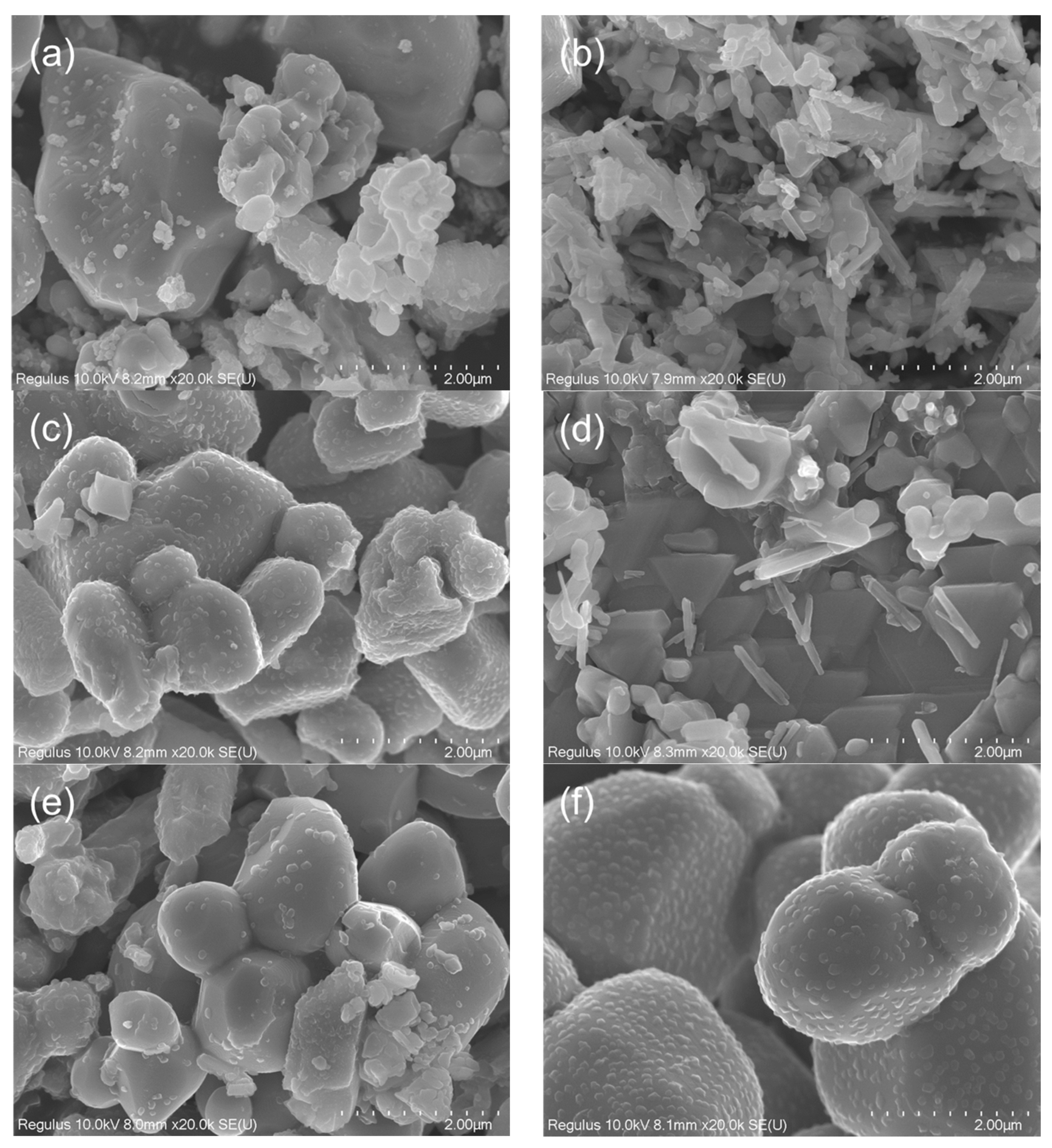
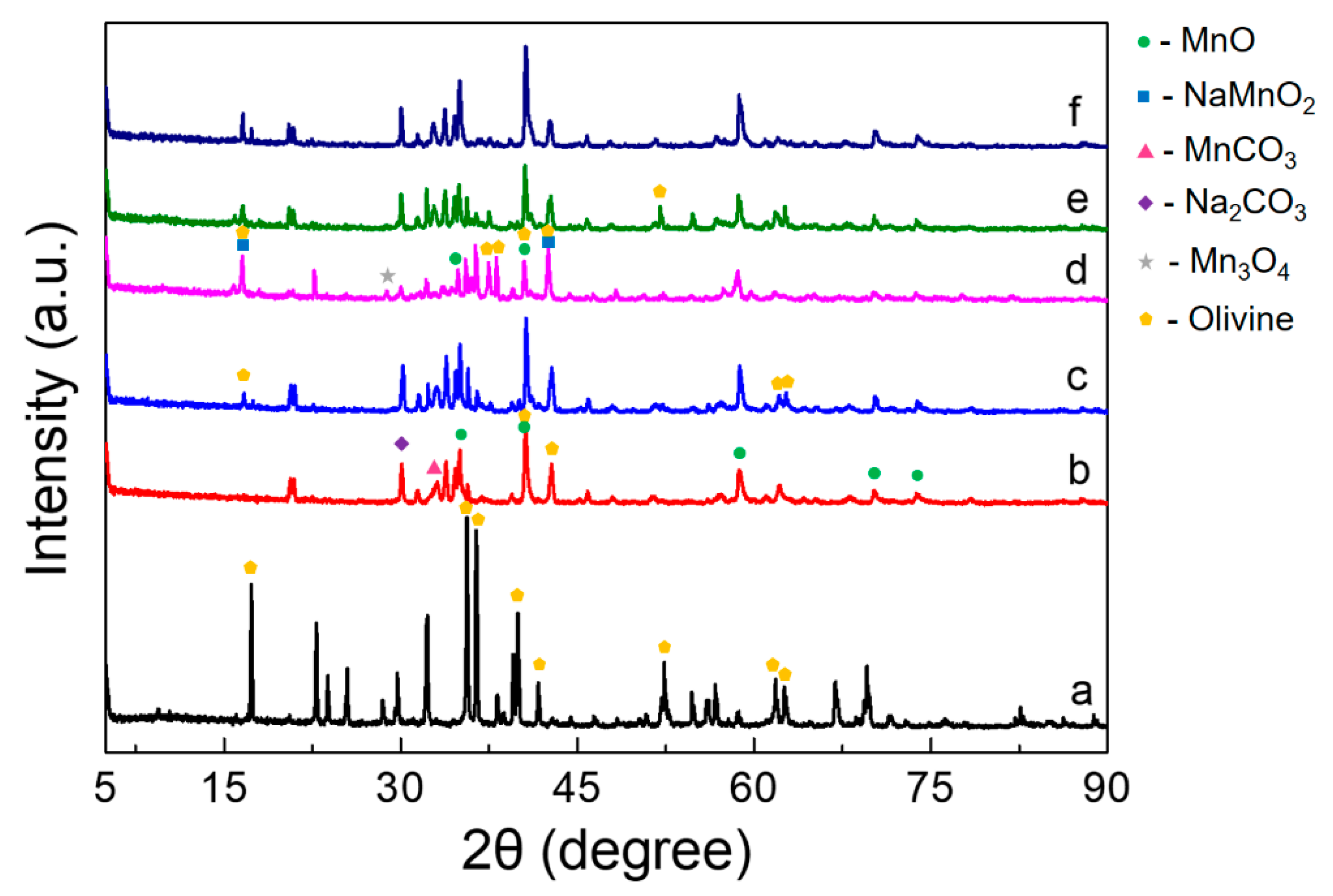
3.2. Morphology of the Mn3O4/MnO/Na2CO3 Reactants
3.3. Solar H2 Production Using the Mn3O4/MnO/Na2CO3 System
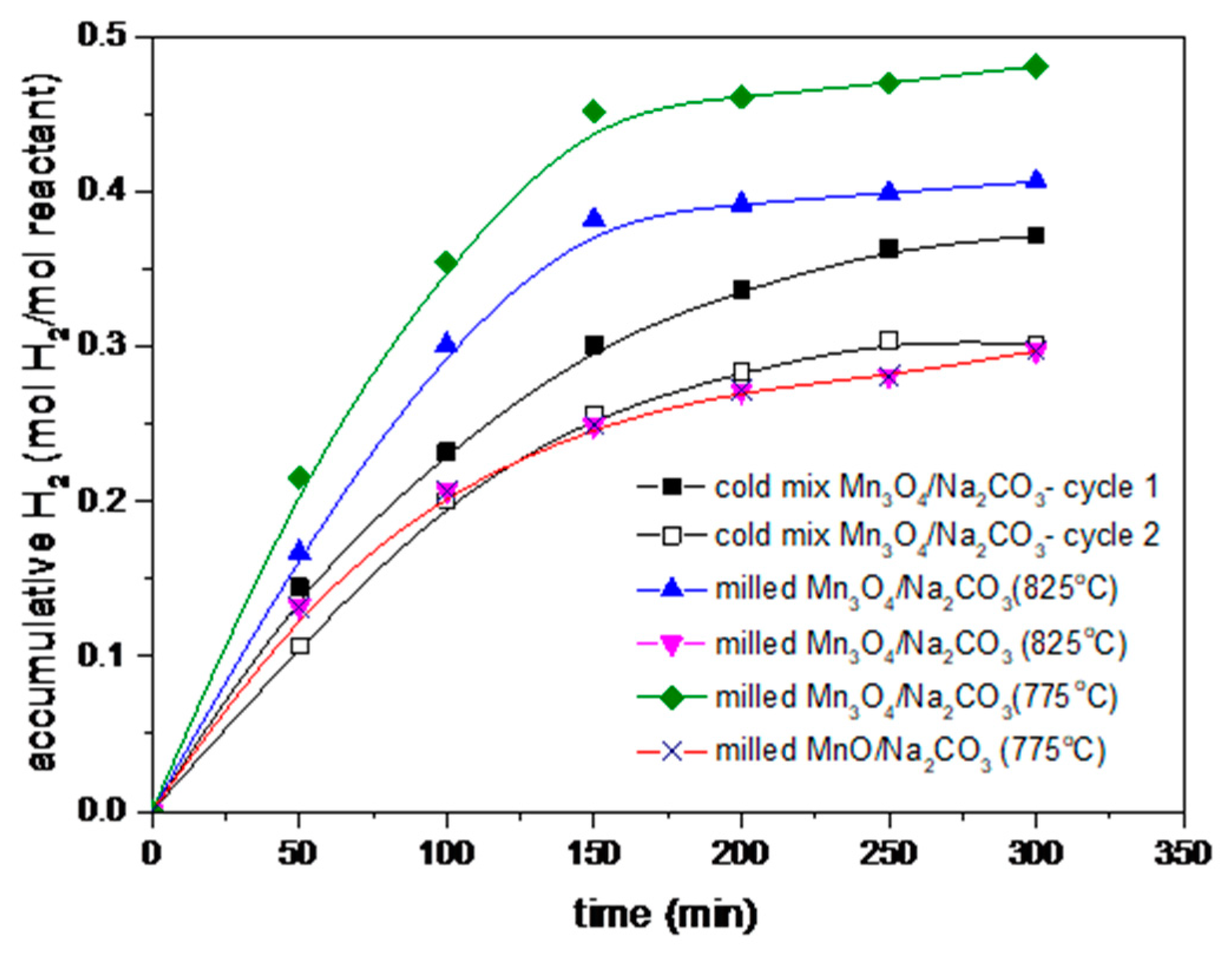
3.4. Cycling Performance
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Kang, Q.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Hydrogen Production: State of Technology. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2020 10th International Conference on Environment Science and Engineering (ICESE 2020) Vienna, Austria, 18–21 May 2020; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 544, p. 012011. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Van Tulden, F.; Li, S.; Yang, M.; Baeyens, J. Hydrogen-enriched natural gas in a decarbonization perspective. Fuel 2022, 318, 123680. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Gabriel, K.S. Synergizing hydrogen and cement industries for Canada’s climate plan—Case study. Energy Sources, Part A Recover. Util. Environ. Eff. 2021, 43, 3151–3165. [Google Scholar] [CrossRef]
- Ipsakis, D.; Varvoutis, G.; Lampropoulos, A.; Papaefthimiou, S.; Marnellos, G.E.; Konsolakis, M. Τechno-economic assessment of industrially-captured CO2 upgrade to synthetic natural gas by means of renewable hydrogen. Renew. Energy 2021, 179, 1884–1896. [Google Scholar] [CrossRef]
- Dudley, B. Statistical Review of World Energy; BP Statistical Review: London, UK, 2021. [Google Scholar]
- Antweiler, W. What Role Does Hydrogen Have in the Future of Electric Mobility? Available online: https://wernerantweiler.ca/blog.php?item=2020-09-28 (accessed on 25 May 2022).
- BP, p.l.c. Hydroelectricity. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/hydroelectricity.html (accessed on 25 May 2022).
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Dewil, R.; Deng, Y.; Li, S. Co-Al and Mn-Fe Catalytic Steam Reforming of CH3OH to H2. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 11th International Conference on Environment Science and Engineering (ICESE 2021) Vienna, Austria, 9–12 September 2021; IOP Publishing Ltd.: Bristol, UK, 2022; Volume 952, p. 012007. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Li, S.; Baeyens, J.; Degrève, J.; Wang, G. Thermo-chemical water splitting: Selection of priority reversible redox reactions by multi-attribute decision making. Renew. Energy 2021, 170, 800–810. [Google Scholar] [CrossRef]
- Xu, B.; Bhawe, Y.; Davis, M.E. Low-temperature, manganese oxide-based, thermochemical water splitting cycle. Proc. Natl. Acad. Sci. USA 2012, 109, 9260–9264. [Google Scholar] [CrossRef] [Green Version]
- Bayón, A.; de la Peña O’Shea, V.A.; Serrano, D.P.; Coronado, J.M. Exploring the alternative MnO-Na2CO3 thermochemical cycle for water splitting. J. CO2 Util. 2020, 42, 101264. [Google Scholar] [CrossRef]
- Kreider, P.B.; Funke, H.H.; Cuche, K.; Schmidt, M.; Steinfeld, A.; Weimer, A.W. Manganese oxide based thermochemical hydrogen production cycle. Int. J. Hydrogen Energy 2011, 36, 7028–7037. [Google Scholar] [CrossRef]
- Bayón, A.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Role of the physicochemical properties of hausmannite on the hydrogen production via the Mn3O4–NaOH thermochemical cycle. Int. J. Hydrogen Energy 2016, 41, 113–122. [Google Scholar] [CrossRef]
- Xu, B.; Bhawe, Y.; Davis, M.E. Spinel Metal Oxide-Alkali Carbonate-Based, Low-Temperature Thermochemical Cycles for Water Splitting and CO2 Reduction. Chem. Mater. 2013, 25, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Alonso, E.; Hutter, C.; Romero, M.; Steinfeld, A.; Gonzalez-Aguilar, J. Kinetics of Mn2O3 –Mn3O4 and Mn3O4–MnO Redox Reactions Performed under Concentrated Thermal Radiative Flux. Energy Fuels 2013, 27, 4884–4890. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Dey, S. Solar thermochemical splitting of water to generate hydrogen. Proc. Natl. Acad. Sci. USA 2017, 114, 13385–13393. [Google Scholar] [CrossRef] [Green Version]
- Mottana, A.; Crespi, R.; Liborio, G. Simon & Schuster’s Guide to Rocks and Minerals; Simon and Schuster: New York, NY, USA, 1991; ISBN 0671244175. [Google Scholar]
- Deng, Y.; Dewil, R.; Appels, L.; Zhang, H.; Li, S.; Baeyens, J. The Need to Accurately Define and Measure the Properties of Particles. Standards 2021, 1, 19–38. [Google Scholar] [CrossRef]
- Murmura, M.A.; Varsano, F.; Padella, F.; La Barbera, A.; Alvani, C.; Annesini, M.C. Hydrogen Production by the Sodium Manganese Ferrite Thermochemical Cycle—Experimental Rate and Modeling. Ind. Eng. Chem. Res. 2014, 53, 10310–10317. [Google Scholar] [CrossRef]
- Deng, Y.; Sabatier, F.; Dewil, R.; Flamant, G.; Le Gal, A.; Gueguen, R.; Baeyens, J.; Li, S.; Ansart, R. Dense upflow fluidized bed (DUFB) solar receivers of high aspect ratio: Different fluidization modes through inserting bubble rupture promoters. Chem. Eng. J. 2021, 418, 129376. [Google Scholar] [CrossRef]
- Huili, Z.; Jan, B.; Shuo, L.; Raf, D.; Yimin, D. Power Generation upon Demand by Using Thermal Energy Storage in Concentrated Solar Power Plants: Recent Developments. In Proceedings of the IEEE 2021 5th International Conference on Power and Energy Engineering (ICPEE), Xiamen, China, 2–4 December 2021; pp. 96–102. [Google Scholar]
- Baeyens, J.; Zhang, H.; Kong, W.; Dumont, P.; Flamant, G. Solar thermal treatment of non-metallic minerals: The potential application of the SOLPART technology. AIP Conf. Proc. 2019, 2126, 180002. [Google Scholar]
- Capossio, J.P.; Fabani, M.P.; Reyes-Urrutia, A.; Torres-Sciancalepore, R.; Deng, Y.; Baeyens, J.; Rodriguez, R.; Mazza, G. Sustainable Solar Drying of Brewer’s Spent Grains: A Comparison with Conventional Electric Convective Drying. Processes 2022, 10, 339. [Google Scholar] [CrossRef]
- Kang, Q.; Flamant, G.; Dewil, R.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Particles in a circulation loop for solar energy capture and storage. Particuology 2019, 43, 149–156. [Google Scholar] [CrossRef]
- Liu, J.; Baeyens, J.; Deng, Y.; Wang, X.; Zhang, H. High temperature Mn2O3/Mn3O4 and Co3O4/CoO systems for thermo-chemical energy storage. J. Environ. Manag. 2020, 267. [Google Scholar] [CrossRef] [PubMed]
- Geldart, D.; Baeyens, J. The design of distributors for gas-fluidized beds. Powder Technol. 1985, 42, 67–78. [Google Scholar] [CrossRef]
- Li, S.; Baeyens, J.; Dewil, R.; Appels, L.; Zhang, H.; Deng, Y. Advances in rigid porous high temperature filters. Renew. Sustain. Energy Rev. 2021, 139, 110713. [Google Scholar] [CrossRef]
- Deng, Y.; Seville, J.P.K.; Bell, S.D.; Ingram, A.; Zhang, H.; Sweygers, N.; Dewil, R.; Baeyens, J.; Appels, L. Reviewing Fundamental CO2 Adsorption Characteristics of Zeolite and Activated Carbon by In-situ Measurements With Radioactively Labelled CO2. Sep. Purif. Rev. 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Baeyens, J.; Deng, Y.; Tan, T.; Zhang, H. The chemical CO2 capture by carbonation-decarbonation cycles. J. Environ. Manag. 2020, 260, 110054. [Google Scholar] [CrossRef] [PubMed]
- Seville, J.P.K.; Deng, Y.; Dawn Bell, S.; Dewil, R.; Appels, L.; Ansart, R.; Leadbeater, T.; Parker, D.; Zhang, H.; Ingram, A.; et al. 11CO2 positron emission imaging reveals the in-situ gas concentration profile as function of time and position in opaque gas-solid contacting systems. Chem. Eng. J. 2021, 404, 126507. [Google Scholar] [CrossRef]



| Reactant | Chemical Formula | Supplier | Purity (%) |
|---|---|---|---|
| Sodium carbonate | Na2CO3 | Lushi Co., Ltd. (Guangzhou, China) | ≥99.9 |
| Manganese (II) carbonate | MnCO3 | Sigma-Aldrich Chemie GmbH (St. Louis, MO, USA) | ≥99.9 |
| Manganese (II–III) oxide | Mn3O4 | Sigma-Aldrich Chemie GmbH | ≥99 |
| Manganese (II) oxide | MnO | Sigma-Aldrich Chemie GmbH | ≥99 |
| Names | Models | Manufacturer |
|---|---|---|
| Syringe water pump | LSP02-1B | LongerPump (Hebei, China) |
| Electronic balance | ME403/02 | Mettler Toledo (Shanghai, China) |
| Multifunctional crusher | 800Y | Yongkang Boou Hardware Products Co. (Zhejiang, China) |
| Tubular furnace reactor | Customized | ZSHIELD Inc. (Cupertino, CA, USA) |
| Particle size distribution | Mastersizer 2000 | Malvern panalytical (Malvern, UK) |
| GC-MS | HAS-301-1474 | DECRA (Berlin, Germany) |
| XRD | RINT2000 | RIGAKU (Tokyo, Japan) |
| SEM | JSM-7800F | JEOL (Tokyo, Japan) |
| BET | ASAP 2020 | Micromeritics (Norcross, GA, USA) |
| Chemicals | dv (μm) | dsv (μm) | ψ |
|---|---|---|---|
| Mn3O4 | 15.12 | 12.70 | 0.84 |
| Na2CO3 | 394.45 | 331.34 | 0.84 |
| MnO | 10.94 | 9.19 | 0.84 |
| α-Al2O3 | 60.4 | 56.95 | 0.85 |
| Olivine (100–150 mesh) | 167.85 | 142.67 | 0.84 |
| Bed Height | Fluidizing Carrier Gas | Reaction T | Superficial Gas Velocity (cm/s) | Vibration Frequency | Vibration Amplitude | Vibration Direction |
|---|---|---|---|---|---|---|
| 0.25 m | N2 | 775–825 °C | <2 | <50 Hz | 0.6 mm | Vertical |
| Reactants | T (°C) | N2 Flow Rate (L/min, 20 °C) | |
|---|---|---|---|
| 1 | Mn3O4 + Na2CO3 | 825 | 0.5 |
| 2 | Milled Mn3O4 + Na2CO3 | 825 | 0.5 |
| 3 | Mn3O4 + Na2CO3 | 775 | 0.5 |
| 4 | Milled Mn3O4 + Na2CO3 | 775 | 0.5 |
| 5 | Milled MnO + Na2CO3 | 775 | 0.5 |
| Reactants | T (°C) | N2 Flow Rate (L/min, 20 °C) | H2 yield | ||||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 5 | Cycle 10 | ||||
| 1 | Mn3O4 + Na2CO3 | 825 | 0.5 | 72.3% | 67.9% | 68.2% | 54.0% |
| 2 | Milled Mn3O4 + Na2CO3 | 825 | 0.5 | 78.3% | - | 71.6% | 60.4% |
| 3 | Mn3O4 + Na2CO3 | 775 | 0.5 | Very low | Not further studied | ||
| 4 | Milled Mn3O4 + Na2CO3 | 775 | 0.5 | 56.4% | Not further studied | ||
| 5 | Milled MnO + Na2CO3 | 775 | 0.5 | 80.2% | - | 77.4% | 61.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, S.; Dewil, R.; Vanierschot, M.; Baeyens, J.; Deng, Y. Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions. Sustainability 2022, 14, 7597. https://doi.org/10.3390/su14137597
Liu J, Li S, Dewil R, Vanierschot M, Baeyens J, Deng Y. Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions. Sustainability. 2022; 14(13):7597. https://doi.org/10.3390/su14137597
Chicago/Turabian StyleLiu, Jia, Shuo Li, Raf Dewil, Maarten Vanierschot, Jan Baeyens, and Yimin Deng. 2022. "Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions" Sustainability 14, no. 13: 7597. https://doi.org/10.3390/su14137597
APA StyleLiu, J., Li, S., Dewil, R., Vanierschot, M., Baeyens, J., & Deng, Y. (2022). Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions. Sustainability, 14(13), 7597. https://doi.org/10.3390/su14137597








