Spatial and Temporal Nutrient Dynamics and Water Management of Huanglongbing-Affected Mature Citrus Trees on Florida Sandy Soils
Abstract
:1. Introduction
2. Materials and Methods
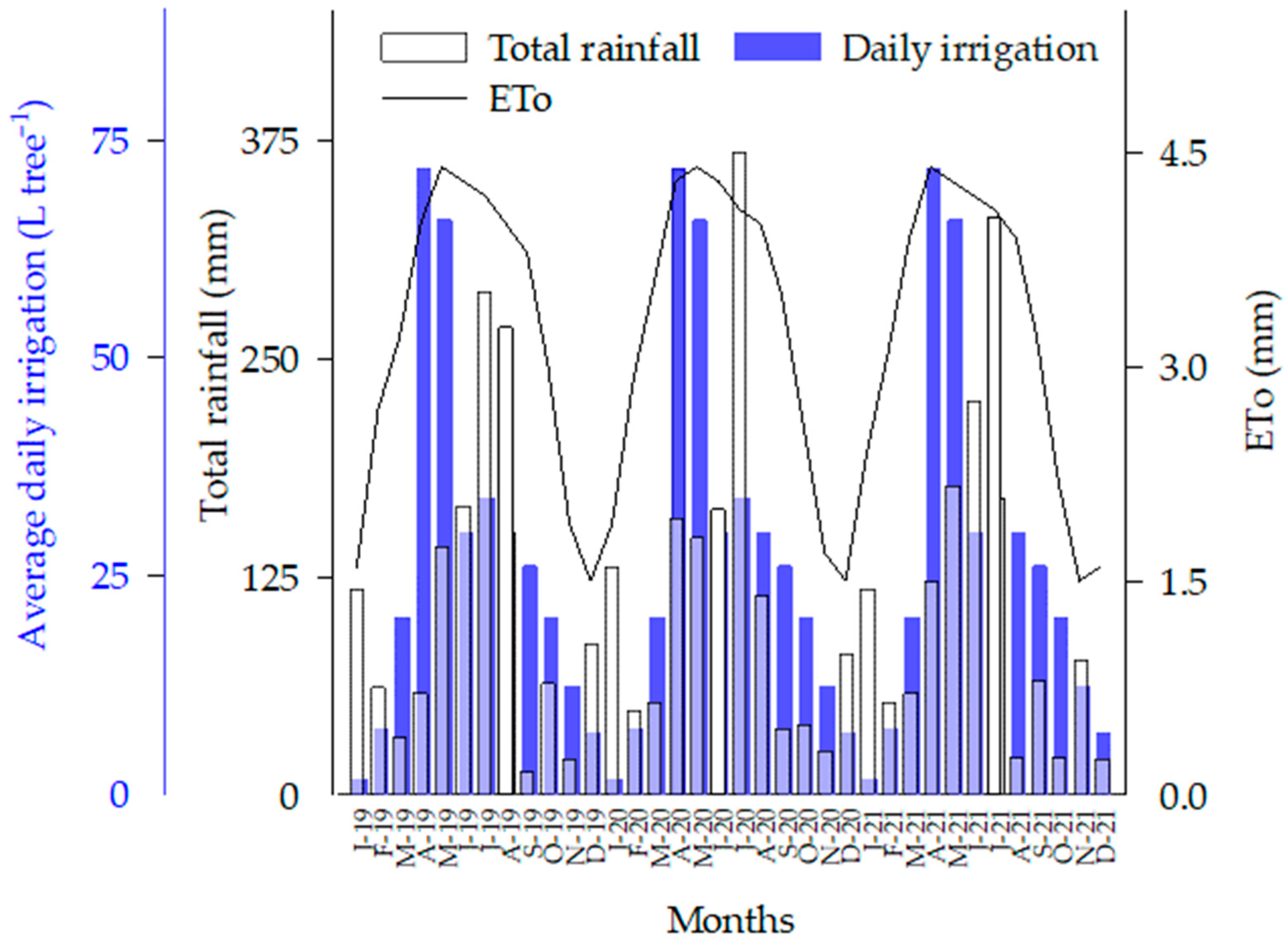
2.1. Study Site Conditions
2.2. Experimental Design and Treatments
2.3. Data Collection and Sample Analyses
2.3.1. Soil Sampling and Analysis
2.3.2. Minirhizotron Root
2.3.3. Water Relations
2.4. Statistical Analysis
3. Results and Discussion
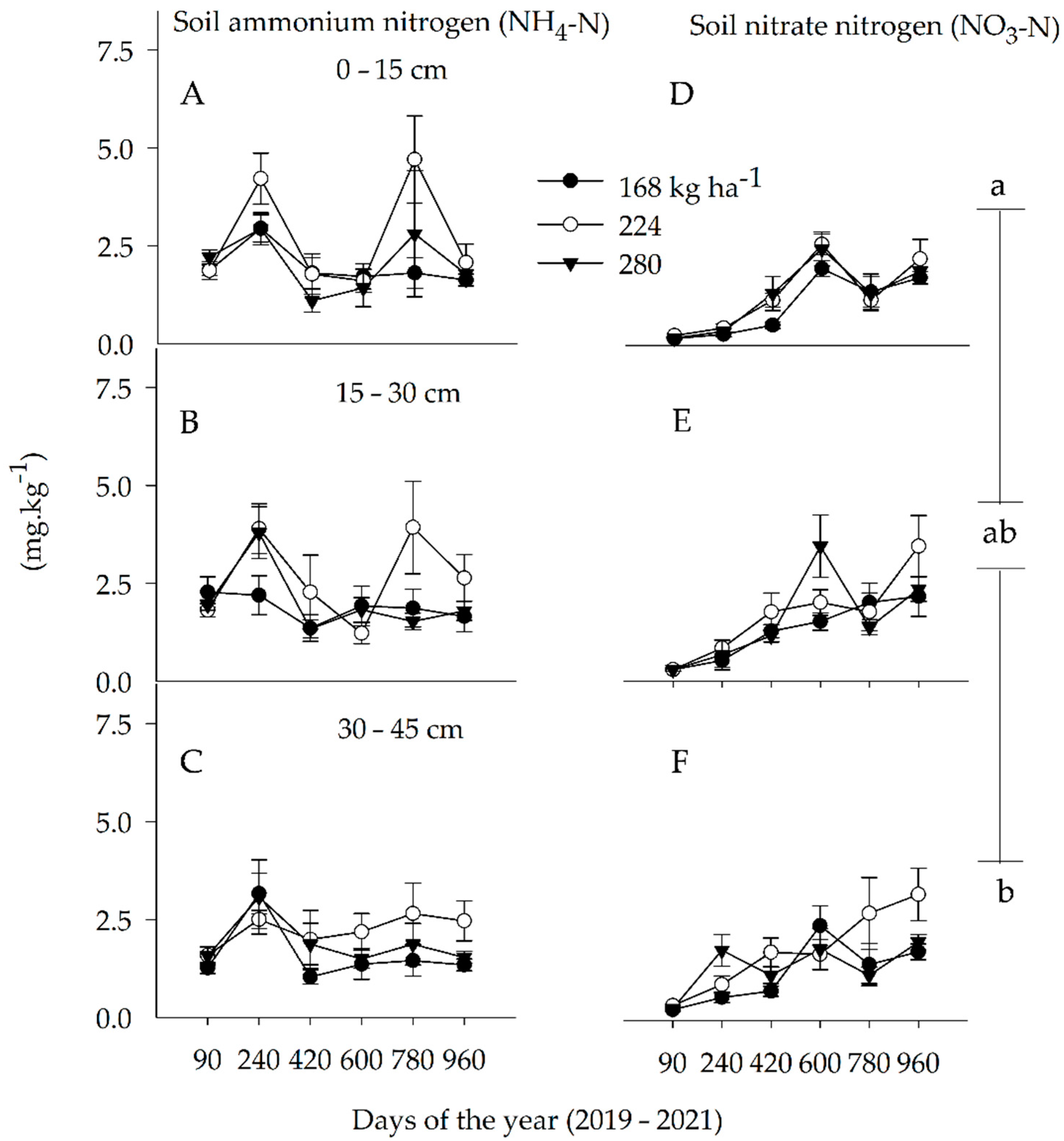
3.1. Soil N Concentration
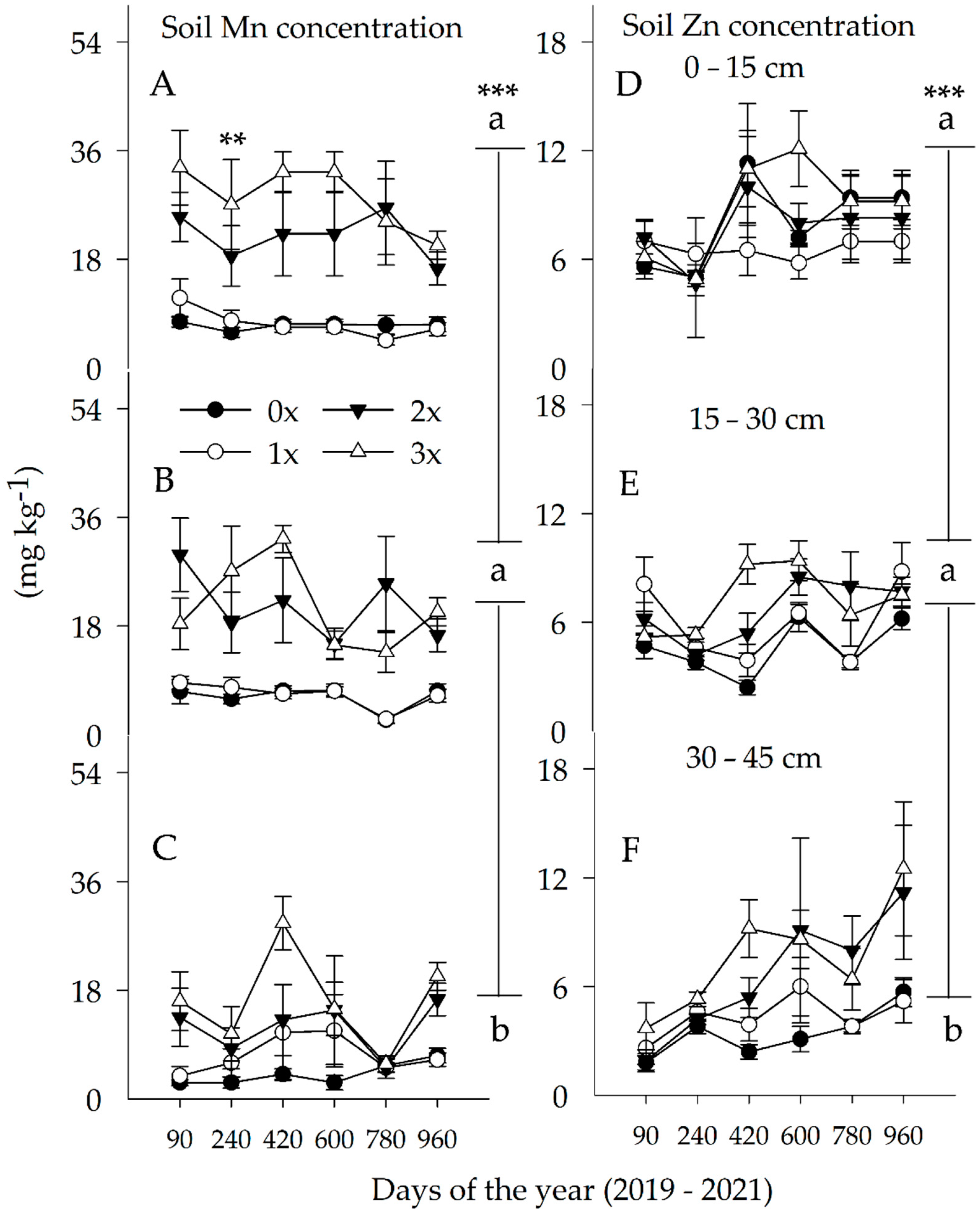
3.2. Soil Mn and Zn Concentrations
3.3. Root Zone Soil pH
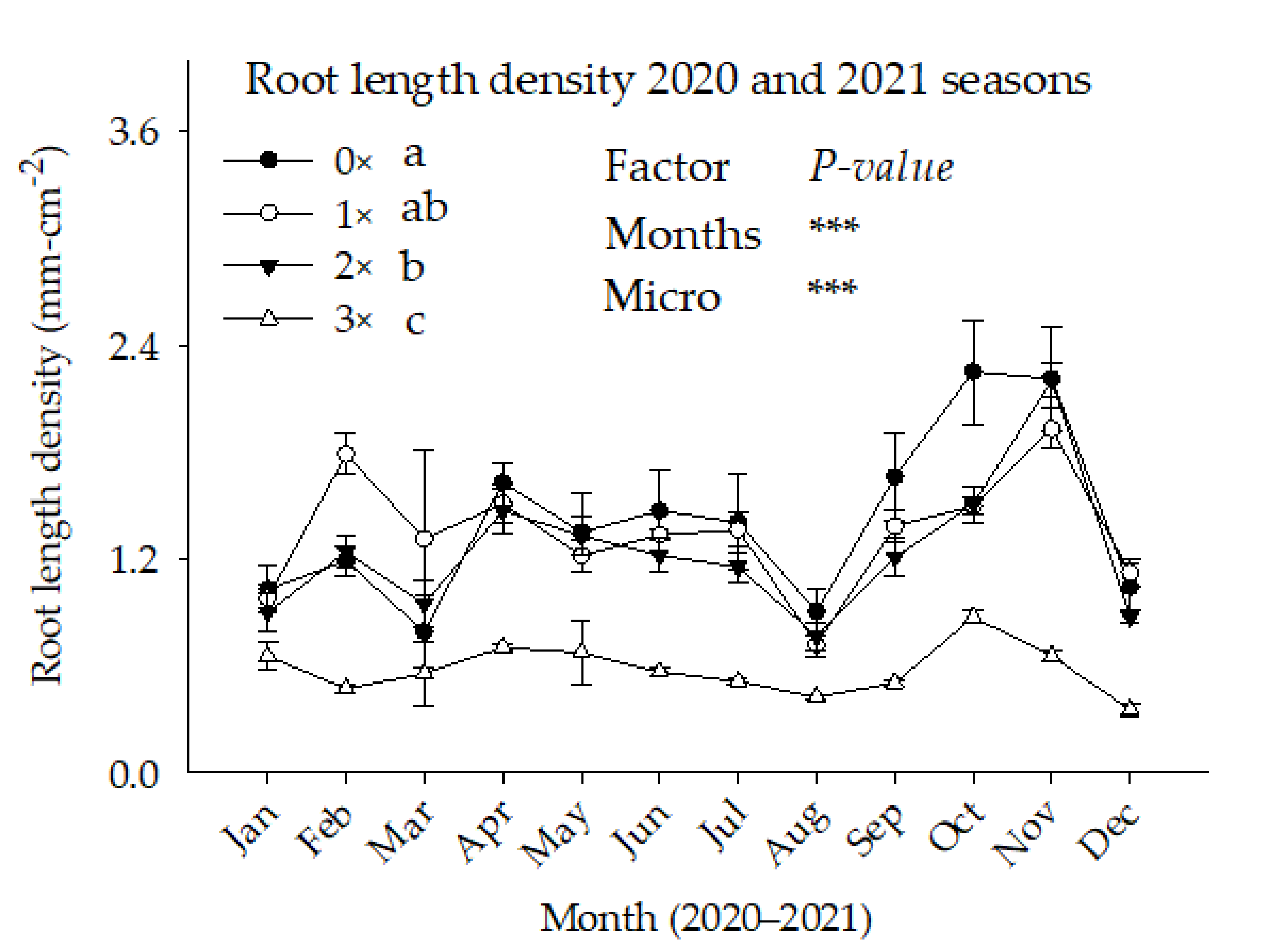
3.4. Root Length Density
3.5. Water Relations
3.5.1. Stem Water Potential (ψstem)
3.5.2. Stomata Conductance (gs)
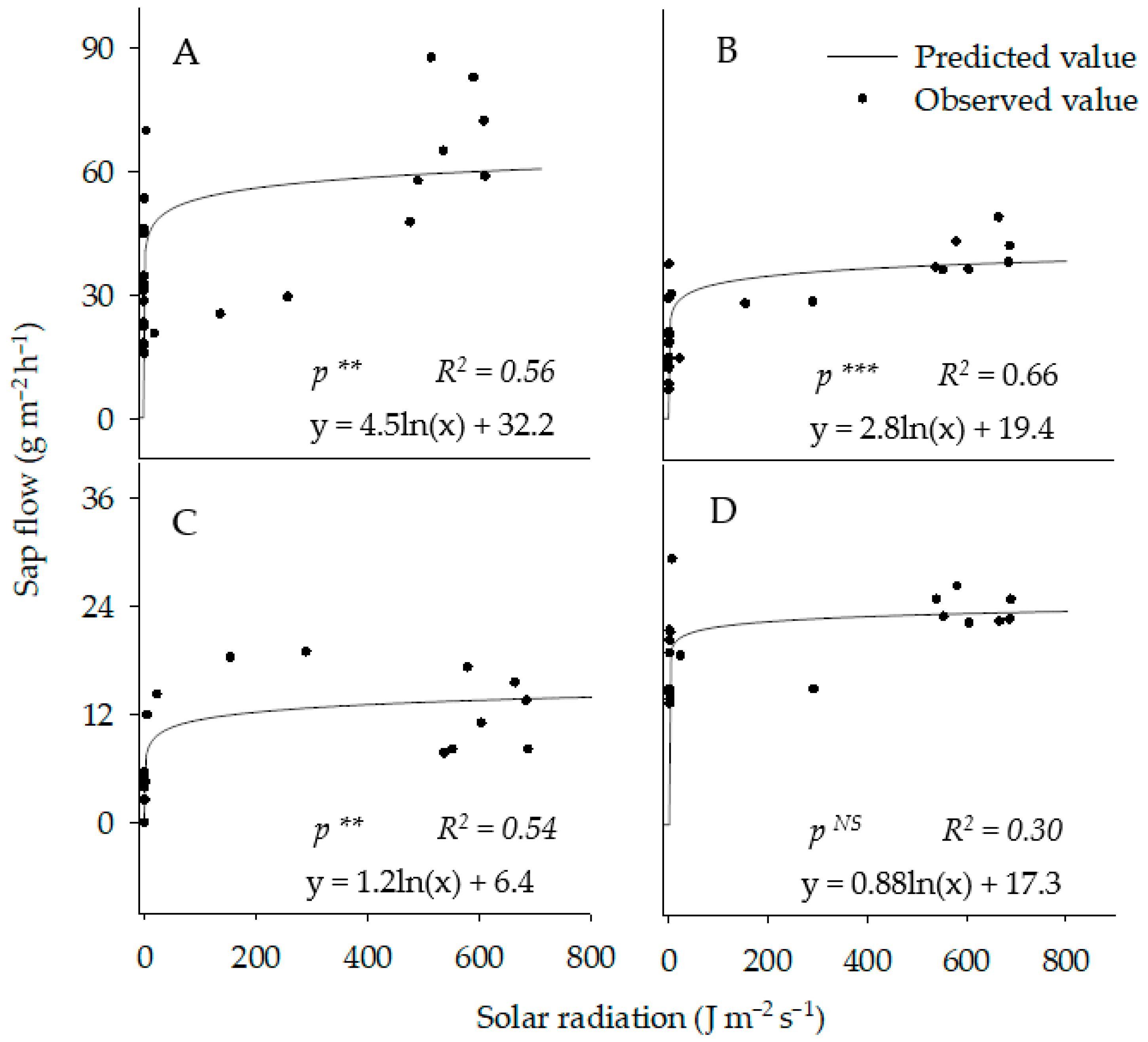
3.5.3. Stem Sap Flow
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottwald, T.R.; Graham, J.H.; Irey, M.S.; McCollum, T.G.; Wood, B.W. Inconsequential effect of nutritional treatments on huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Prot. 2012, 36, 73–82. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar applications of essential nutrients on growth and yield of ‘Valencia’ sweet orange infected with huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Christos, D. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar]
- Cao, J.; Cheng, C.; Yang, J.; Wang, Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Sci. Rep. 2015, 5, 14675. [Google Scholar] [CrossRef] [Green Version]
- Uthman, Q.O.; Kadyampakeni, D.; Nkedi-Kizza, P.; Barlas, N.; Atta, A.; Morgan, K. Comparative response of Huanglongbing-Affected sweet orange trees to nitrogen and zinc fertilization under microsprinkler irrigation. Agriculture 2020, 10, 489. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Mahmoud, K.A. The effect of foliar and ground-applied essential nutrients on Huanglongbing-affected mature citrus trees. Plants 2021, 10, 925. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T. Effect of various irrigation rates on growth and root development of young citrus trees in high-density planting. Plants 2020, 9, 1462. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Mahmoud, K. Ammonium and nitrate distribution in soil using drip and microsprinkler irrigation for citrus production. Soil Sci. Soc. Am. J. 2014, 78, 645–654. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T. The effect of irrigation rate on the water relations of young citrus trees in high-density planting. Sustainability 2021, 13, 759. [Google Scholar] [CrossRef]
- Morgan, K.T.; Kadyampakemi, D. Open field hydroponics: Concept and application. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 271–280. [Google Scholar] [CrossRef]
- Fu, X.; Xing, F.; Cao, L.; Chun, C.; Ling, L. Effects of foliar application of various zinc fertilizers with organosilicone on correcting citrus zinc deficiency. HortScience 2016, 51, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M.; Mahmoud, K.A. Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients. Agronomy 2020, 10, 1485. [Google Scholar] [CrossRef]
- Graham, J.H.; Johnson, E.G.; Gottwald, T.R.; Irey, M.S. Presymptomatic fibrous root decline in citrus trees caused by huanglongbing and potential interaction with phytophthora spp. Plant Dis. 2013, 97, 1195–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, N.; Sishodia, R.P.; Shukla, S.; Hansen, K.M. Improved water and economic sustainability with low-input compact bed plasticulture and precision irrigation. J. Irrig. Drain. Eng. 2019, 145, 04019013. [Google Scholar] [CrossRef]
- Siebert, S.; Burke, J.; Faures, J.M.; Frenken, K.; Hoogeveen, J.; Döll, P.; Portmann, F.T. Groundwater use for irrigation—A global inventory. Hydrol. Earth Syst. Sci. 2010, 14, 1863–1880. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.A.; Morgan, K.T.; Mahmoud, K.A. Split application of nutrients improve growth and yield of Huanglongbing-affected citrus trees. Soil Sci. Soc. Am. J. 2021, 85, 2040–2053. [Google Scholar] [CrossRef]
- Zambon, F.T.; Kadyampakeni, D.M.; Grosser, J.W. Ground application of overdoses of manganese have a therapeutic effect on sweet orange trees infected with Candidatus Liberibacter asiaticus. HortScience 2019, 54, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Stansly, P.A.; Arevalo, H.A.; Qureshi, J.A.; Jones, M.M.; Hendricks, K.; Roberts, P.D.; Roka, F.M. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Manag. Sci. 2014, 70, 415–426. [Google Scholar] [CrossRef]
- Aubert, B. Huanglongbing (HLB) a Diagnosis and Strategies for Control in Reunion Island a Case Study. IRCHLB Proc. 2008, 1–34. Available online: https://swfrec.ifas.ufl.edu/hlb/database/pdf/22_IRCHLB_08.pdf (accessed on 2 April 2022).
- Pustika, A.B.; Subandiyah, S.; Holford, P.; Beattie, G.A.C.; Iwanami, T.; Masaoka, Y. Interactions between plant nutrition and symptom expression in mandarin trees infected with the disease huanglongbing. Australas. Plant Dis. Notes 2008, 3, 112. [Google Scholar] [CrossRef]
- Kiggundu, N.; Migliaccio, K.W.; Schaffer, B.; Li, Y.; Crane, J.H. Water savings, nutrient leaching, and fruit yield in a young avocado orchard as affected by irrigation and nutrient management. Irrig. Sci. 2012, 30, 275–286. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Obreza, T.A. Water use in drip- and microsprinkler-irrigated citrus trees. Soil Sci. Soc. Am. J. 2014, 78, 1351. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.; Alfred, L.; Nkedi-kizza, P. Phosphorus and potassium distribution and adsorption on two florida sandy soils. Soil Sci. Soc. Am. J. 2014, 78, 325–334. [Google Scholar] [CrossRef]
- Obreza, T.A.; Morgan, K.T. Recommended fertilizer rates and timing. In Nutrition of Florida Citrus Trees; Elec. Data Info. Source, SL 253; University of Florida Institute of Food and Agricultural Sciences: Gainesville, GL, USA, 2008. [Google Scholar]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of essential nutrients on roots growth and lifespan of huanglongbing affected citrus trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G.; Road, S.R.; Pierce, F.; Albrigo, L.G. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.A.; Hall, D.G.; Goenaga, R. Diaphorina citri (Hemiptera: Liviidae) abundance in Puerto Rico declines with elevation. J. Econ. Entomol. 2015, 108, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ringuet, S.; Sassano, L.; Johnson, Z.I. A suite of microplate reader-based colorimetric methods to quantify ammonium, nitrate, orthophosphate and silicate concentrations for aquatic nutrient monitoring. J. Environ. Monit. 2011, 13, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, C. Soil Sampling and Methods of Analysis, 7th ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; p. 1224. ISBN 978-0-8593-3586-0. [Google Scholar]
- Abrisqueta, J.M.; Mounzer, O.; Álvarez, S.; Conejero, W.; García-Orellana, Y.; Tapia, L.; Vera, J. Root dynamics of peach trees submitted to partial rootzone drying and continuous deficit irrigation. Agric. Water Manag. 2008, 95, 959–967. [Google Scholar] [CrossRef]
- Box, J.E.; Smucker, A.J.M.; Ritchie, J.T. Minirhizotron installation techniques for investigating root responses to drought and oxygen stresses. Soil Sci. Soc. Am. J. 1989, 53, 115–118. [Google Scholar] [CrossRef]
- Keel, S.G.; Pepin, S.; Leuzinger, S.; Körner, C. Stomatal conductance in mature deciduous forest trees exposed to elevated CO2. Trees-Struct. Funct. 2007, 21, 151–159. [Google Scholar] [CrossRef] [Green Version]
- McCutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 2019, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Hamido, S.A.; Ebel, R.C.; Morgan, K.T. Interaction of huanglongbing and foliar applications of copper on water relations of Citrus sinensis cv. Valencia. Plants 2019, 8, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamido, S.A.; Morgan, K.T.; Ebel, R.C.; Kadyampakeni, D.M. Improved irrigation management of sweet orange with Huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Sato, S.; Morgan, K.T.; Ozores-Hampton, M.; Simonne, E.H. Spatial and temporal distributions in sandy soils with seepage irrigation: I. ammonium and nitrate. Soil Sci. Soc. Am. J. 2009, 73, 1044–1052. [Google Scholar] [CrossRef]
- Paramasivam, S.; Alva, A.K.; Fares, A.; Sajwan, K.S. Fate of nitrate and bromide in an unsaturated zone of a sandy soil under citrus production. J. Environ. Qual. 2002, 31, 671–681. [Google Scholar] [CrossRef]
- Quiñones, A.; Martínez-Alcántara, B.; Legaz, F. Influence of irrigation system and fertilization management on seasonal distribution of N in the soil profile and on N-uptake by citrus trees. Agric. Ecosyst. Environ. 2007, 122, 399–409. [Google Scholar] [CrossRef]
- Uthman, Q.O.; Kadyampakeni, D.M.; Nkedi-Kizza, P. Manganese adsorption, availability, and uptake in citrus under microsprinkler irrigation. Agrosyst. Geosci. Environ. 2020, 3, e20061. [Google Scholar] [CrossRef]
- Hippler, F.W.R.; Boaretto, R.M.; Quaggio, J.A.; Boaretto, A.E.; Abreu-Junior, C.H.; Mattos, D., Jr. Uptake and distribution of soil applied zinc by Citrus trees—Addressing fertilizer use efficiency with 68Zn labeling. PLoS ONE 2015, 10, e0116903. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S. Zinc nutrition, a global concern for sustainable citrus production. J. Sustain. Agric. 2015, 25, 5–42. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T. Nutrition of Florida Citrus Trees, in Special Situations; Elec. data info. Source, SL465; University of Florida Institute of Food and Agricultural Sciences: Gainesville, GL, USA, 2008. [Google Scholar]
- Morgan, K.T.; Graham, J.H. Nutrient status and root density of Huanglongbing-affected trees: Consequences of irrigation water bicarbonate and soil pH mitigation with acidification. Agronomy 2019, 9, 746. [Google Scholar] [CrossRef] [Green Version]
- Brunner, I.; Sperisen, C. Aluminum exclusion and aluminum tolerance in woody plants. Front. Plant Sci. 2013, 4, 172. [Google Scholar] [CrossRef] [Green Version]
- Ebel, R.C.; Hamido, S.; Morgan, K.T.; Bov, F. Interaction of huanglongbing and foliar applications of copper on growth and nutrient acquisition of Citrus sinensis cv. Valencia. HortScience 2019, 54, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.M.; Xia, R.X.; Xiao, Z.Y.; Huang, R.H.; Tan, M.L.; Wang, M.Y.; Wu, Q.S. Reduced leaf photosynthesis at midday in citrus leaves growing under field or screenhouse conditions. J. Hortic. Sci. Biotechnol. 2007, 82, 387–392. [Google Scholar] [CrossRef]
- Annamalainathan, K.; Joseph, J.; Alam, B.; Satheesh, P.R.; Jacob, J. Seasonal changes in xylem sap flow rate in mature rubber plants. J. Plantaion Crop. 2013, 41, 343–349. [Google Scholar]
- Fassio, C.; Heath, R.; Arpaia, M.L.; Castro, M. Sap flow in ‘Hass’ avocado trees on two clonal rootstocks in relation to xylem anatomy. Sci. Hortic. 2009, 120, 8–13. [Google Scholar] [CrossRef]


| Soil Depth | pH a | NO3-N | NH4-N | P | K | Ca | Mg | Mn | Zn | B | Cu | Fe | Organic Matter 2 | Cation Exchange Capacity 2 | Bulk Density 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | ------------------------------ (mg kg−1) 1 ----------------------------------- | % | cmolc kg−1 | g cm−3 | |||||||||||
| 0–15 | 5.67 | 0.81 | 2.79 | 19 | 33 | 195 | 17 | 8 | 4 | 2.0 | 17 | 52 | 0.61 | 7.63 | 1.62 |
| 15–30 | 5.93 | 0.99 | 2.68 | 15 | 31 | 187 | 17 | 10 | 4 | 2.0 | 15 | 50 | 0.41 | 0.74 | 1.62 |
| 30–45 | 6.29 | 1.24 | 2.07 | 10 | 22 | 131 | 10 | 4 | 3 | 3.0 | 11 | 50 | 0.49 | 0.33 | 1.59 |
| Method and Rate of Application (kg ha−1 year−1) | |||||||
|---|---|---|---|---|---|---|---|
| Soil 2 | Foliar 3 | Soil 4 | |||||
| Treatments 1 | N | Mn | Zn | B | Mn | Zn | B |
| 0× | 168 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ |
| 224 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ | |
| 280 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ | |
| 1× | 168 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ |
| 224 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ | |
| 280 | 9 | 9 | 2.3 | ̶ | ̶ | ̶ | |
| 2× | 168 | 9 | 9 | 2.3 | 9 | 9 | 2.3 |
| 224 | 9 | 9 | 2.3 | 9 | 9 | 2.3 | |
| 280 | 9 | 9 | 2.3 | 9 | 9 | 2.3 | |
| 3× | 168 | 9 | 9 | 2.3 | 18 | 18 | 4.6 |
| 224 | 9 | 9 | 2.3 | 18 | 18 | 4.6 | |
| 280 | 9 | 9 | 2.3 | 18 | 18 | 4.6 | |
| Soil Nutrient Concentration (mg kg−1) 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | |||||||||
| Treatments 1 | NO3-N | NH4-N | P | K | Ca | Mg | B | Cu | Fe |
| 0× | 0.8 ± 0.1 | 1.8 ± 0.2 b | 19 ± 1.7 | 33 ± 1.1 | 195 ± 14 | 17 ± 1.4 | 1.1 ± 0.1 | 15 ± 1.2 b | 52 ± 3.0 |
| 1× | 0.7 ± 0.1 | 2.0 ± 0.2 ab | 12 ± 1.1 | 27 ± 1.9 | 165 ± 12 | 13 ± 1.0 | 1.2 ± 0.1 | 18 ± 1.4 a | 36 ± 2.4 |
| 2× | 0.9 ± 0.2 | 2.7 ± 0.4 a | 14 ± 1.0 | 28 ± 1.2 | 172 ± 13 | 9 ± 0.5 | 1.4 ± 0.1 | 15 ± 0.7 ab | 36 ± 2.7 |
| 3× | 0.7 ± 0.1 | 2.2 ± 0.2 ab | 16 ± 1.7 | 32 ± 1.7 | 143 ± 12 | 16 ± 1.3 | 1.6 ± 0.2 | 20 ± 1.2 ab | 46 ± 1.8 |
| Model Effect | ANOVA | ||||||||
| Nitrogen | ** | NS | NS | NS | NS | NS | NS | NS | NS |
| Micronutrient | NS | * | NS | NS | NS | NS | NS | * | NS |
| Depth | NS | ** | ** | *** | NS | NS | * | * | NS |
| Nitogen × Depth | NS | NS | ** | NS | NS | *** | NS | NS | * |
| Summer | |||||||||
| 0× | 1.6 ± 0.2 | 2.7 ± 0.2 | 11 ± 1.3 | 22 ± 1.9 | 252 ± 16 ab | 18 ± 1.4 a | 0.9 ± 0.0 | 6.1 ± 0.4 | 36 ± 1.9 |
| 1× | 1.3 ± 0.1 | 2.3 ± 0.1 | 14 ± 1.2 | 20 ± 1.7 | 306 ± 25 a | 16 ± 1.2 ab | 1.3 ± 0.3 | 6.9 ± 0.6 | 33 ± 2.5 |
| 2× | 1.3 ± 0.1 | 1.8 ± 0.1 | 11 ± 1.6 | 13 ± 1.1 | 224 ± 28 b | 12 ± 1.2 ab | 0.8 ± 0.1 | 4.8 ± 0.4 | 23 ± 2.3 |
| 3× | 1.3 ± 0.1 | 2.3 ± 0.1 | 17 ± 2.4 | 16 ± 1.3 | 231 ± 23 ab | 16 ± 1.4 | 1.1 ± 0.1 | 6.8 ± 0.6 | 40 ± 2.8 |
| Model Effect | ANOVA | ||||||||
| Nitrogen | * | * | NS | NS | * | * | ** | * | NS |
| Micronutrient | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Depth | * | NS | NS | *** | ** | ** | NS | * | NS |
| Nitogen × Depth | NS | NS | ** | NS | NS | NS | *** | NS | ** |
| Spring | Summer | |||||
|---|---|---|---|---|---|---|
| Depths (cm) | ||||||
| Micro 1 | 0–15 | 15–30 | 30–45 | 0–15 | 15–30 | 30–45 |
| 0× | 6.1 ± 0.13 a 2 | 6.3 ± 0.12 a | 6.6 ± 0.09 a | 5.6 ± 0.07 a | 5.9 ± 0.11 a | 6.3 ± 0.10 a |
| 1× | 5.8 ± 0.08 a | 6.3 ± 0.10 a | 6.6 ± 0.11 a | 5.7 ± 0.08 a | 5.8 ± 0.10 a | 6.2 ± 0.11 a |
| 2× | 5.4 ± 0.09 b | 5.6 ± 0.13 b | 6.0 ± 0.15 b | 5.3 ± 0.11 ab | 5.2 ± 0.18 b | 5.6 ± 0.18 b |
| 3× | 5.2 ± 0.07 b | 5.4 ± 0.12 b | 5.7 ± 0.18 b | 5.0 ± 0.09 b | 5.1 ± 0.08 b | 5.5 ± 0.11 b |
| p-value | *** | *** | *** | *** | *** | *** |
| N rate (Kg ha−1) | ||||||
| Depth (cm) 3 | 168 | 224 | 280 | 168 | 224 | 280 |
| 0–15 | 5.6 ± 0.15 a | 5.6 ± 0.12 a | 5.6 ± 0.14 a | 5.5 ± 0.13 a | 5.3 ± 0.11 a | 5.4 ± 0.11 a |
| 15–30 | 5.9 ± 0.15 ab | 5.9 ± 0.17 ab | 5.9 ± 0.14 ab | 5.6 ± 0.14 ab | 5.5 ± 0.15 ab | 5.4 ± 0.17 ab |
| 30–45 | 6.2 ± 0.15 b | 6.2 ± 0.17 b | 6.3 ± 0.2 b | 5.9 ± 15 b | 5.9 ± 0.15 b | 5.8 ± 0.18 b |
| p-value | * | * | * | * | ** | * |
| Model effect 4 | ANOVA | |||||
| Depth | *** | ** | ||||
| Micronutrient | *** | *** | ||||
| Nitogen × Depth | NS | ** | ||||
| Stem Water Potential (Mpa) | Stomata Conductance (mmol m−2 s−1) | |||
|---|---|---|---|---|
| Micro 1 | Spring | Summer | Spring | Summer |
| 0× | 0.94 ± 0.04 2 | 1.18 ± 0.05 a | 313 ± 13 a | 185 ± 18 |
| 1× | 0.93 ± 0.03 | 1.25 ± 0.06 ab | 383 ± 20 b | 205± 13 |
| 2× | 0.98 ± 0.04 | 1.25 ± 0.05 ab | 349 ± 15 ab | 197 ± 16 |
| 3× | 1.03 ± 0.03 | 1.32 ± 0.0 8b | 349 ± 17 ab | 188 ± 18 |
| p-value | NS | ** | * | NS |
| Model effect 3 | ANOVA | |||
| Season | ** | *** | ||
| Micronutrient | ** | * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M. Spatial and Temporal Nutrient Dynamics and Water Management of Huanglongbing-Affected Mature Citrus Trees on Florida Sandy Soils. Sustainability 2022, 14, 7134. https://doi.org/10.3390/su14127134
Atta AA, Morgan KT, Kadyampakeni DM. Spatial and Temporal Nutrient Dynamics and Water Management of Huanglongbing-Affected Mature Citrus Trees on Florida Sandy Soils. Sustainability. 2022; 14(12):7134. https://doi.org/10.3390/su14127134
Chicago/Turabian StyleAtta, Alisheikh A., Kelly T. Morgan, and Davie M. Kadyampakeni. 2022. "Spatial and Temporal Nutrient Dynamics and Water Management of Huanglongbing-Affected Mature Citrus Trees on Florida Sandy Soils" Sustainability 14, no. 12: 7134. https://doi.org/10.3390/su14127134
APA StyleAtta, A. A., Morgan, K. T., & Kadyampakeni, D. M. (2022). Spatial and Temporal Nutrient Dynamics and Water Management of Huanglongbing-Affected Mature Citrus Trees on Florida Sandy Soils. Sustainability, 14(12), 7134. https://doi.org/10.3390/su14127134








