Seed Mucilage Promotes Dispersal of Plantago asiatica Seeds by Facilitating Attachment to Shoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Walking Experiment
2.3. Data Analysis
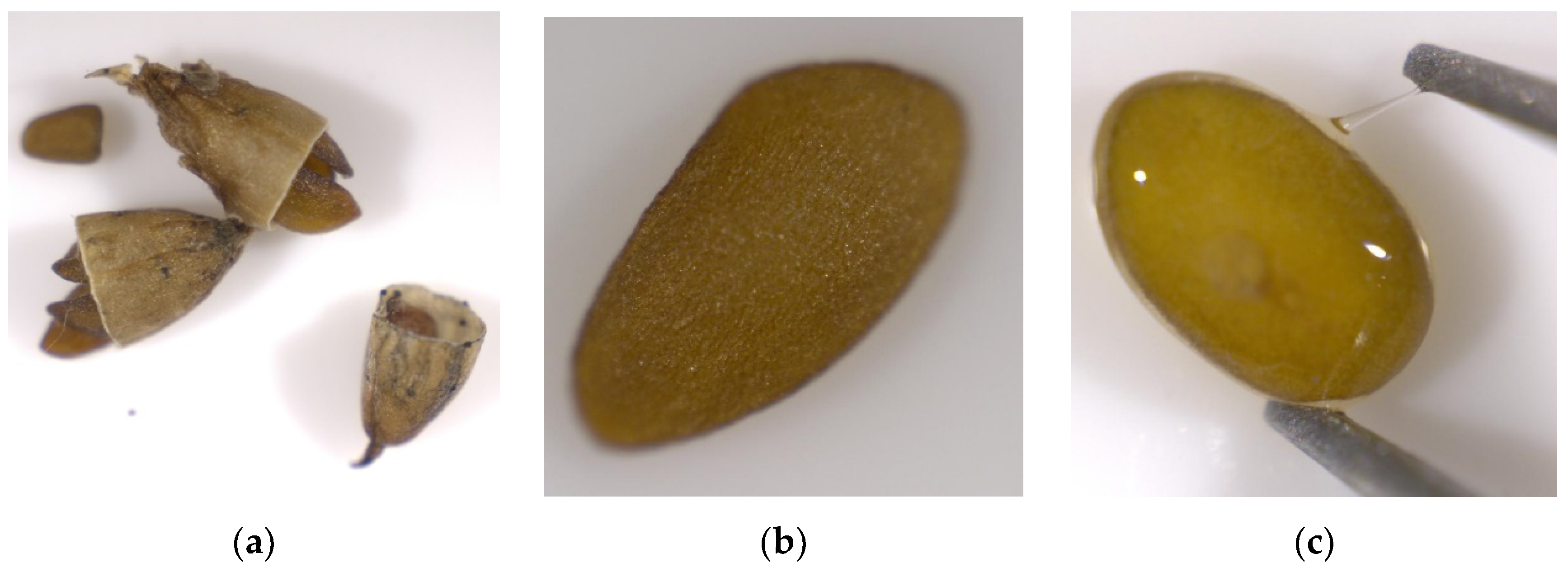
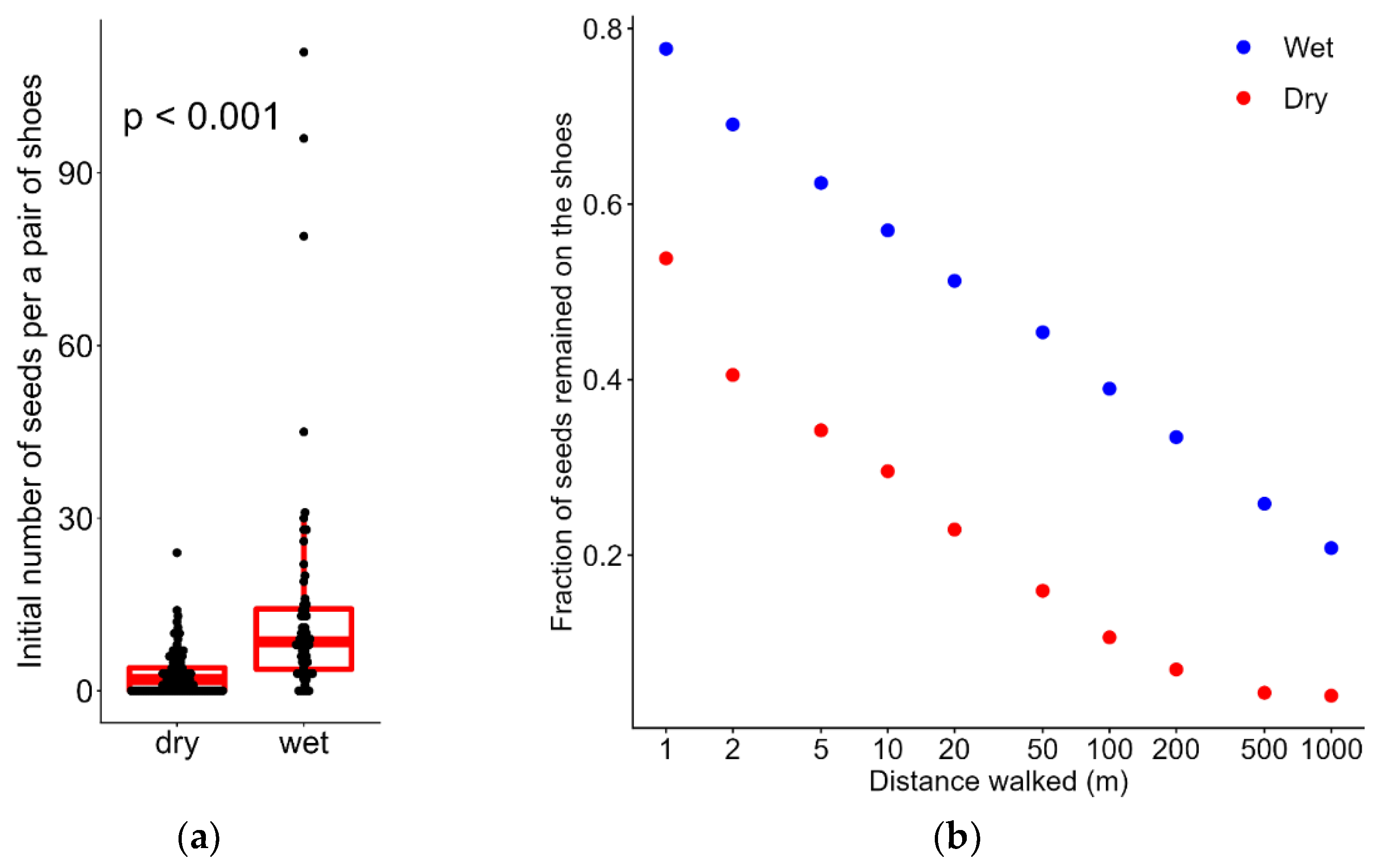
3. Results
4. Discussion
4.1. Role of Diaspore Mucilage in Human-Mediated Dispersal
4.2. Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bullock, S.H.; Primack, R.B. Comparative experimental study of seed dispersal on animals. Ecology 1977, 58, 681–686. [Google Scholar] [CrossRef]
- Carey, P.; Watkinson, A. The dispersal and fates of seeds of the winter annual grass Vulpia ciliata. J. Ecol. 1993, 81, 759–767. [Google Scholar] [CrossRef]
- Bruun, H.H.; Poschlod, P. Why are small seeds dispersed through animal guts: Large numbers or seed size per se? Oikos 2006, 113, 402–411. [Google Scholar] [CrossRef]
- Bullock, J.M.; Wichmann, M.C.; Hails, R.S.; Hodgson, D.J.; Alexander, M.J.; Morley, K.; Knopp, T.; Ridding, L.E.; Hooftman, D.A.P. Human-mediated dispersal and disturbance shape the metapopulation dynamics of a long-lived herb. Ecology 2020, 101, e03087. [Google Scholar] [CrossRef]
- Liu, Z.; Evans, M. Effect of tree density on seed production and dispersal of birch (Betula pendula Roth and Betula pubescens Ehrhs). Forests 2021, 12, 929. [Google Scholar] [CrossRef]
- Martínez-López, V.; García, C.; Zapata, V.; Robledano, F.; De la Rúa, P. Intercontinental long-distance seed dispersal across the Mediterranean Basin explains population genetic structure of a bird-dispersed shrub. Mol. Ecol. 2020, 29, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Al-Qthanin, R.N.; Alharbi, S.A. Spatial structure and genetic variation of a mangrove species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests 2020, 11, 1287. [Google Scholar] [CrossRef]
- Whinam, J.; Chilcott, N.; Bergstrom, D.M. Subantarctic hitchhikers: Expeditioners as vectors for the introduction of alien organisms. Biol. Conserv. 2005, 121, 207–219. [Google Scholar] [CrossRef]
- Scott, K.A. Potential for the dispersal of weed seeds on clothing: An example with Gamba Grass in northern Australia. Ecol. Manag. Restor. 2009, 10, 71–73. [Google Scholar] [CrossRef]
- Ansong, M.; Pickering, C. Long-distance dispersal of Black Spear Grass (Heteropogon contortus) seed on socks and trouser legs by walkers in Kakadu National Park. Ecol. Manag. Restor. 2013, 14, 71–74. [Google Scholar] [CrossRef]
- von der Lippe, M.; Bullock, J.M.; Kowarik, I.; Knopp, T.; Wichmann, M. Human-Mediated Dispersal of Seeds by the Airflow of Vehicles. PLoS ONE 2013, 8, e52733. [Google Scholar] [CrossRef]
- Pickering, C.; Ansong, M. Human-mediated dispersal of the seeds of Australian weeds. Victorian Nat. 2020, 137, 269–275. [Google Scholar] [CrossRef]
- Runghen, R.; Bramon Mora, B.; Godoy-Lorite, A.; Stouffer, D.B. Assessing unintended human-mediated dispersal using visitation networks. J. Appl. Ecol. 2021, 58, 777–788. [Google Scholar] [CrossRef]
- Wróbel, A.; Kurek, P.; Bogdziewicz, M.; Dobrowolska, D.; Zwolak, R. Avian dispersal of an invasive oak is modulated by acorn traits and the presence of a native oak. For. Ecol. Manag. 2022, 505, 119866. [Google Scholar] [CrossRef]
- Yang, M.; Pickering, C.M.; Xu, L.; Lin, X. Tourist vehicle as a selective mechanism for plant dispersal: Evidence from a national park in the eastern Himalaya. J. Environ. Manag. 2021, 285, 112109. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Masaki, T.; Motooka, M.; Hino, D.; Ueda, K. Highly toxic seeds of the Japanese star anise Illicium anisatum are dispersed by a seed-caching bird and a rodent. Ecol. Res. 2018, 33, 495–504. [Google Scholar] [CrossRef]
- Tsuji, Y.; Campos-Arceiz, A.; Prasad, S.; Kitamura, S.; McConkey, K.R. Intraspecific differences in seed dispersal caused by differences in social rank and mediated by food availability. Sci. Rep. 2020, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Núñez, R.C.; Alvarez-Alvarez, E.A.; Sierra-Morales, P.; Rodríguez-Godínez, R. Fruit Size and Structure of Zoochorous Trees: Identifying Drivers for the Foraging Preferences of Fruit-Eating Birds in a Mexican Successional Dry Forest. Animals 2021, 11, 3343. [Google Scholar] [CrossRef]
- Almeida, A.P.; Gomes, M.; Rabaça, J.E.; Ramos, J.A. Songbirds promote connectivity between riparian galleries and adjacent habitats. Ecol. Res. 2021, 36, 45–56. [Google Scholar] [CrossRef]
- Araki, N.; Hirayama, K. Differences in the fruit removal patterns of Cleyera japonica by frugivorous birds in two forest stands at different developmental stages in a warm-temperate region. Ecol. Res. 2021, 36, 189–201. [Google Scholar] [CrossRef]
- Ben-Zvi, G.; Seifan, M.; Giladi, I. Ant guild identity determines seed fate at the post-removal seed dispersal stages of a desert perennial. Insects 2021, 12, 147. [Google Scholar] [CrossRef]
- Blanco, G.; Romero-Vidal, P.; Carrete, M.; Chamorro, D.; Bravo, C.; Hiraldo, F.; Tella, J.L. Burrowing parrots Cyanoliseus patagonus as long-distance seed dispersers of keystone algarrobos, genus Prosopis, in the Monte Desert. Diversity 2021, 13, 204. [Google Scholar] [CrossRef]
- Selås, V.; Framstad, E.; Rolstad, J.; Sonerud, G.A.; Spidsø, T.K.; Wegge, P. Bilberry seed production explains spatiotemporal synchronicity in bank vole population fluctuations in Norway. Ecol. Res. 2021, 36, 409–419. [Google Scholar] [CrossRef]
- Tanaka, K.; Tokuda, M. Road preference of ants in a Japanese warm temperate forest and its implications for the regeneration of myrmecochorous sedges. Ecol. Res. 2021, 36, 629–636. [Google Scholar] [CrossRef]
- Teixido, A.L.; Fuzessy, L.F.; Souza, C.S.; Gomes, I.N.; Kaminski, L.A.; Oliveira, P.C.; Maruyama, P.K. Anthropogenic impacts on plant-animal mutualisms: A global synthesis for pollination and seed dispersal. Biol. Conserv. 2022, 266, 109461. [Google Scholar] [CrossRef]
- Vazquez, M.S.; Rodriguez-Cabal, M.A.; Amico, G.C. The forest gardener: A marsupial with a key seed-dispersing role in the Patagonian temperate forest. Ecol. Res. 2022, 37, 270–283. [Google Scholar] [CrossRef]
- Gómez, J.M.; Schupp, E.W.; Jordano, P. Synzoochory: The ecological and evolutionary relevance of a dual interaction. Biol. Rev. 2019, 94, 874–902. [Google Scholar] [CrossRef]
- Wyatt, G.E.; Hamrick, J.L.; Trapnell, D.W. The role of anthropogenic dispersal in shaping the distribution and genetic composition of a widespread North American tree species. Ecol. Evol. 2021, 11, 11515–11532. [Google Scholar] [CrossRef]
- Wichmann, M.C.; Alexander, M.J.; Soons, M.B.; Galsworthy, S.; Dunne, L.; Gould, R.; Fairfax, C.; Niggemann, M.; Hails, R.S.; Bullock, J.M. Human-mediated dispersal of seeds over long distances. P. Roy. Soc. B-Biol. Sci. 2009, 276, 523–532. [Google Scholar] [CrossRef]
- Ansong, M.; Pickering, C. Weed seeds on clothing: A global review. J. Environ. Manag. 2014, 144, 203–211. [Google Scholar] [CrossRef]
- Ansong, M.; Pickering, C. The effects of seed traits and fabric type on the retention of seed on different types of clothing. Basic Appl. Ecol. 2016, 17, 516–526. [Google Scholar] [CrossRef]
- Healy, A.J. Seed dispersal by human activity. Nature 1943, 151, 140. [Google Scholar] [CrossRef]
- Faliński, J. Anthropochory in xerothermic grasslands in the light of experimental data. Acta. Soc. Bot. Pol. 1972, 41, 357–368. [Google Scholar] [CrossRef]
- Mount, A.; Pickering, C.M. Testing the capacity of clothing to act as a vector for non-native seed in protected areas. J. Environ. Manag. 2009, 91, 168–179. [Google Scholar] [CrossRef]
- Pickering, C.M.; Mount, A.; Wichmann, M.C.; Bullock, J.M. Estimating human-mediated dispersal of seeds within an Australian protected area. Biol. Invasions 2011, 13, 1869–1880. [Google Scholar] [CrossRef]
- Ansong, M.; Pickering, C. What’s a weed? Knowledge, attitude and behaviour of park visitors about weeds. PLoS ONE 2015, 10, e0135026. [Google Scholar] [CrossRef]
- Ansong, M.; Pickering, C.; Arthur, J.M. Modelling seed retention curves for eight weed species on clothing. Austral. Ecol. 2015, 40, 765–774. [Google Scholar] [CrossRef]
- Clifford, H.T. Seed dispersal on footwear. P. Bot. Soc. Brit. Isles 1956, 2, 129–131. [Google Scholar]
- Higashino, P.; Guyer, W.; Stone, C. The Kilauea wilderness marathon and crater rim runs: Sole searching experiences. Hawaii. Bot. Soc. 1983, 22, 25–28. [Google Scholar]
- Clifford, H.T. Seed Dispersal by Motor Vehicles. J. Ecol. 1959, 47, 311–315. [Google Scholar] [CrossRef]
- Hodkinson, D.J.; Thompson, K. Plant dispersal: The role of man. J. Appl. Ecol. 1997, 34, 1484–1496. [Google Scholar] [CrossRef]
- Manzano, P.; Malo, J.E. Extreme long-distance seed dispersal via sheep. Front. Ecol. Environ. 2006, 4, 244–248. [Google Scholar] [CrossRef]
- Woodruffe-Peacock, E.A. A Fox-Covert Study. J. Ecol. 1918, 6, 110–125. [Google Scholar] [CrossRef]
- Baiges, J.; Espadaler, X.; Blanché, C. Seed dispersal in W Mediterranean Euphorbia species. Bot. Chron. 1991, 10, 697–705. [Google Scholar]
- Grubert, M. Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 1974, 8, 315–352. [Google Scholar]
- van der Pijl, L. Principles of Dispersal in Higher Plants, 3rd Revised and Expanded ed.; Springer: Berlin, Germany, 1982. [Google Scholar]
- Alcántara, J.M.; Rey, P.J. Conflicting selection pressures on seed size: Evolutionary ecology of fruit size in a bird-dispersed tree, Olea europaea. J. Evol. Biol. 2003, 16, 1168–1176. [Google Scholar] [CrossRef]
- Nunez, C.V.; de Oliveira, M.L.; Lima, R.D.; Diaz, I.E.C.; Sargentini, É.; Pereira, O.L.; Araújo, L.M. Chemical analyses confirm a rare case of seed dispersal by bees. Apidologie 2008, 39, 618–626. [Google Scholar] [CrossRef]
- Wallace, H.M.; Howell, M.G.; Lee, D.J. Standard yet unusual mechanisms of long-distance dispersal: Seed dispersal of Corymbia torelliana by bees. Divers. Distrib. 2008, 14, 87–94. [Google Scholar] [CrossRef]
- Case, S.B.; Tarwater, C.E. Functional traits of avian frugivores have shifted following species extinction and introduction in the Hawaiian Islands. Funct. Ecol. 2020, 34, 2467–2476. [Google Scholar] [CrossRef]
- Valenta, K.; Nevo, O. The dispersal syndrome hypothesis: How animals shaped fruit traits, and how they did not. Funct. Ecol. 2020, 34, 1158–1169. [Google Scholar] [CrossRef]
- Koyama, K.; Tashiro, M. No effect of selective maturation on fruit traits for a bird-dispersed species, Sambucus racemosa. Plants 2021, 10, 376. [Google Scholar] [CrossRef]
- Phan, J.L.; Tucker, M.R.; Khor, S.F.; Shirley, N.; Lahnstein, J.; Beahan, C.; Bacic, A.; Burton, R.A. Differences in glycosyltransferase family 61 accompany variation in seed coat mucilage composition in Plantago spp. J. Exp. Bot. 2016, 67, 6481–6495. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. “Sticky invasion”—the physical properties of Plantago lanceolata L. seed mucilage. Beilstein. J. Nanotech. 2016, 7, 1918–1927. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Gorb, S.N. How does the cell wall ‘stick’ in the mucilage? A detailed microstructural analysis of the seed coat mucilaginous cell wall. Flora 2017, 229, 9–22. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. Plant seed mucilage as a glue: Adhesive properties of hydrated and dried-in-contact seed mucilage of five plant species. Int. J. Mol. Sci. 2021, 22, 1443. [Google Scholar] [CrossRef]
- Cowley, J.M.; Burton, R.A. The goo-d stuff: Plantago as a myxospermous model with modern utility. New Phytol. 2021, 229, 1917–1923. [Google Scholar] [CrossRef]
- Viudes, S.; Burlat, V.; Dunand, C. Seed mucilage evolution: Diverse molecular mechanisms generate versatile ecological functions for particular environments. Plant Cell Environ. 2020, 43, 2857–2870. [Google Scholar] [CrossRef]
- Ryding, O. Myxocarpy in the Nepetoideae (Lamiaceae) with notes on myxodiaspory in general. Syst. Geogr. Plants 2001, 71, 503–514. [Google Scholar] [CrossRef]
- LoPresti, E.F.; Pan, V.; Goidell, J.; Weber, M.G.; Karban, R. Mucilage-bound sand reduces seed predation by ants but not by reducing apparency: A field test of 53 plant species. Ecology 2019, 100, e02809. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Haase, E.; Gorb, S.N. The role of mucilage envelope in the endozoochory of selected plant taxa. Sci. Nat. 2020, 108, 2. [Google Scholar] [CrossRef]
- Pan, V.S.; Girvin, C.; LoPresti, E.F. Anchorage by seed mucilage prevents seed dislodgement in high surface flow: A mechanistic investigation. Ann. Bot. 2022, mcac045. [Google Scholar] [CrossRef]
- Carlquist, S. The biota of long-distance dispersal. V. Plant dispersal to Pacific Islands. B. Torrey Bot. Club 1967, 94, 129–162. [Google Scholar] [CrossRef]
- Swarbrick, J.T. External mucilage production by the seeds of British plants. Bot. J. Linn. Soc. 1971, 64, 157–162. [Google Scholar] [CrossRef]
- Fahn, A.; Werker, E. Anatomical mechanisms of seed dispersal. In Seed Biology: Importance, Development, and Germination; Academic Press: New York, NY, USA, 1972; Volume 1, pp. 151–221. [Google Scholar]
- Sorensen, A.E. Seed dispersal by adhesion. Annu. Rev. Ecol. Syst. 1986, 17, 443–463. [Google Scholar] [CrossRef]
- Norton, D.A.; Delange, P.J.; Garnock-Jones, P.J.; Given, D.R. The role of seabirds and seals in the survival of coastal plants: Lessons from New Zealand Lepidium (Brassicaceae). Biodivers. Conserv. 1997, 6, 765–785. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Vallès, J. Achene morphology and slime structure in some taxa of Artemisia L. and Neopallasia L. (Asteraceae). Flora 2007, 202, 570–580. [Google Scholar] [CrossRef]
- Kreitschitz, A. Biological properties of fruit and seed slime envelope: How to live, fly, and not die. In Functional Surfaces in Biology: Little Structures with Big Effects Volume 1; Gorb, S.N., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 11–30. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. Slipping vs. sticking: Water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomater. 2015, 17, 152–159. [Google Scholar] [CrossRef]
- Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients 2021, 13, 3354. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Benton, R.A. The behaviour of seeds in soil: II. The germination of seeds on the surface of a water supplying substrate. J. Ecol. 1966, 54, 151–166. [Google Scholar] [CrossRef]
- Young, J.A.; Evans, R.A. Mucilaginous seed coats. Weed Sci. 1973, 21, 52–54. [Google Scholar] [CrossRef]
- Tomoda, M.; Yokoi, M.; Ishikawa, K. Plant mucilages. XXIX. Isolation and characterization of a mucous polysaccharide, ‘Plantago-mucilage A’ from the seeds of Plantago major var. asiatica. Chem. Pharm. Bull. 1981, 29, 2877–2884. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, G.; Gowthamarajan, K.; Rao, B.; Suresh, B. Evaluation of binding properties of Plantago ovata and Trigonella foenum graecum mucilages. Indian Drugs 2002, 39, 422–425. [Google Scholar]
- Malviya, R.; Srivastava, P.; Kulkarni, G. Applications of mucilages in drug delivery-a review. Adv. Biol. Res. 2011, 5, 1–7. [Google Scholar]
- Western, T.L. The sticky tale of seed coat mucilages: Production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 2011, 22, 1–25. [Google Scholar] [CrossRef]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohyd. Polym. 2017, 165, 132–141. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Guo, X. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef]
- Mukherjee, T.; Lerma-Reyes, R.; Thompson, K.A.; Schrick, K. Making glue from seeds and gums: Working with plant-based polymers to introduce students to plant biochemistry. Biochem. Mol. Biol. Edu. 2019, 47, 468–475. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Paunescu, A. Plantago media L.—Explored and potential applications of an underutilized plant. Plants 2021, 10, 265. [Google Scholar] [CrossRef]
- Tsai, A.Y.-L.; McGee, R.; Dean, G.H.; Haughn, G.W.; Sawa, S. Seed Mucilage: Biological Functions and Potential Applications in Biotechnology. Plant Cell Physiol. 2021, 62, 1847–1857. [Google Scholar] [CrossRef]
- Cowley, J.M.; O’Donovan, L.A.; Burton, R.A. The composition of Australian Plantago seeds highlights their potential as nutritionally-rich functional food ingredients. Sci. Rep. 2021, 11, 12692. [Google Scholar] [CrossRef]
- The Global Biodiversity Information Facility. Available online: http://www.gbif.org (accessed on 14 February 2022).
- Yin, J.-Y.; Chen, H.-H.; Lin, H.-X.; Xie, M.-Y.; Nie, S.-P. Structural features of alkaline extracted polysaccharide from the seeds of Plantago asiatica L. and its rheological properties. Molecules 2016, 21, 1181. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yoshida, M.; Ohnuna, S. Germinated plants from seeds carried by tourist’s shoes into Mt. Tateyama, Toyama Prefecture, Central Japan. Bull. Bot. Gard. Toyama 2008, 13, 15–21. [Google Scholar]
- Pickering, C.; Mount, A. Do tourists disperse weed seed? A global review of unintentional human-mediated terrestrial seed dispersal on clothing, vehicles and horses. J. Sustain. Tour. 2010, 18, 239–256. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: https://www.jma.go.jp (accessed on 25 March 2022).
- R Core Team. R: A Language and Environment for Satistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Wilke, C.O. cowplot: Streamlined plot theme and plot annotations for ‘ggplot2’. CRAN Repos 2016, 2, R2. [Google Scholar]
- Clarke, E.; Sherrill-Mix, S. ggbeeswarm: Categorical Scatter (Violin Point) Plots. 2017. Available online: https://cran.r-project.org/web/packages/ggbeeswarm/index.html (accessed on 17 January 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Staab, M.; Pereira-Peixoto, M.H.; Klein, A.-M. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 2020, 194, 465–480. [Google Scholar] [CrossRef]
- Iwabe, R.; Koyama, K.; Komamura, R. Shade avoidance and light foraging of a clonal woody species, Pachysandra terminalis. Plants 2021, 10, 809. [Google Scholar] [CrossRef]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lan. 2013, 68, 255–278. [Google Scholar] [CrossRef]
- Schaber, C.F.; Kreitschitz, A.; Gorb, S.N. Friction-active surfaces based on free-standing anchored cellulose nanofibrils. ACS Appl. Mater. Inter. 2018, 10, 37566–37574. [Google Scholar] [CrossRef] [PubMed]
- North, H.M.; Berger, A.; Saez-Aguayo, S.; Ralet, M.-C. Understanding polysaccharide production and properties using seed coat mutants: Future perspectives for the exploitation of natural variants. Ann. Bot. 2014, 114, 1251–1263. [Google Scholar] [CrossRef]
- García-Fayos, P.; Cerda, A. Seed losses by surface wash in degraded Mediterranean environments. Catena 1997, 29, 73–83. [Google Scholar] [CrossRef]
- García-Fayos, P.; Bochet, E.; Cerdà, A. Seed removal susceptibility through soil erosion shapes vegetation composition. Plant Soil 2010, 334, 289–297. [Google Scholar] [CrossRef]
- Huang, Z.; Boubriak, I.; Osborne, D.J.; Dong, M.; Gutterman, Y. Possible role of pectin-containing mucilage and dew in repairing embryo DNA of seeds adapted to desert conditions. Ann. Bot. 2008, 101, 277–283. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Tadele, Z.; Gola, E.M. Slime cells on the surface of Eragrostis seeds maintain a level of moisture around the grain to enhance germination. Seed Sci. Res. 2009, 19, 27–35. [Google Scholar] [CrossRef]
- Huang, Z.; Yitzchak, G.; Zhenghai, H. Structure and function of mucilaginous achenes of Artemisia monosperma inhabiting the Negev desert of Israel. Isr. J. Plant Sci. 2000, 48, 255–266. [Google Scholar] [CrossRef]
- Pan, V.S.; McMunn, M.; Karban, R.; Goidell, J.; Weber, M.G.; LoPresti, E.F. Mucilage binding to ground protects seeds of many plants from harvester ants: A functional investigation. Funct. Ecol. 2021, 35, 2448–2460. [Google Scholar] [CrossRef]
- Vazačová, K.; Münzbergová, Z. Simulation of seed digestion by birds: How does it reflect the real passage through a pigeon’s gut? Folia Geobot. 2013, 48, 257–269. [Google Scholar] [CrossRef]
- Lee, K.; Kreitschitz, A.; Lee, J.; Gorb, S.N.; Lee, H. Localization of phenolic compounds at an air–solid interface in plant seed mucilage: A strategy to maximize its biological function? ACS Appl. Mater. Inter. 2020, 12, 42531–42536. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Zhang, W.; Huang, Z. Degradation of seed mucilage by soil microflora promotes early seedling growth of a desert sand dune plant. Plant Cell Environ. 2012, 35, 872–883. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, N.; Koyama, K.; Okamoto, A.; Katayama, K.; Kato, Y.; Mimura, N.; Okoshi, S.; Tanaka, Y. Seed Mucilage Promotes Dispersal of Plantago asiatica Seeds by Facilitating Attachment to Shoes. Sustainability 2022, 14, 6909. https://doi.org/10.3390/su14116909
Abe N, Koyama K, Okamoto A, Katayama K, Kato Y, Mimura N, Okoshi S, Tanaka Y. Seed Mucilage Promotes Dispersal of Plantago asiatica Seeds by Facilitating Attachment to Shoes. Sustainability. 2022; 14(11):6909. https://doi.org/10.3390/su14116909
Chicago/Turabian StyleAbe, Nanako, Kohei Koyama, Azumi Okamoto, Kowa Katayama, Yura Kato, Natsuki Mimura, Shoji Okoshi, and Yuki Tanaka. 2022. "Seed Mucilage Promotes Dispersal of Plantago asiatica Seeds by Facilitating Attachment to Shoes" Sustainability 14, no. 11: 6909. https://doi.org/10.3390/su14116909
APA StyleAbe, N., Koyama, K., Okamoto, A., Katayama, K., Kato, Y., Mimura, N., Okoshi, S., & Tanaka, Y. (2022). Seed Mucilage Promotes Dispersal of Plantago asiatica Seeds by Facilitating Attachment to Shoes. Sustainability, 14(11), 6909. https://doi.org/10.3390/su14116909





