1. Introduction
The global consumption of textile fibers is increasing annually, with polyester (PET) accounting for more than half of the total fiber volume [
1]. With increased consumption follows increased waste generation, and currently textile waste is predominately sent to landfill or incineration. Globally, less than 1% of all textile waste is recycled into fibers for use in new textile products [
2]. However, there are often clear environmental benefits, e.g., reduced carbon footprint and reduced energy consumption, to reuse or recycle textiles [
3].
An attractive recycling route for PET waste textiles is chemical recycling through depolymerization. Depolymerization of PET textile waste produces monomers, which may substitute the use of monomers synthesized from fossil resources. PET produced by polymerization of monomers from such chemical recycling has the potential to hold an equal quality to virgin materials. The polyester derived monomers can also substitute fossil-derived chemicals in the production of other value-added materials, such as alkyd resins [
4], reactive polyester resins [
5], or polyurethanes [
6]. This is to be compared with thermomechanical recycling, in which PET is melted and reshaped, which typically yields materials with lowered molecular weight and inferior mechanical properties [
7]. Chemical recycling has also proven feasible for the separation of PET from blended textiles. Selective depolymerization of PET from cellulose/PET blends has been reported as an attractive opportunity to separate and recover the blend components for further processing into value-added products [
8,
9].
Depolymerization of polyester yields the monomers of the textile. The majority of polyester textiles are composed of poly(ethylene terephthalate) (PET), formed by a condensation reaction between terephthalic acid (TPA) and ethylene glycol (EG). The textile nomenclature, however, does not specify that polyester textile must be composed of PET, but also allows other types of polyesters. Further, co-monomers might be present in the polymer chain to infer certain properties, such as increased dyeability [
10] or flame retardance [
11]. The scientific literature available on depolymerization of textile PET is sparse, while there is a large body of literature on PET packaging depolymerization, predominantly from bottle waste [
12,
13]. Textile recycling is heavily underdeveloped and new solutions are urgently needed, especially considering that the volumes of polyester produced for textile purposes are twice as high as PET produced for packaging purposes. About 55 million tonnes of PET staple fibers were put on the market 2019 [
14], while PET for packaging amounted to about 25 million tonnes [
15]. Studies on recycling of textile PET are, hence, highly relevant.
In chemical recycling, the ester bond of polyesters is commonly cleaved by solvolytic methods; the main ones being glycolysis, methanolysis, and hydrolysis. These solvolytic methods all render small-molecule building blocks of PET with slightly different chemistries. Recently, novel methods, including enzymatic degradation of PET [
16] and solid-state reaction methods [
17], have been proposed; however, these are still at an early research state.
Industrially, glycolysis is the oldest and most common method for PET depolymerization [
18]. It should be noted, however, that its widespread use is limited by the low profitability of PET glycolysis, or any solvolytic recycling method, due to competition with cheap virgin petrochemical feedstock [
19]. Glycolysis of PET in EG produces bis(hydroxyethyl terephthalate) (BHET), which is an intermediate in the PET polymerization reaction. While it is possible to polymerize PET from BHET, it is not commonly done and manufacturers favor TPA as a feedstock for PET production due to its greater process performance [
19]. The yields of the hydrolysis reaction are typically high, approaching 100% while both glycolysis and methanolysis typically have lower yields [
18,
20]. Furthermore, hydrolysis can be conducted under mild conditions, renders monomers of high purity, and can tolerate contaminated waste streams.
The present research covers depolymerization of textile PET using alkaline hydrolysis. Alkaline hydrolysis of textile cellulose/PET blends has the advantage that the aqueous alkaline environment causes hydrolytic cleavage of the ester bond of PET, while cellulose is relatively resistant to alkali degradation [
21]. Hence, cellulose can be recovered in its polymeric form, suitable for recycling into regenerated fibers or microcrystalline cellulose, a topic discussed in Part I of this publication series. While several studies exist on the reaction conditions, kinetics and mechanism of PET depolymerization using alkaline hydrolysis [
18,
22,
23,
24,
25] textile PET is neglected. Studies typically use granulates of virgin PET or post-consumer PET in the form of flakes or powders as the polyester source. Processing of PET into fibers, however, renders an increased crystallinity and a different morphology compared to bulk PET [
26]. Further, PET used for textile applications has a lower molecular weight compared to PET used for bottles [
27].
From the literature on nontextile polyester, there are indications that hydrolysis takes place at the external surface of the solid-state PET and that the major decomposition reaction occurs at the ends of the polymer chains [
25]. López-Fonseca et al. found that the residual particle size of PET-particles decreased during hydrolysis while no structural differences were observed [
22,
28]. Similarly, Mishra et al. found that the molecular-weight distribution (MWD) of PET was not changed over hydrolysis times of 90 min at temperatures ranging from 25 to 150 °C [
29]. A large impact of particle size on the rate of hydrolysis, with a decrease in hydrolysis efficacy as a function of increased particle size, has been reported. The rection rate of hydrolysis has been shown to be proportional to the particle surface area both for alkaline [
28,
29] and acidic hydrolysis [
30,
31].
In the present study, hydrolysis of a PET fabric is studied under mild conditions: 90 °C, 5 wt% NaOH, and atmospheric pressure. While this publication deals exclusively with the depolymerization of textile PET, the reaction conditions are optimized for separation and recovery of a cellulose/PET blend. The hydrolysis rate is explored as a function of PET accessible area, while comparing laundered and non-laundered fabric, as well as shredded fabric. The reaction products, as well as residual PET, is thoroughly characterized, using both spectroscopic and chromatographic methods.
2. Materials and Methods
2.1. Materials
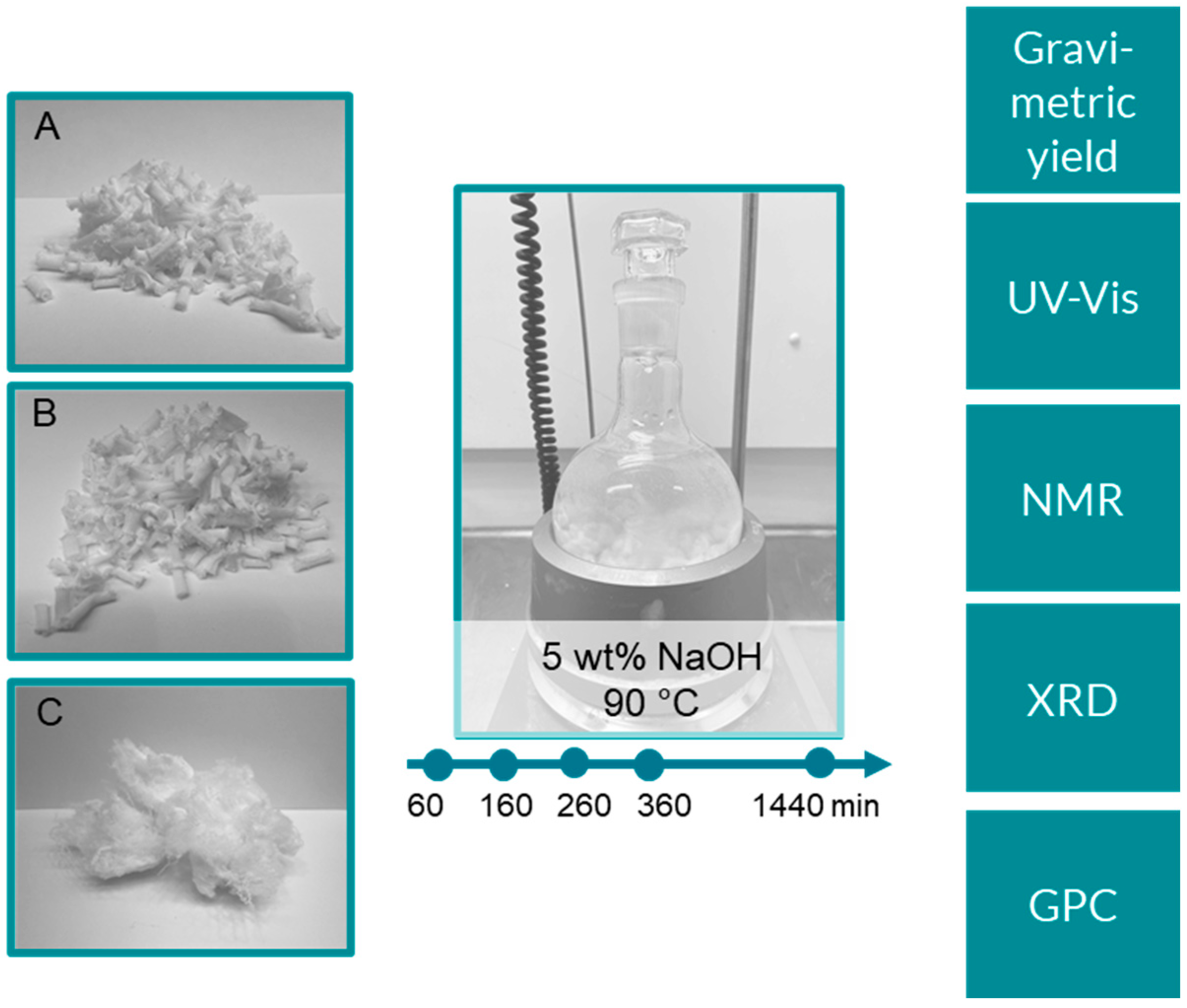
The never-used PET tricot fabric was supplied by a Swedish fashion company. The fabric was either used as received or laundered, and subsequently cut into approximately 1 × 1 cm pieces. Washing was performed at 60 °C, according to Swedish standard SS-EN ISO 6330:2012. A portion of the washed fabric was also shredded (New Shunxing, NSX-QT 310). Sodium hydroxide (NaOH, 50%) and sulfuric acid (H2SO4, reagent grade, 95–97%) were obtained from Sigma-Aldrich, and acetic acid (glacial, 99%,) was obtained from Fisher Scientific and used as received.
2.2. Hydrolysis of PET
Alkaline hydrolysis of PET was performed in a 500 mL glass reactor. The NaOH concentration was 5 wt% and the weight ratio of sample to NaOH solution was kept at 1:100. The aqueous NaOH was heated to 90 °C before adding 5.0 g of oven dry (2 h, 105 °C) sample to the reaction vessel. Hydrolysis was performed for the selected time (60–1440 min). The reaction was quenched by immersing the reactor in an ice bath. Post reaction, the solid residue was separated from the reaction solution by filtration, using a tight-knit PET wire fabric as the barrier. The solids were neutralized in 5% acetic acid and thereafter washed with water and dried at 105 °C for 4 h to calculate the gravimetric yield. The liquid phase was kept in the freezer for further analysis.
2.3. Characterization of Fabric
All analyses on the fabric were made on the laundered sample. The fabric was first dried at 50 °C for 2 h and thereafter conditioned for at least 16 h at 25 °C, 65% relative humidity.
The density (GSM) was determined from 100 cm2 samples punched out from different sampling areas on the fabric. Samples were weighed and the density (g m−2) was calculated. A total of 5 sampling points were used.
The thickness of the fabric was measured according to SS-EN ISO 5084, using a Shirley thickness tester. The sample area was 100 cm2, and the pressure was 1 kPa. A total of 5 sampling points were used.
The structure of yarns and fibers were studied using a microscope (Nikon Eclipse). Prior to examination the fabric was disintegrated by means of carding to reveal the individual yarns and fibers.
2.4. Characterization of Solid Residue
Molecular-weight determination was performed by Smithers, UK. A total of 20 mg of solid residue was dissolved in hexafluoropropan-2-ol overnight. The solution was then filtered through a 0.45 µm PTFE membrane into autosampler vials and inserted into the column (Malvern/Viscotek TDA 301) equipped with a refractive index detector. Poly(methyl methacrylate) was used for calibration.
Wide-angle X-ray scattering (WAXS) measurement was performed at Chalmers University of Technology (Mat:Nordic, SAXSLAB) with a 0.9 mm beam diameter Rigaku 003+ high brilliance microfocus Cu-radiation source at 130 mm distance with the sample irradiated over 20 min.
2.5. Characterization of Filtrate
The TPA-concentration in the filtrate was measured using UV-absorption at 242 nm by UV spectrophotometry (UV spectrometer, Analytik Jena). A linear calibration curve was constructed from 5 measurements of 2 to 10‰ TPA in 5% NaOH.
Further, a portion of each filtrate was carefully weighed and acidified to pH 2–3 by the addition of 4 M H2SO4, which causes TPA to precipitate. The TPA precipitates were separated using a glass microfiber filter and rinsed with distilled water, and then the weight of TPA was determined after drying for 4 h at 105 °C.
To study the degradation products, nuclear magnetic resonance (NMR) spectroscopy was used. A small-scale hydrolysis of PET in 5% NaOH and D2O was performed to reduce the water signal obtained in the spectra. The reaction conditions were, in other aspects, identical to the large-scale experiments, i.e., 90 °C, 5 wt% NaOH and reaction times between 60 and 1440 min. The hydrolysis filtrate was analyzed with NMR spectroscopy. The NMR measurements were conducted on a Varian 400-MR spectrometer operating at 9.4 T, equipped with a OneNMRProbe. The 1H-NMR spectrum parameters included a 5 ms 1H-detection pulse, 2.5 s acquisition time, 2 s recycle delay, and 32 scans. The samples were studied using D2O as solvent, and the chemical shifts were referenced to the residual solvent signal.
3. Results and Discussion
Three textile samples, with different accessibility, were prepared from the same virgin, never-laundered PET tricot fabric and subjected to alkaline hydrolysis (
Figure 1). Sample A was made by cutting the never-laundered tricot into 1 × 1 cm squares. Sample B was made by laundering the sample at 60 °C to remove surface finishing and other processing aids remnant on the fabric. The sample was subsequently sectioned in the same way as sample A. Sample C was made by shredding the laundered fabric in an industrial textile shredder. During this process, the fabric is disintegrated, and the individual threads and fibers liberated, increasing the surface area. As such, samples A–C with an increasing accessibility were produced. Further, an attempt was made to grind the fabric into even smaller fragments (powder) using a rotor mill. However, this sample was not included in the hydrolysis series as, apart from the desired fine fibril grinds, part of the sample melted and formed small crumbles. The sample was excluded as this two-phase material would not be representative.
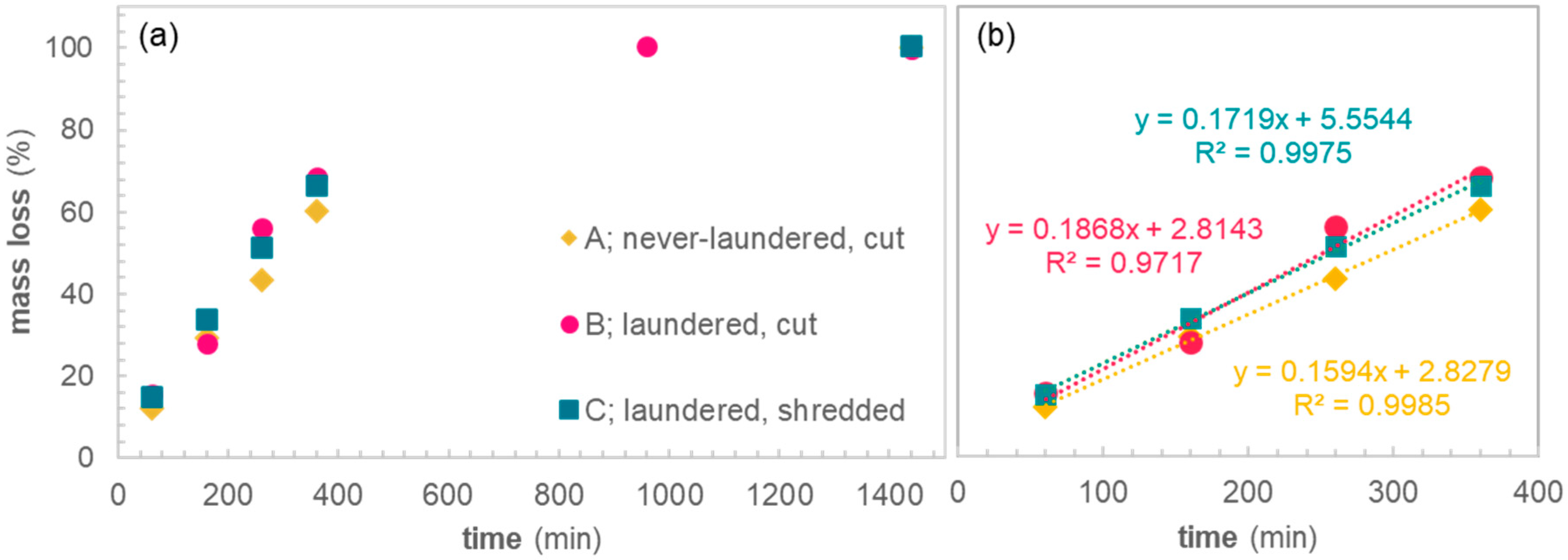
Alkaline hydrolysis was performed under mild conditions during 60, 160, 260, 360, or 1440 min. As expected, the mass loss of samples increases with hydrolysis time (
Figure 2a). After 1440 min reaction time, all samples show 100% mass loss. For sample B, an additional datapoint was taken at 960 min, which also shows 100% mass loss. We assumed an increasing accessibility along samples A–C, due to removal of finishing agents and an increase in surface area. However, the differences in mass loss between the samples at each of the measuring points is small, which was surprising.
The initial hydrolysis rate of the never-laundered sample is slightly lower compared to the laundered samples, which is signified by the flatter slope of the linear fit of sample A as compared to sample B and C (
Figure 2b). This is expected as it is known that surface finishing agents or processing aids remnant on the fabric surface often decrease the rate of hydrolysis [
32]. However, the accessibility of fibers in terms of surface area does not seem to influence the hydrolysis rate. This indicates that mechanical pretreatment of the PET textile to increase the surface area prior to alkaline hydrolysis does not yield a higher rate of hydrolysis. These results were somewhat surprising, as hydrolysis of PET is considered a surface specific reaction with a strong correlation between particle size and reaction rate.
We went on to study the yarn and fabric structure to explain the negligible impact of shredding on the reaction rate. The unraveled yarn was studied in a light microscope and assigned as a non-twisted, texturized multifilament. Texturing is typically performed on synthetic filaments and refers to the formation of crimp, loops, coils, or crinkles in the filament. This treatment decreases the density of yarns spun from texturized filaments and creates air pockets, which gives a different handle of fabrics, as well as improved ventilation and absorbency. The thickness of the sample at a pressure of 1 kPa was 0.6 ± 0.02 mm while the density was 132 ± 2 g m2, which is in the lower range of knit fabrics. Given the texturing of the yarn and the low density of the fabric, the accessibility of the textile to hydrolysis should not be considerably impacted by shredding, which is in line with our findings. Texturing of synthetic yarns is a common practice throughout the fashion industry, hence, shredding of PET fabrics can tentatively be omitted without impacting the kinetics of the hydrolysis reaction. This is advantageous for the recycling scheme of textiles as it reduces the number of pretreatment steps and consequently the energy consumption.
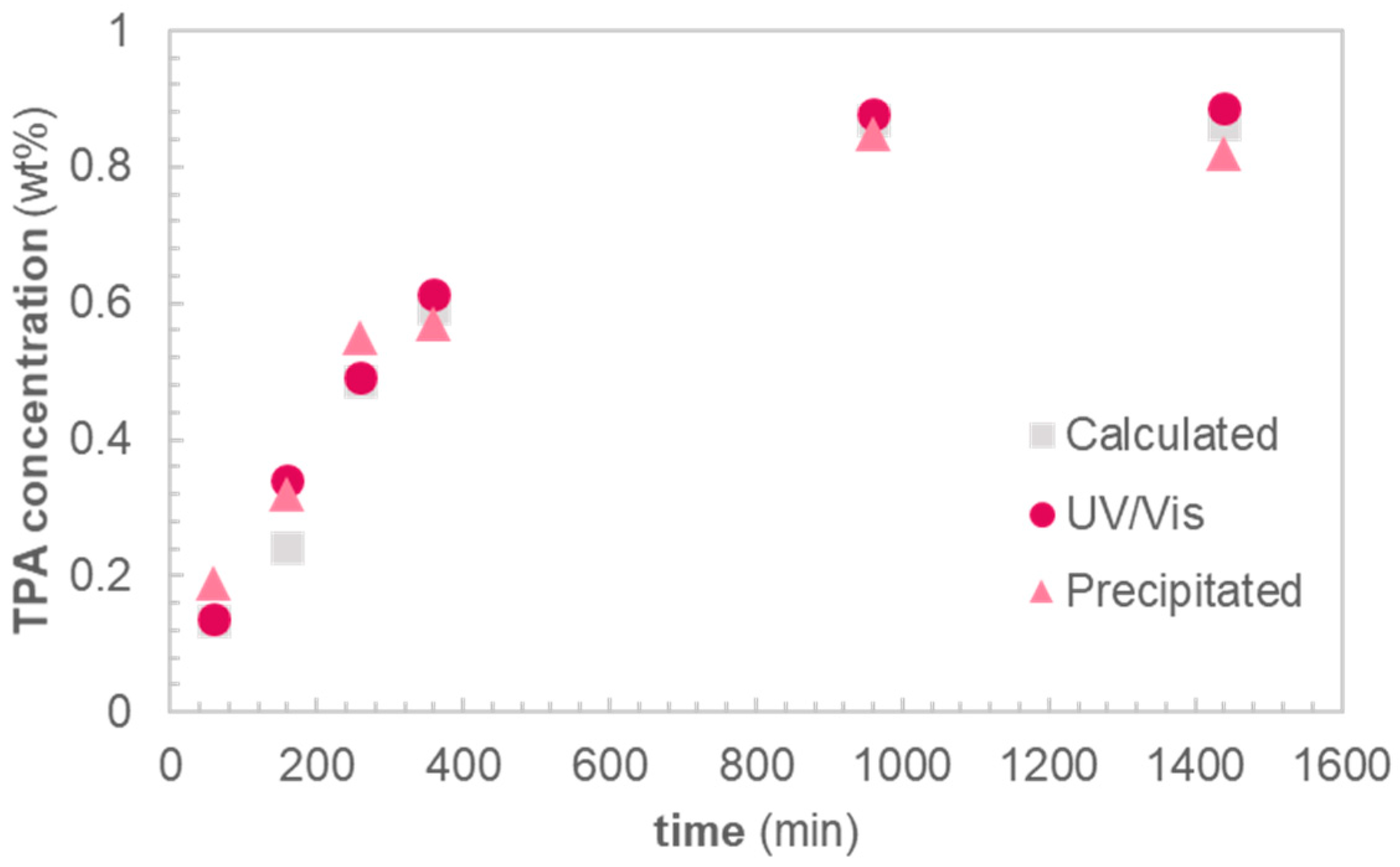
Next, the yield of TPA was compared with the mass loss of PET, in order to determine whether PET is completely depolymerized into TPA and EG, or also yields other degradation products. The theoretical yield was calculated based on the mass loss at each time interval, assuming that PET is formed by the esterification of equimolar amounts of TPA and EG. The calculated theoretical yield is compared to the experimental yield of TPA obtained via gravimetric or spectroscopic means (c.f. Experimental). Gravimetric yields were obtained by precipitation of TPA from carefully weighed portions of the reaction filtrate. Spectroscopic yields were obtained by UV-Vis spectroscopy of the reaction filtrate at 242 nm, a wavelength where the anionic form of TPA, present under alkaline conditions, shows a strong UV absorbance.
There is an excellent agreement between the theoretical yield and the yield obtained from UV absorption spectroscopy, indicating complete degradation of PET into TPA and EG (
Figure 3). Gravimetric data on the mass of precipitated TPA from the reaction filtrate also show good agreement with the theoretical yields. The conversion from degraded PET to TPA at each timepoint, hence, is close to 100%.
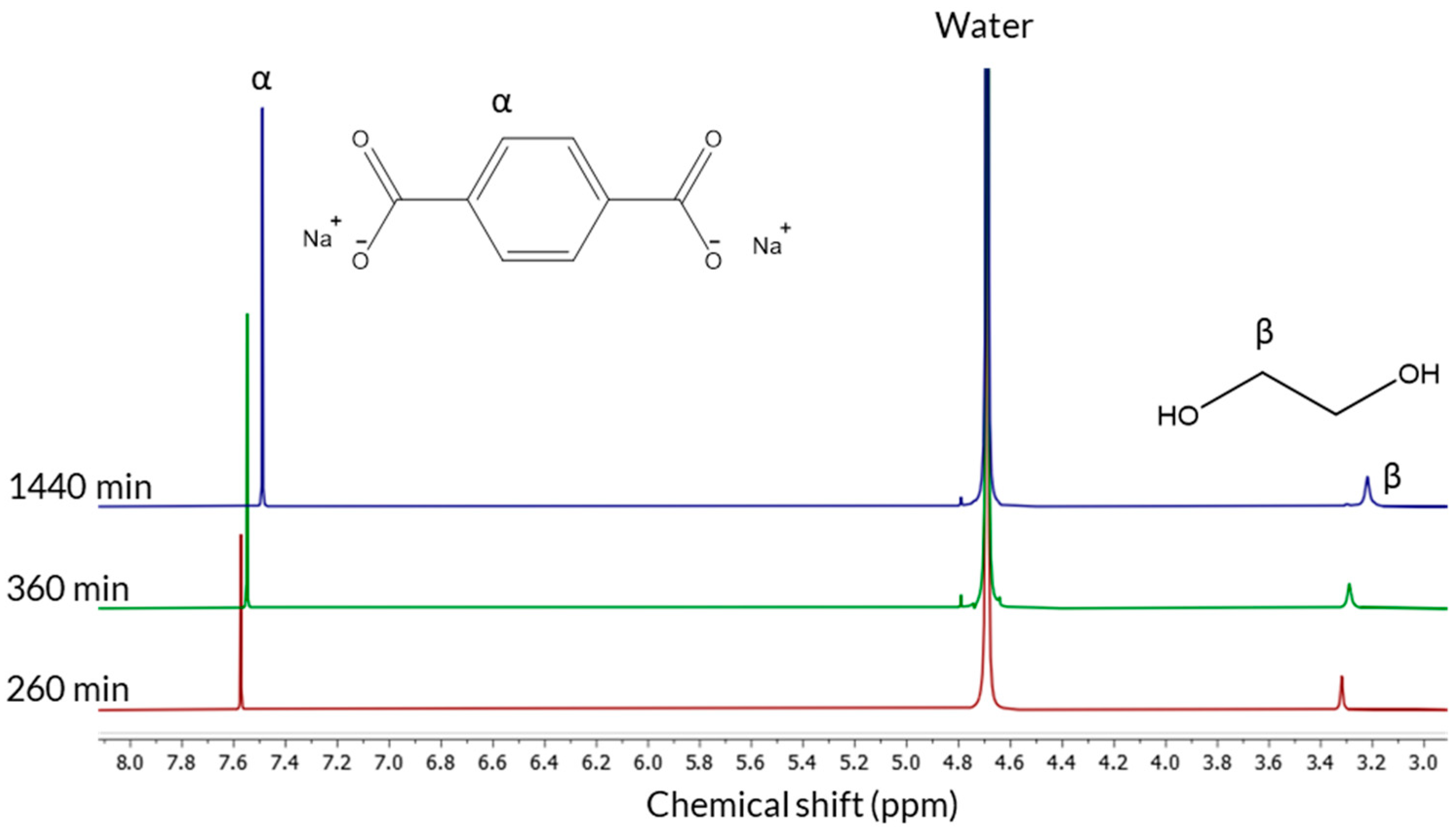
Nuclear magnetic resonance (NMR) spectroscopy was used to further verify the reaction course and purity of products. In
Figure 4 1H-NMR spectra of samples obtained after 260, 360, and 1440 min are shown.
For all spectra, in addition to the residual solvent signal, two singlets can be seen, one around 7.5 ppm and one around 3.3 ppm, assigned to originate from TPA and EG, respectively. An expected increase in the integrals for both components can be seen with increased hydrolysis time. For each time point, the integral of the signals from the protons complies well with each other indicating a 1:1 molar ratio of the two components, as expected from the starting material.
The presence of two singlets is distinct proof that only monomers of TPA and EG are present in the sample. If oligomers were present, a much more complicated splitting structure of the signals from the TPA and EG protons would be seen. One could imagine an oligomer where one, or the other, signal would appear as a singlet; however, the other cannot in that case appear as a singlet. One could also imagine that for a large polymer the protons will appear as two singlets; however, in addition to the fact that such a polymer would not be soluble, the narrow line width of the singlets indicates a small molecule, such as a monomer, rather than a large polymer. The end groups would, in that case, also be visible in the spectrum, which is not the case. The slight change in the chemical shift of the two components between different time points could be attributed to a change in pH, as expected upon consumption of hydroxide ions during depolymerization.
Once the effectiveness of alkaline hydrolysis on textile PET and the impact of accessibility was established, we moved on to study the degradation mechanism. TPA yields were close to the theoretical maximum at each time point during hydrolysis, indicating that the polymer is depolymerized from the chain ends via a peeling reaction, in agreement with earlier results from Kumar and Guria [
25]. Further, NMR analysis showed pure TPA, with no visible traces of oligomers or other degradation products, which further supports a peeling reaction from the chain ends.
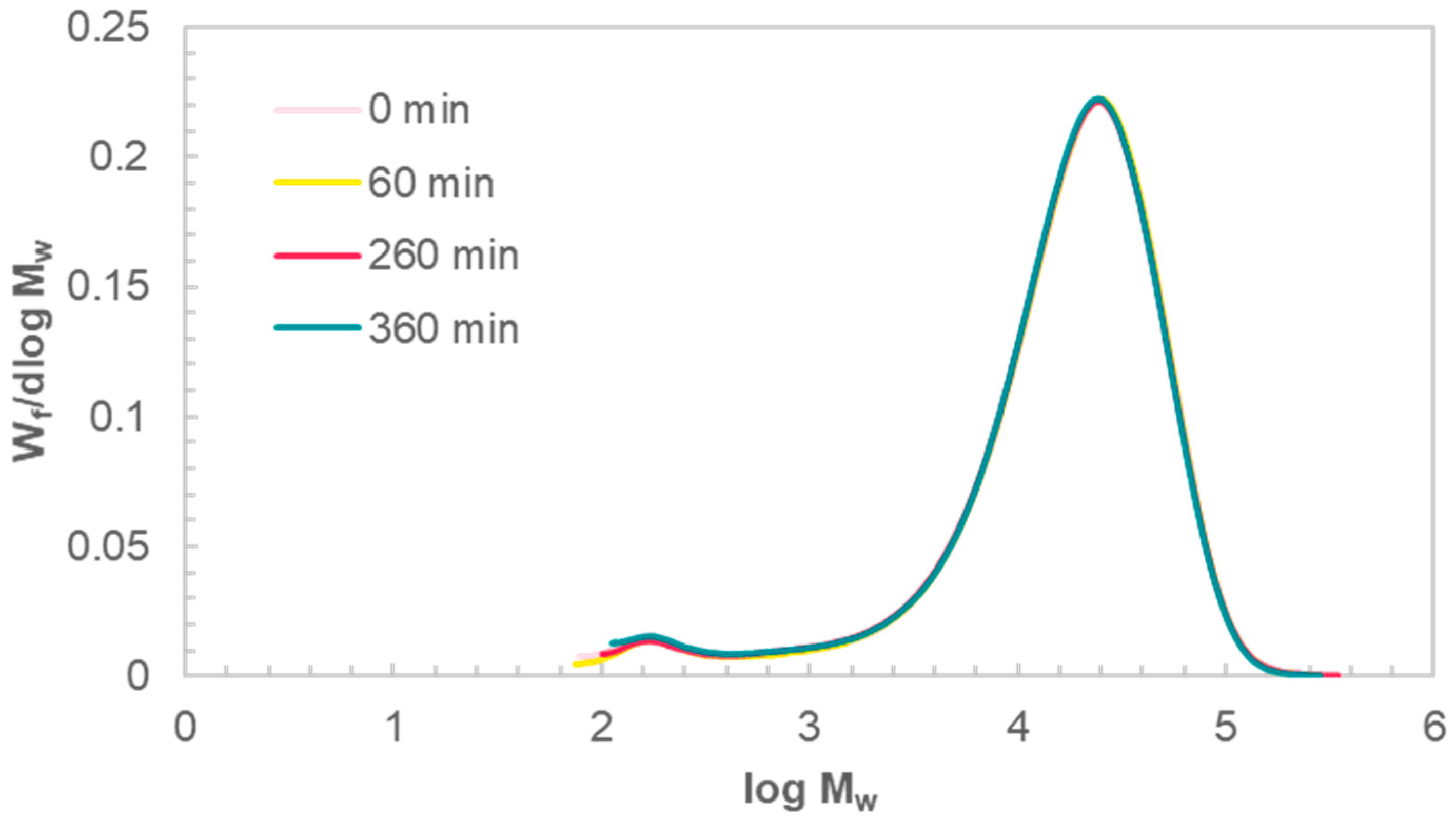
Molecular-weight distributions (MWD) were obtained by GPC for the solid residue of sample B after hydrolysis for different times. The MWD is identical for samples run for 60, 260, or 360 min (
Figure 5). GPC of the sample run for 1440 min could not be performed as no solid residue was left. The overlapping curves show no changes to the MWD over the course of reaction. This indicates that chain scission, which would drastically decrease the molecular weight, is not prevalent.
Further, it confirms that the reaction proceeds predominantly through endwise peeling and is surface specific. Water and the hydrated OH
− catalyzing the hydrolysis have a low penetration into PET, also at the reaction temperature, 90 °C. At 90 °C, PET is still below the glass transition temperature, the temperature at which the polymer chains in the amorphous regions gain considerable mobility. However, even above the glass transition temperature, water penetration into the PET matrix is minimal, with typical water regains of below 0.5% [
26]. The low penetration of the reaction medium into the polymer, together with the identical MWD over the course of reaction, confirms that the bulk of PET is not affected by hydrolysis but the reaction is surface specific.
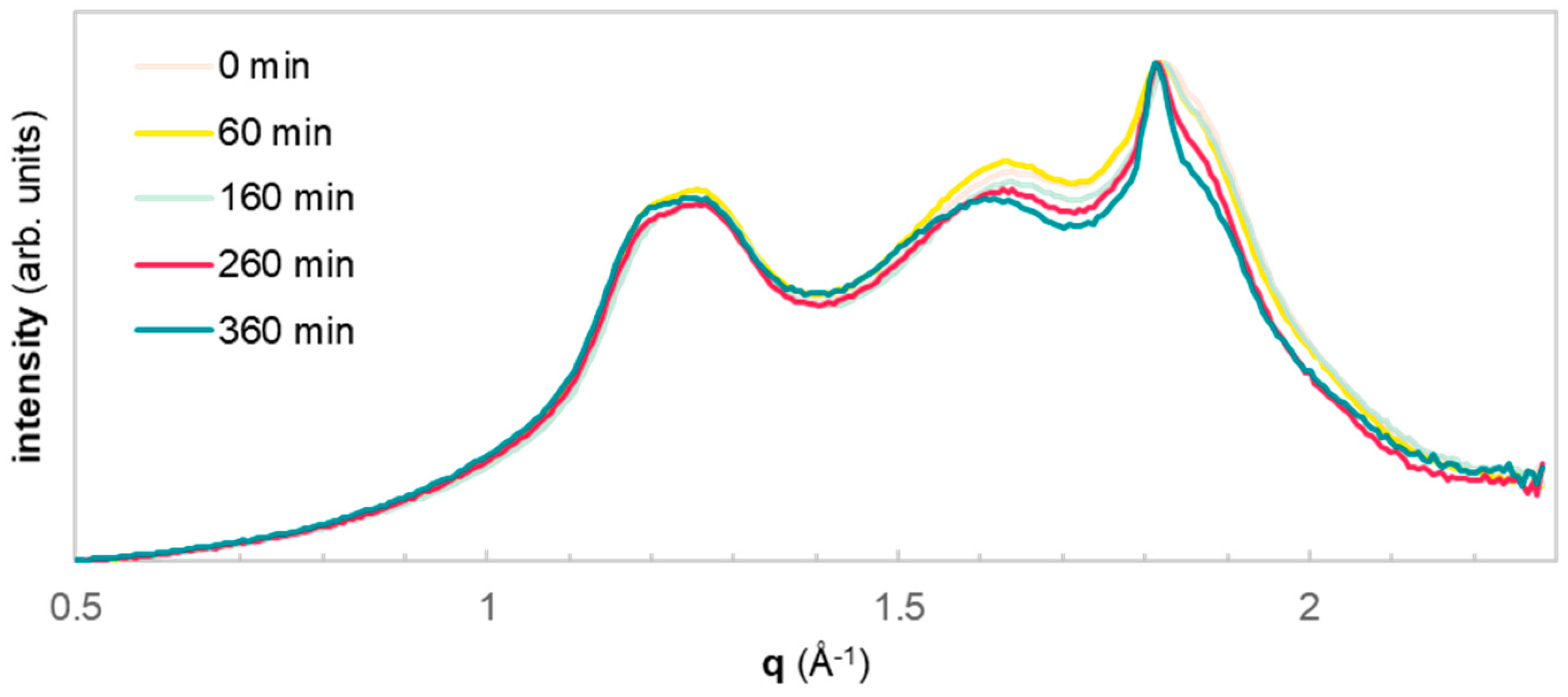
Further, the microstructure of samples was assessed using WAXS (
Figure 6). The peaks centered at 1.25, 1.63, and 1.82 refer to crystal planes (010), (−110), and (100) [
33]. In terms of resolution, the peaks at higher q-number show a slight increase with hydrolysis time. This sharpening of peaks may be related to a slight increase in crystallinity. Similarly, to the GPC chromatograms, the curves are overlapping, indicating that the morphology is not significantly changed over the course of the reaction, in line with a surface specific reaction.