The Relationship between Hydrological Connectivity Changes Inside and Outside Biodiversity Hotspots and Its Implication for Sustainable Environmental Management
Abstract
:1. Introduction
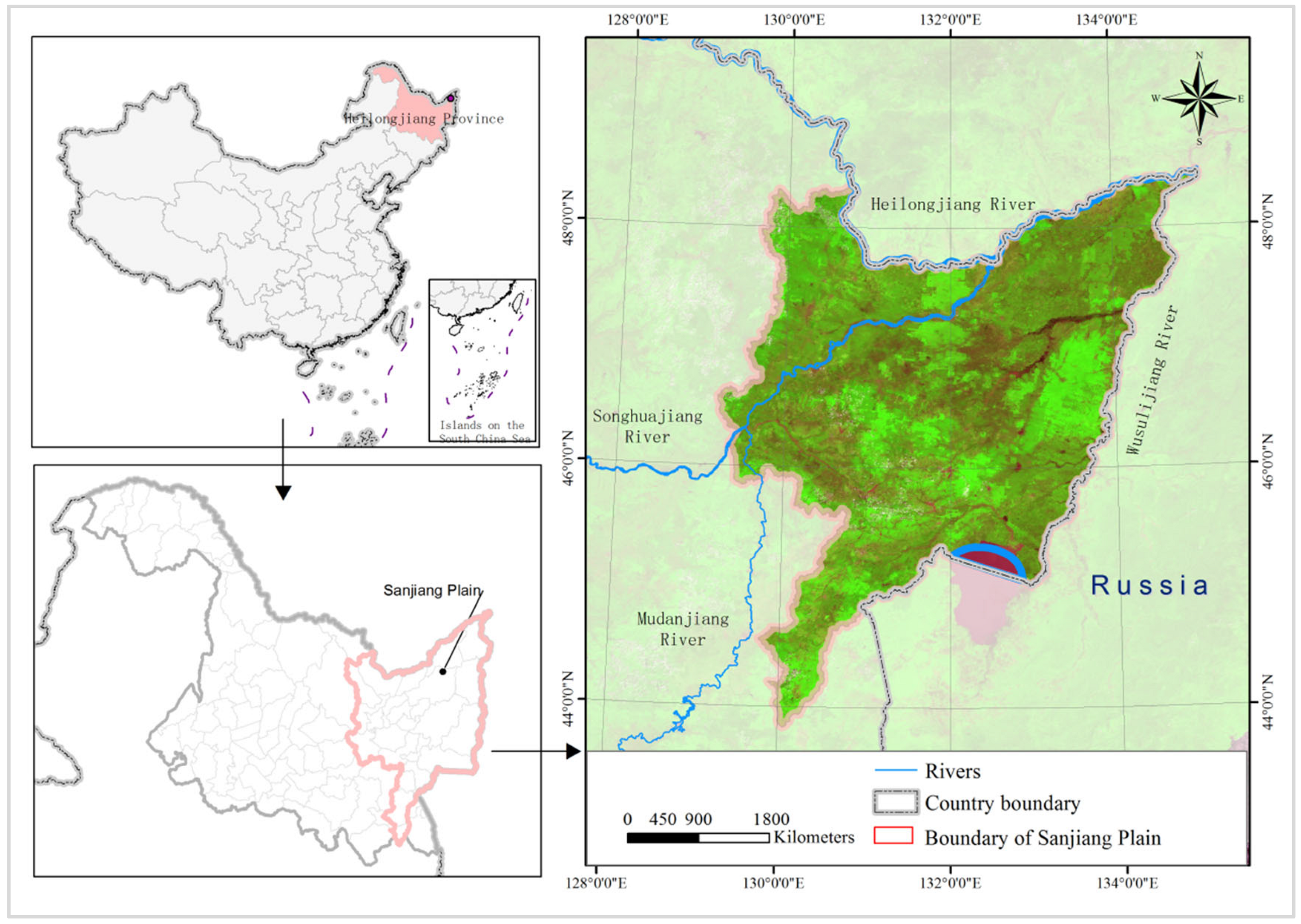
2. Overview of the Study Area
3. Research Methods
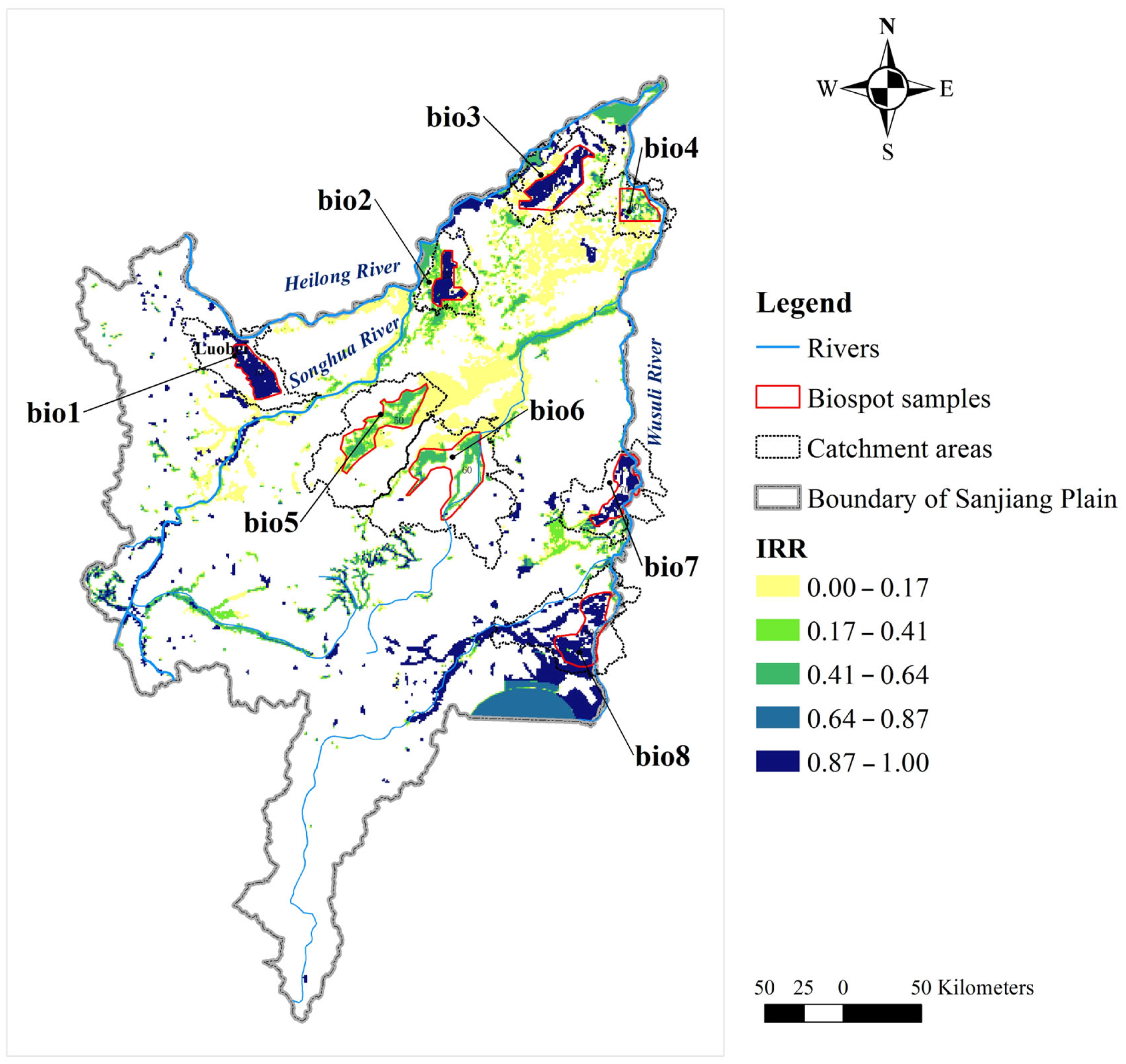
3.1. Identification of Biodiversity Hotspots
3.2. Relationship between Hydrological Connectivity and Pattern Changes Inside and Outside of Biodiversity Hotspots
3.2.1. Extraction of Wetland Distribution Based on NDWI
3.2.2. Changing Trends of Hydrological Connectivity Inside Biodiversity Hotspots
3.2.3. Relationship between Hydrological Connectivity and Pattern Changes Inside and Outside of Biodiversity Hotspots
4. Results
4.1. Identification of Biodiversity Hotspots
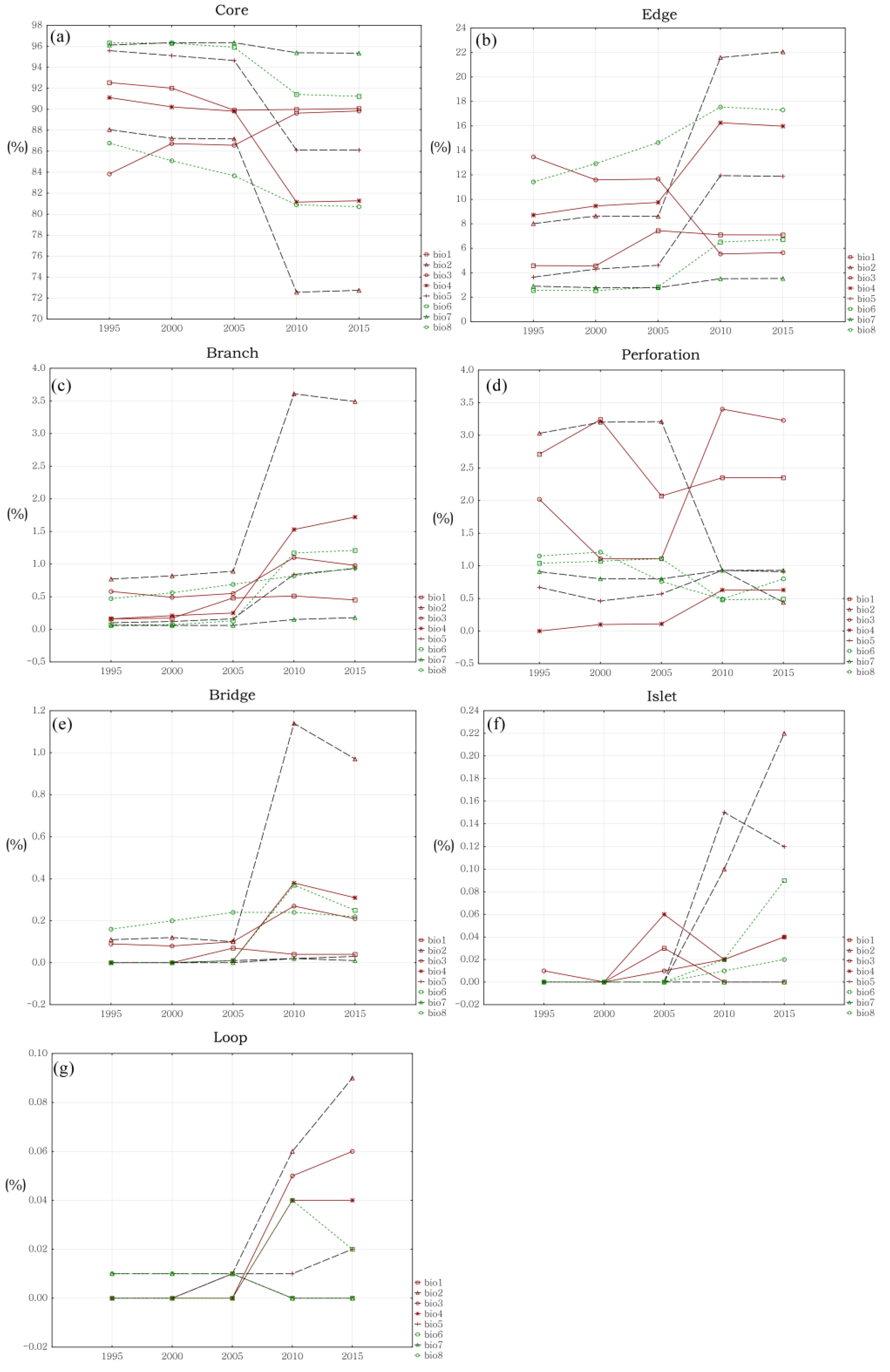
4.2. Changing Trends of Hydrological Connectivity Inside Biodiversity Hotspots
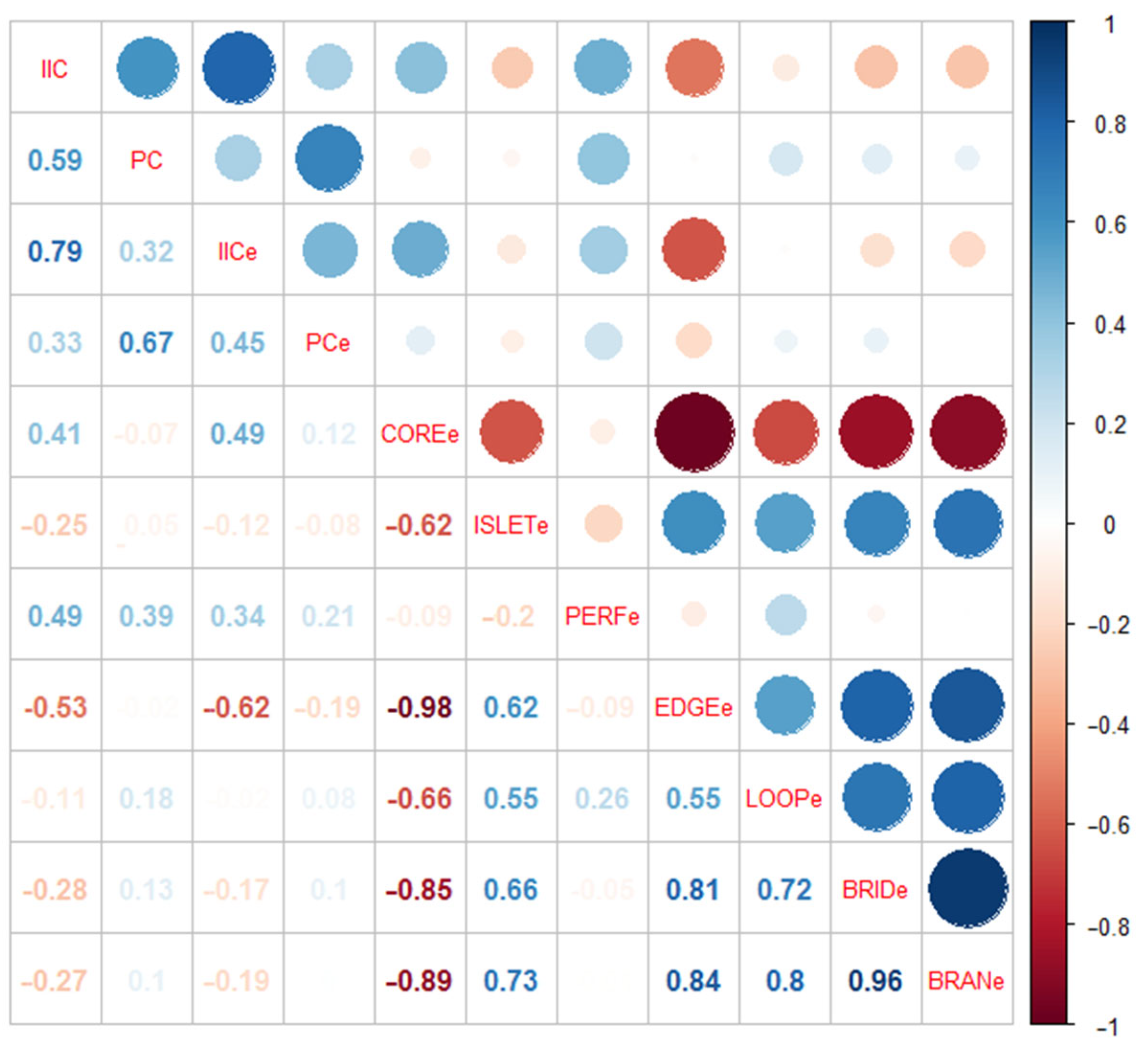
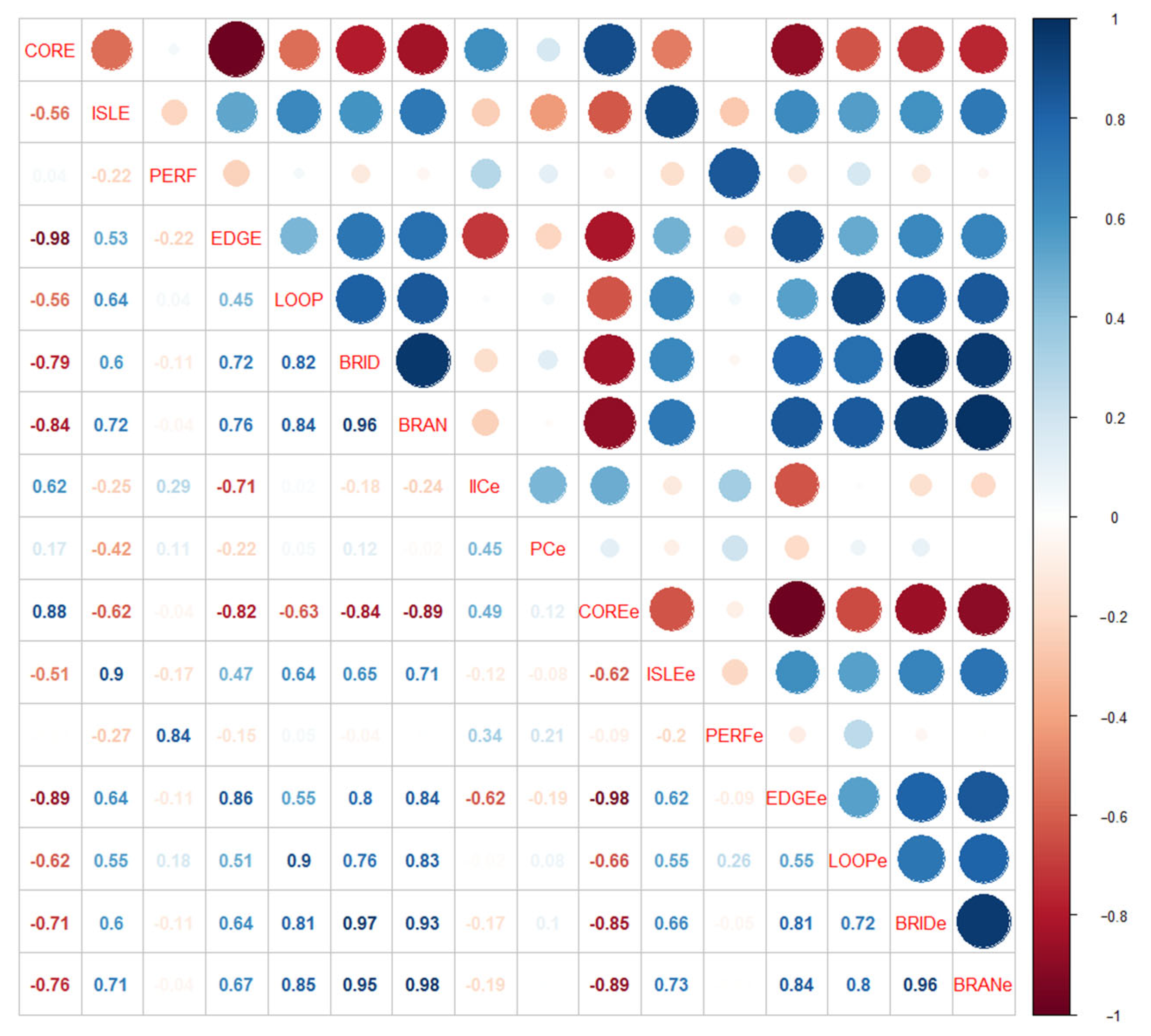
4.3. Relationship between Hydrological Connectivity Changes Inside and Outside of Biodiversity Hotspots
4.3.1. Effects of External Hydrological Connectivity on Internal Structural Hydrological Connectivity
4.3.2. Effects of External Hydrological Connectivity on Internal Functional Hydrological Connectivity
5. Discussions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Liu, Y.; Ou, C.; Gabor, S. Examining water quality effects of riparian wetland loss and restoration scenarios in a southern Ontario watershed. J. Environ. Manag. 2016, 174, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F. Cumulative Hydrologic Impact of Wetland Loss: Numerical Modeling Study of the Rideau River Watershed, Canada. J. Hydrol. Eng. 2014, 19, 593–606. [Google Scholar] [CrossRef]
- Quesnelle, P.E.; Fahrig, L.; Lindsay, K. Effects of habitat loss, habitat configuration and matrix composition on declining wetland species. Biol. Conserv. 2013, 160, 200–208. [Google Scholar] [CrossRef]
- Freeman, M.C.; Pringle, C.M.; Jackson, R.C. Hydraulic connectivity and the contribution of stream headwaters to ecological integrity at regional scales. J. Am. Water Resour. Assoc. 2007, 43, 5–14. [Google Scholar] [CrossRef]
- Bracken, L.J.; Wainwright, J.; Ali, G.A.; Tetzlaff, D.; Smith, M.W.; Reaney, S.M.; Roy, A.G. Concepts of hydrological connectivity: Research approaches, pathways and future agendas. Earth Sci. Rev. 2013, 119, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indexes: Towards the priorization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Pedroli, B.; Pinto-Correia, T.; Cornish, P. Landscape—What’s in it? Trends in European Landscape Science and Priority Themes for Concerted Research. Landsc. Ecol. 2006, 21, 421–430. [Google Scholar] [CrossRef]
- Cui, Z.; Shen, H.; Zhang, G.X. Changes of Landscape Patterns and Hydrological Connectivity of Wetlands in Momoge National Natural Wetland Reserve and Their Driving Factors for Three Periods. Wetl. Sci. 2016, 14, 866–873. [Google Scholar]
- Zhang, M.M.; Wu, X.Q. Changes in hydrological connectivity and spatial morphology of Baiyangdian wetland over the last 20 years. Acta Ecol. Sin. 2018, 38, 4205–4213. [Google Scholar]
- Amoros, C.; Bornette, G. Connectivity and biocomplexity in waterboies of riverine floodplain. Freshw. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Simioni, J.P.D.; Guasselli, L.A.; Etchelar, C.B. Connectivity among Wetlands of EPA of Banhado Grande, RS. RBRH 2017, 22, UNSP e15. [Google Scholar] [CrossRef] [Green Version]
- Khoi, D.N.; Nguyen, V.T.; Sam, T.T.; Nhi, P.T.T. Evaluation on Effects of Climate and Land-Use Changes on Streamflow and Water Quality in the La Buong River Basin, Southern Vietnam. Sustainability 2019, 11, 7221. [Google Scholar] [CrossRef] [Green Version]
- Nie, W.; Yuan, Y.; Kepner, W.; Nash, M.S.; Jackson, M.; Erickson, C. Assessing impacts of Landuse and Landcover changes on hydrology for the upper San Pedro watershed. J. Hydrol. 2011, 407, 105–114. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain system. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Tockner, K.; Schiemer, F.; Ward, J.V. Conservation by restoration: The management concept for a river-floodplain system on the Danube river in Austria. Aquat. Conserv. Mar. Freshw. Ecosyst. 1998, 8, 71–86. [Google Scholar] [CrossRef]
- Thoms, M.C.; Southwell, M.; McGinness, H. Floodplain–river ecosystems: Fragmentation and water resources development. Geomorphology 2005, 71, 126–138. [Google Scholar] [CrossRef]
- Ruiz, L.; Parikh, N.; Heintzman, L.J.; Collins, S.D.; Starr, S.M.; Wright, C.K.; Henebry, G.M.; van Gestel, N.; McIntyre, N.E. Dynamic connectivity of temporary wetlands in the southern Great Plains. Landsc. Ecol. 2014, 29, 507–516. [Google Scholar] [CrossRef]
- McDonough, O.T.; Lang, M.; Hosen, J.; Palmer, M.A. Surface Hydrologic Connectivity Between Delmarva Bay Wetlands and Nearby Streams Along a Gradient of Agricultural Alteration. Wetlands 2015, 35, 41–53. [Google Scholar] [CrossRef]
- Xia, G. Effects of Landscape Pattern on Bird Community Diversity—A Case Study of Jinhai Wetland in Shanghai; Shanghai Normal University: Shanghai, China, 2015. [Google Scholar]
- Li, W.; Kerrylee, R.; Neil, S.; Joanne, L. The influences of climate and hydrology on population dynamics of waterbirds in the lower Murrumbidgee Rive floodplains in Southeast Australia: Implication for environmental water management. Ecol. Model. 2011, 222, 154–163. [Google Scholar]
- Karim, F.; Kinsey-Henderson, A.; Wallace, J.; Arthington, A.H.; Pearson, R.G. Modelling wetland connectivity during overbank flooding in a tropical floodplain in north Queensland, Australia. Hydrol. Process. 2012, 26, 2710–2723. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Y.; Dong, Z.R.; Zhai, Z.L.; Sun, D.Y. Evaluation method for river floodplain system connectivity based on graph theory. J. Hydraul. Eng. 2011, 42, 537–543. [Google Scholar]
- Meerkerk, A.L.; van Wesemael, B.; Bellin, N. Application of connectivity theory to model the impact of terrace failure on runoff in semi-arid catchments. Hydrol. Process. 2009, 23, 2792–2803. [Google Scholar] [CrossRef]
- Cui, B.; Wang, C.; Tao, W.; You, Z.Y. River channel network design for drought and flflood control: A case study of Xiaoqinghe River basin, Jinan City, China. J. Environ. Manag. 2009, 90, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.; Riitters, K. GuidosToolbox: Universal digital image object analysis. Eur. J. Remote Sens. 2017, 50, 352–361. [Google Scholar] [CrossRef]
- Margules, C.R.; Pessey, R.L. Systematic conservation Planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Pressey, R.L.; Cabeza, M.; Watts, M.E.; Richard, M.; Cowling, R.M.; Wilson, K.A. Conservation planning in a changing world. Trends Ecol. Evol. 2007, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. China Red Data Book of Endangered Animals; Science Press: Beijing, China, 1998. [Google Scholar]
- Wang, S.; Xie, Y. Chinese Species Red List; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Lagabrielle, E.; Rouget, M.; Bourgeois, T.L.; Payet, K.; Durieux, L.; Baret, S.; Dupont, J.; Strasberg, D. Integrating conservation, restoration and land-use planning in islands—An illustrative case study in Réunion Island (Western Indian Ocean). Landsc. Urban Plan. 2011, 2, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.V.; Bryer, M.T.; Khoury, M.L.; Fitzhugh, T.W. A Freshwater Classification Approach for Biodiversity Conservation Planning. Conserv. Biol. 2005, 19, 432–445. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, G.; Luo, C.; Zeng, X.; Zhang, H.; Murray, N.J.; Xu, N. Identifying restoration priorities for wetlands based on historical distributions of biodiversity features and restoration suitability. J. Environ. Manag. 2019, 231, 1222–1231. [Google Scholar] [CrossRef]
- Carwardine, J.; Rochester, W.A.; Richardson, K.S.; Williams, K.J.; Pressey, R.L.; Possingham, H.P. Conservation planning with irreplaceability: Does the method matter? Biodivers. Conserv. 2007, 16, 245–258. [Google Scholar] [CrossRef]
- Ferrier, S.; Pressey, R.L.; Barrett, T.W. A new predictor of the irreplaceability of areas for achieving a conservation goal, its application to real-world planning, and a research agenda for further refinement. Biol. Conserv. 2000, 93, 303–326. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. Conefor Sensinode2.2 User’ s Manual; University of Lleida: Lleida, Spain, 2007; pp. 23–24. [Google Scholar]
- Saura, S.; Torné, J. Conefor 2.6 User Manual; Universidad Politécnica de Madrid: Madrid, Spain, 2012; Available online: www.conefor.org (accessed on 1 April 2021).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2021; Available online: https://www.rstudio.com/ (accessed on 10 April 2021).
- Naskar, M.; Roy, K.; Karnatak, G.; Nandy, S.K.; Roy, A. Quantifying climate change induced threats to wetland fisheries: A stakeholder-driven approach. Environ. Dev. Sustain. 2018, 20, 2811–2830. [Google Scholar] [CrossRef]
- Chignell, S.M.; Laituri, M.J.; Young, N.E.; Evangelista, P.H. Afroalpine Wetlands of the Bale Mountains, Ethiopia: Distribution, Dynamics, and Conceptual Flow Model. Ann. Am. Assoc. Geogr. 2019, 109, 791–811. [Google Scholar] [CrossRef]
- Li, T.X.; Fu, Q.; Meng, F.X. Analysis of evolution trend and abrupt changes of annual precipitation in Sanjiang Plain from 1959 to 2013. China Rural. Water Hydropower 2016, 9, 201–204. [Google Scholar]
- Zhou, H.Y.; Diao, L.M.; Zheng, S.F. Remote Sensing Monitoring of Paddy Field Expansion in Fuyuan During 1990–2013. Chin. Agric. Sci. Bull. 2018, 34, 140–146. [Google Scholar]
- Liu, H.Y.; Zhang, S.; Li, Z.F. Impacts on wetlands of large-scale land-use changes by agricultural development: The small Sanjiang Plain, China. Ambio 2004, 33, 306–310. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Zeng, X.; Luo, C.; Zhang, H.; Qu, Y.; Xu, N. The Relationship between Hydrological Connectivity Changes Inside and Outside Biodiversity Hotspots and Its Implication for Sustainable Environmental Management. Sustainability 2022, 14, 6654. https://doi.org/10.3390/su14116654
Bao W, Zeng X, Luo C, Zhang H, Qu Y, Xu N. The Relationship between Hydrological Connectivity Changes Inside and Outside Biodiversity Hotspots and Its Implication for Sustainable Environmental Management. Sustainability. 2022; 14(11):6654. https://doi.org/10.3390/su14116654
Chicago/Turabian StyleBao, Wenhui, Xingyu Zeng, Chunyu Luo, Hongqiang Zhang, Yi Qu, and Nan Xu. 2022. "The Relationship between Hydrological Connectivity Changes Inside and Outside Biodiversity Hotspots and Its Implication for Sustainable Environmental Management" Sustainability 14, no. 11: 6654. https://doi.org/10.3390/su14116654
APA StyleBao, W., Zeng, X., Luo, C., Zhang, H., Qu, Y., & Xu, N. (2022). The Relationship between Hydrological Connectivity Changes Inside and Outside Biodiversity Hotspots and Its Implication for Sustainable Environmental Management. Sustainability, 14(11), 6654. https://doi.org/10.3390/su14116654




