1. Introduction
Soil stabilization with chemical additives is a widely used technique in the construction industry. It is particularly suitable for the improvement of the engineering properties of soils containing clay minerals, mainly for the increase in the bearing capacity of fine particle soils, for the construction of pavement layers. This way, the consumption of aggregates in pavement layers’ construction decreases, and these low-bearing soils are valorized. The stabilization of a soil also reduces plasticity and swelling, among other usual problems of many clayey soils, favoring the use of these inadequate soils in civil engineering applications such as road construction or urbanization works. Soil stabilization is usually carried out by the use of calcium-based additives such as lime or cement [
1]. These additives hydrate in the presence of water, and CaO transforms into Ca(OH)
2. The release of OH
− ions in the soil solution produces a pH increase up to 12.4 and clay mineral flocculation due to Ca
2+ ions’ availability. In these conditions, calcium reacts with the silicon and aluminum, supplied by the additive or extracted from the clay matrix, to form cementitious gels that are the responsible of the improvement of the soil properties [
2].
Despite the demonstrated suitability of the use of lime and cement as stabilizers for clayey soils, stabilization may cause adverse effects that may even lead to the destruction of the treated soil [
3]. One of the most common causes of this type of failure is the presence in the stabilized soil of sulfate (
). Sulfate is a very common saline component in many types of natural soils, mainly in the form of gypsum. It can also be present in the water used to compact pavement layers, or can even come from the infiltration of water from the surrounding terrain. The reaction of the Ca
2+ ions supplied by the additive, aluminum from the clay minerals and the sulfate, with water availability, forms ettringite (Ca
6Al
2(SO
4)
3(OH)
12∙26H
2O), a highly hydrated and expansive mineral. The adequate conditions for the formation of ettringite are: 1. high pH, 2. availability of reactive aluminum, 3. availability of reactive calcium, 4. presence of sulfate and 5. water availability [
4]. Ettringite formation is accelerated by high temperatures (higher than 60–70 °C) [
5,
6,
7,
8], and with adequate conditions, ettringite can appear even during the mixing of the materials. Ouhadi and Yong [
4,
9] established the formation of this mineral over one month in one case, and between the mixture of the soil with lime and 48 h in another experiment. Once the ettringite has been formed, the soil is volumetrically stable, and it has been observed that this mineral contributes to improving the soil bearing capacity [
5,
10,
11].
Because of their wide distribution around the world, sulfate soils’ stabilization continues to be a necessity, with technical and environmental implications. Sulfate soil stabilization continues being a technical challenge because of the risks associated with the formation of ettringite in the stabilized soil. From an environmental point of view, the stabilization of sulfate soils could contribute to limiting the environmental impact of the construction industry by means of saving natural resources they could substitute. This way, different authors are currently researching this field: Diaz Caselles et al. [
12], stabilized a sulfate soil with different hydraulic binders based on calcium. They found that the most effective treatment was obtained with binders rich in GGBS. Although ettringite was observed in this treatment, no volume expansion was observed on the samples. Raja et al. [
13] stabilized an expansive soil with a sulfate-resistant cement, rich in GGBS. They found that when the treated soil was exposed to a solution of sulfate, ettringite formed and the soil strength and dry density decreased. Eyo et al. [
14] stabilized soils with different sulfate contents using cement and GGBS. They observed the beneficial effect against sulfate attack caused by the substitution of the cement by GGBS. This effect increased as the GGBS content did, mainly for high substitution rates, up to 80%. Other authors have demonstrated the ability of the magnesium-based hydraulic binders for the stabilization of sulfate soils. Seco et al. [
3] stabilized five different sulfate soils with lime and a magnesium oxide by-product alone and combined with GGBS. They found that the most effective binder to increase the mechanical strength of the sulfate soils was the mix of GGBS and the magnesium oxide by-product. They also found that the five soils showed increased swelling when they were treated with lime and maintained in water immersion. Four of the five soil samples showed ettringite formation. On the other hand, the soil samples treated with the magnesium oxide showed a decrease in their natural swelling and no expansive minerals were identified on them. Seco et al. [
15] studied the ability of a sulfate soil stabilized with cement, sulfate-resistant cement, magnesium oxide by-products and GGBS, to act as a subgrade for pavement construction. They found that cement and a binder composed of magnesium oxide and GGBS were the most effective additives for the improvement of the bearing capacity of the sulfate soil. They also observed unexpected delayed swelling increases when the sulfate soil treated with cement and sulfate-resistant cement was kept in water for more than 20 days.
This work aims to contribute towards knowledge about the now-available technological solutions for the stabilization of soils containing sulfate. For this, a two-phase stabilization method is proposed. The first phase consists of the consumption of the natural sulfate present in the soil through its controlled transformation into ettringite. This way, ettringite would form before the use of the soil as a construction material, becoming a volumetrically stable modified soil with, based on the literature, improved mechanical properties. In the second phase, the treated soil’s mechanical properties were improved by the use of additives rich in oxides of calcium or magnesium, combined with by-products or waste materials containing reactive aluminum or silicon oxides. These additives were used like binary hydraulic binders for the development in the treated soil of hydraulic calcium or magnesium cementitious gels based on [
1,
3,
15,
16,
17,
18,
19]. This way, the proposed soil stabilization method would contribute not only to the sulfate soil’s valorization, diminishing the risks associated with the delayed formation of the ettringite, but also to the valorization of the recycled additives considered in this investigation.
Section 2 introduces the sulfate soil and the additives considered, and shows the sample manufacturing and sample testing methods carried out in the experimental investigation.
Section 3 shows the investigation results and the discussion.
Section 4 explains the investigation’s conclusions.
Highlights
A two-phase sulfate soil stabilization treatment was developed;
Ettringite formed in a sulfate soil treated with lime and coal bottom ash;
The modified soil showed improved engineering properties;
Binary binders based on rice husk fly ash and lime or magnesium oxide improved the modified soil mechanical properties;
Lime and alumina filler formed ettringite in the modified soil.
3. Results and Discussion
3.1. Determination of Optimum Dosage of Additives, and Time for Formation of Ettringite
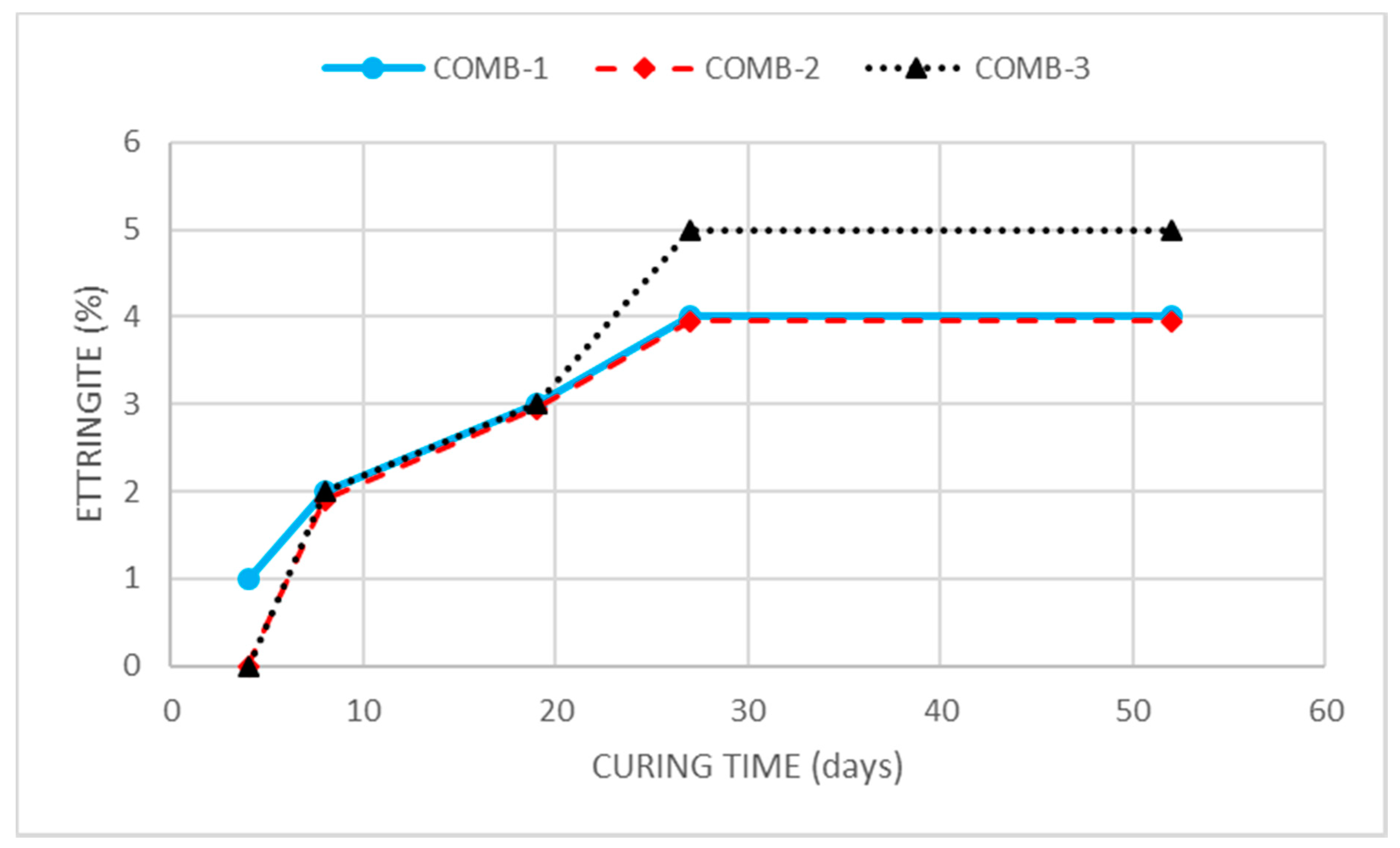
Three dosages of lime + CBA were considered for the consumption of the soil sulfate through ettringite formation: 3%lime + 5%CBA (COMB-1), 5%lime + 5%CBA (COMB-2) and 8%lime + 5%CBA (COMB-3). The evolution of the presence of ettringite in the treated soil samples is shown in
Figure 1.
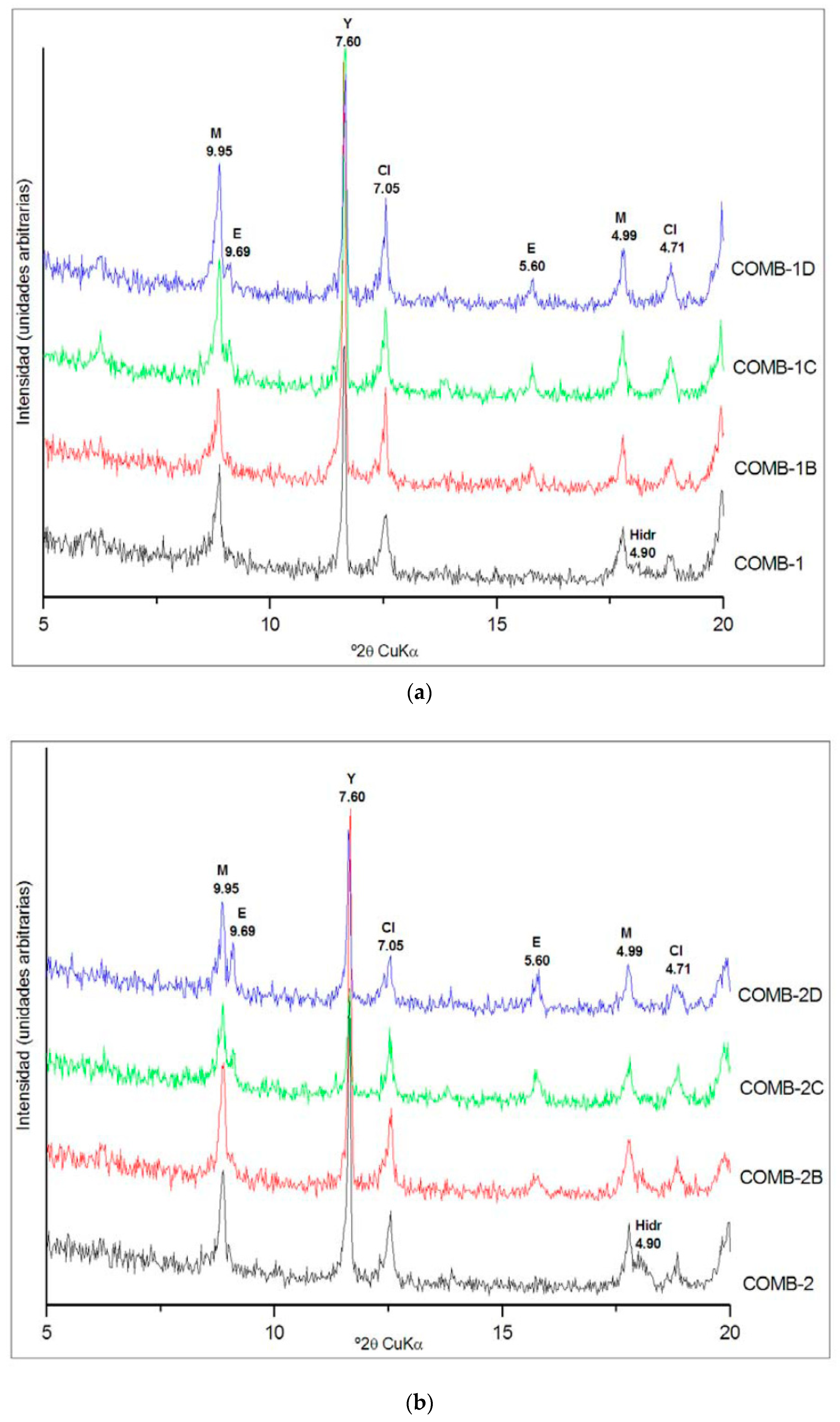
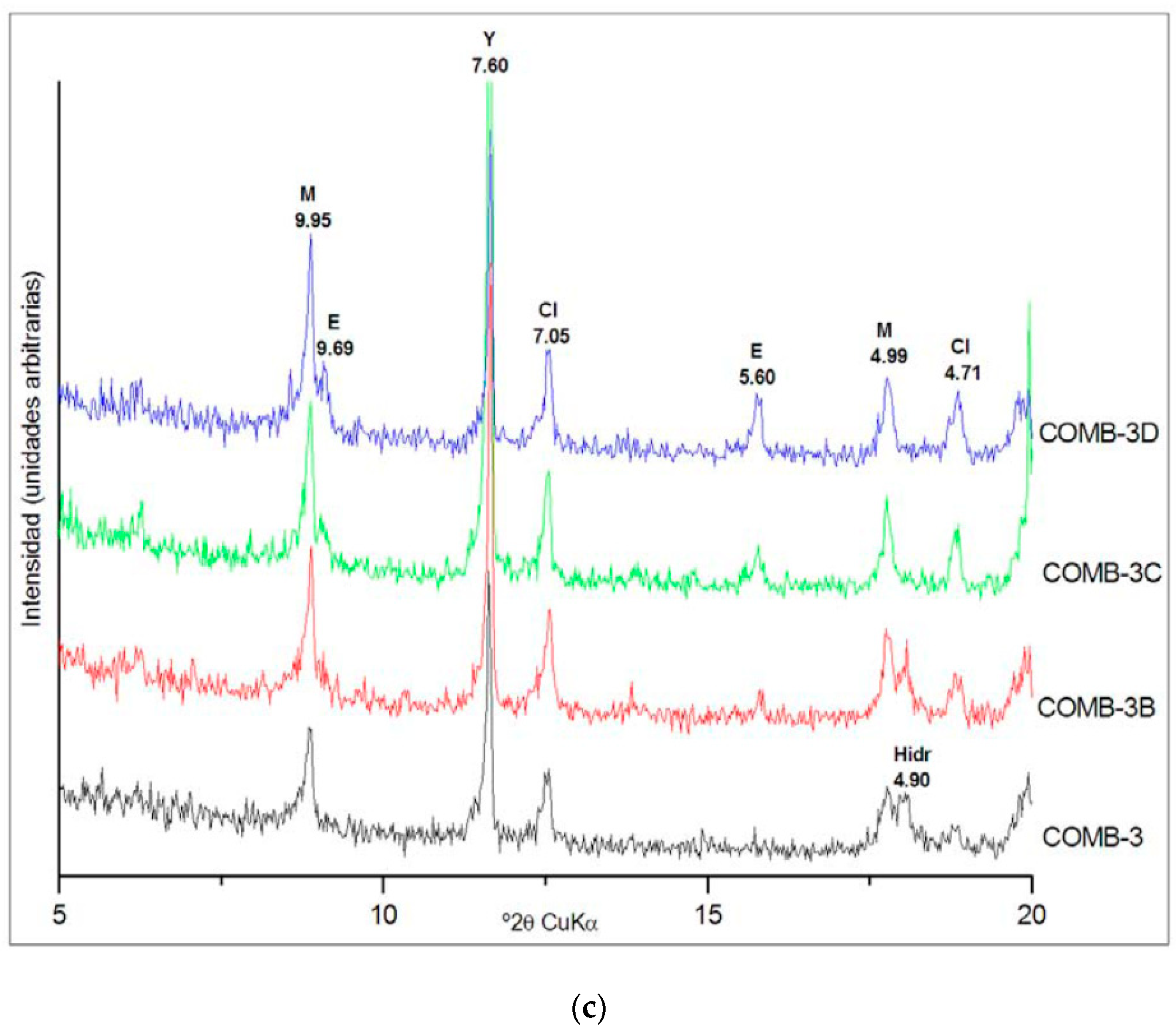
Figure 2 shows the XRD diffractograms of the tests carried out in the three combinations considered, at the different curing ages, with the main peaks corresponding to the ettringite marked with an (E).
For the three dosages considered, ettringite formed in the soil up to the age of 27 days and then remained stable. The total amount of ettringite produced by the three combinations was very similar, with contents estimated by XRD of between 4 and 5%. Based on these results, the treatment with 5% lime and 5% of CBA was chosen as standpoint of the second part of the stabilization treatment. Despite the fact that combinations 1 and 2 produced the same quantity of ettringite, combination 2 was chosen for the soil modification to prevent the uncertainties in the realization of this treatment and to be sure that all the soil sulfate is consumed. On the other hand, the formation of only 1% more ettringite was not considered sufficient to justify increasing the dosage up to 8% of lime. The curing conditions for the soil modification, 27 days at 40 °C with water saturation, were chosen based on the XRD results, because in the three combinations tested, the formation of ettringite was completed before 27 days. Once the sample of soil required for the second treatment was cured, it was maintained in the stove at 40 °C for 24 h to dry it before its characterization and the realization of the treatment for the improving of its mechanical properties. According to the SP test, the modified soil compared to the natural soil showed a reduction in maximum density from 1.79 g/cm3 to 1.37 kg/cm3 and an increase in optimum moisture content from 14.2% to 30.5%. This reduction in the soil density was attributed to the formation of ettringite and its lower density because of its high degree of hydration. The new optimum humidity was determined by drying the samples at 100 °C, which justifies the increase in humidity obtained, since, in addition to the free water, the water of mineral constitution integrated in the formed ettringite was as well-eliminated. The behavior of the modified soil was non-plastic, and the free swelling in the oedometer test was 0.0%. The compressive strength of the modified soil at 7, 14 and 28 days, reached 0.46, 0.49 and 0.61 MPa, respectively, showing a slight increase compared to the natural soil.
3.2. Improvement of Mechanical Properties of the Soil
Once the sulfate was transformed into ettringite, the modified soil received a second treatment in order to increase its mechanical properties. The combinations and dosages of the additives tested are shown in
Table 3.
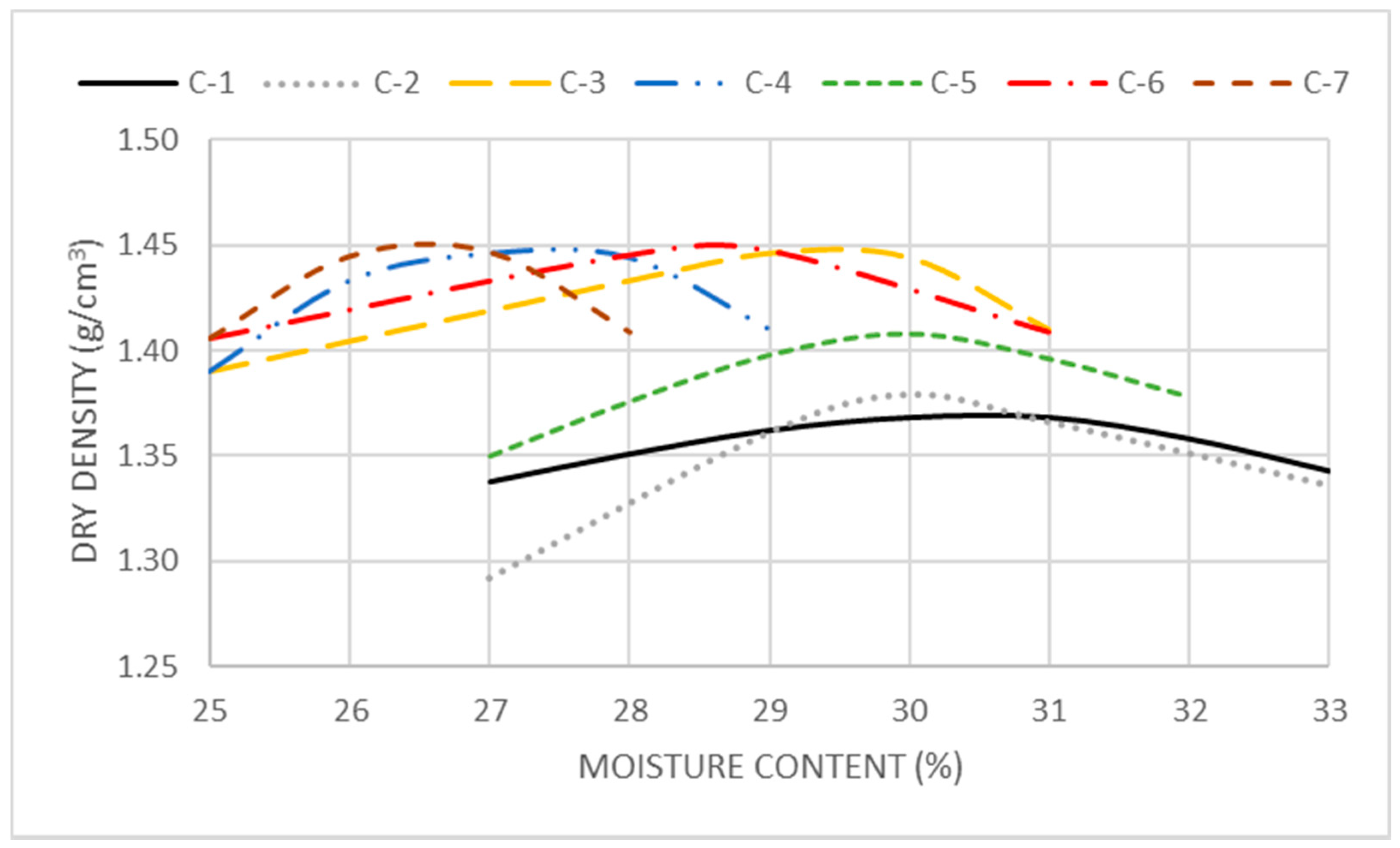
The maximum density and optimum moisture of each of the combinations was determined according to the SP test, and its compressive strength was determined at 7, 14 and 28 days after the second treatment for the stabilization of the soil.
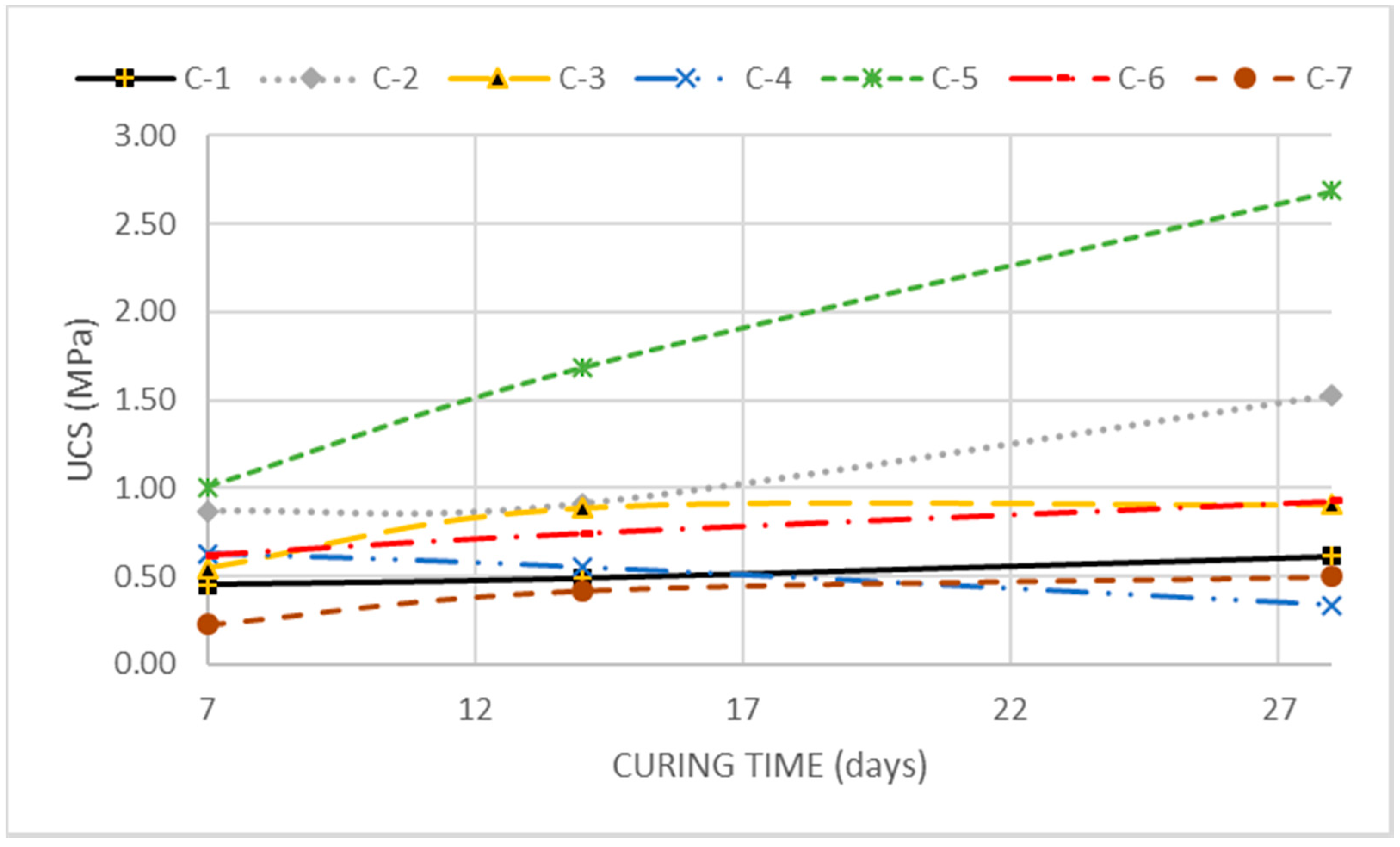
Figure 3 shows the curves of the SP tests of all combinations tested. The use of the additives in this second treatment increased the maximum density obtained and the optimum moisture for all the combinations tested. Combinations 2 and 5 did not change their optimum moisture content compared to the treated soil without additives and reached different maximum densities. This demonstrates that in these combinations the differences observed depend mainly on the RHFA. With a different behavior, combinations 3, 4, 6 and 7 reached very close maximum density results and showed a reduction in their manufacturing optimum moisture content. This reduction is more evident for the PC-8 than for the lime and for the FA than for the CBA, demonstrating the influence of both binder constituents on the combinations’ optimum moisture content.
Figure 4 shows the results of unconfined compression tests for each combination at each of the ages considered. The combination formed by the modified soil gave a strength of 0.45 MPa at 7 days, 0.49 MPa at 14 days, and 0.61 MPa at 28 days. This reflects an increase in the unconfined compressive strength of the modified soil compared to the strength of the natural soil. The small increase in resistance observed along the curing time is likely due to the cementitious effect of Ca
2+ ions not consumed for the formation of ettringite, which generate pozzolanic reactions in the modified soil.
The samples modified treated with lime + RHFA blend (combination 2) and lime + CBA (combination 3) showed unconfined compressive strengths of 0.87 and 0.55 MPa at 7 days, 0.91 and 0.89 MPa at 14 days, and 1.53 and 0.91 MPa at 28 days, respectively. These increases in resistance were attributed to cementitious gel formation in the soil, due to the availability of oxides of calcium, silicon and aluminum. Something similar occurs with the combinations of PC-8 + RHFA blend (combination 5) and PC-8 + CBA blend (combination 6), which reached unconfined compressive strengths of 1.01 and 0.62 MPa at 7 days, 1.69 and 0.74 MPa at 14 days and 2.69 and 0.93 MPa at 28 days, respectively. Combinations PC-8 + RHFA achieved better UCS results than lime + RHFA, demonstrating an improved behavior of the MgO kiln dust compared to lime as an activator of Si and Al sources for the formation of cementitious hydraulic gels, in accordance with Seco et al. [
3].
Combinations with AF showed anomalous results compared to those obtained with other sources of Si and Al. In the case of combination 4 (lime + AF blend), the values of unconfined compressive strength decreased from 0.63 MPa at 7 days to 0.55 MPa at 14 days and 0.33 MPa at 28 days. Given that aluminum oxide is one of the minerals involved in the formation of ettringite, these anomalously low values of compressive strength have been attributed to the formation of more ettringite, producing a swelling effect in the sample. For combination 7 (PC-8 + AF blend), the compressive strength values reached 0.23 MPa at 7 days, 0.42 MPa at 14 days and 0.50 MPa at 28 days. The increase in the UCS values of this combination demonstrate the effectiveness of the MgO in the formation of cementitious gels, without expansive behavior, even in high-aluminum environments.
Although there are differences between the sulfate soils studied, the binder kinds considered or the binder dosages, the UCS results obtained in this investigation agree with those obtained by other authors. P. Sriram Karthick Raja and T. Thyagaraj [
13] reached 3 MPa of UCS at 28 days when they stabilized a sulfate soil with 10% of cement. Eyo et al. [
14] obtained up to 1.50 MPa of UCS in soils with sulfate contents in the range 4–12% when they used 9% of cement or a mix of cement and GGBS. On the other hand, the results obtained in this investigation are lower than those obtained by Seco et al. [
3], who achieved UCS values above 10 MPa at 28 days for different sulfate soils stabilized with 10% of a binary binder composed of a magnesium oxide by-product and GGBS. These results agree with those of Seco et al. [
15], who also reached UCS at 28 days above 10 MPa when they stabilized a sulfate soil with a mix of magnesium oxide and GGBS. These results highlight the convenience of the GGBS as source of silicon and aluminum compared with those used in this investigation, RHFA, CBA and AF. Despite the convenience of the GGBS as stabilizer additive and the fact that it is a by-product of pig iron manufacturing, GGBS is a scarce and expensive product, being nowadays almost entirely consumed by the cement industry. Nevertheless, RHFA, CBA and AF are wastes that in many cases lack effective valorization methods. Indeed, RHFA, the most effective source of reactive silicon considered in this investigation, is available around the world. This way, the use of any waste as a soil stabilizer additive can help to increase the sustainability of the stabilization. On the other hand, combinations 2 and 5 achieve the 1.5 MPa of UCS considered by the Spanish Road Regulation [
20] for the construction of road subgrades, demonstrating the convenience of both combinations for civil engineering applications. As the PC-8 is a by-product, combination 5 is not only the one that achieved the highest UCS results but also a combination in which the binary binder is 100% made of recycled constituents.
4. Conclusions
This experiment showed how a sulfate soil with low mechanical properties can be stabilized for its use as a construction material in civil engineering applications. This valorization process first required sulfate transformation from gypsum to ettringite, and secondly the improvement of the mechanical strength of the modified soil. The formation of ettringite in the considered soil, when the necessary conditions (use of additives rich in the minerals involved in the formation of ettringite, adequate temperature and water availability) were fulfilled, was rapid, with a formation time of less than 1 month in this experiment. As a result of applying this treatment, a soil with modified engineering characteristics was obtained: lower maximum density and greater optimum moisture in SP test, no plasticity and improved UCS. The stabilization of this modified soil with additives (mainly waste materials and industry by-products) rich in oxides of calcium or magnesium, aluminum and silicon, enabled the development of cementitious gels mainly for the combinations treated with PC-8 + RHFA and to a lesser extent for the combination with lime + RHFA. This demonstrated the effectiveness of silicon to form cementitious compounds and the improved ability of magnesium compared to calcium as an activator. This experiment allows for the validation of a two-phase stabilization process and the non-conventional additives used, even for high-bearing-requirement pavement layers’ construction. The addition of significant amounts of aluminum caused a significant loss of unconfined compressive strength of the combination containing lime and AF, highlighting the importance of the calcium and aluminum for the formation of ettringite, the most likely source of the loss of resistance and swelling of sulfate soils. Although the results obtained in this investigation are promising, more investigations are required to state the convenience of the proposed stabilization method in other sulfate soils. Depending on the local availability, other additives rich in calcium, magnesium, silicon and aluminum, wastes or by-products preferably, can be tested for sulfate soils’ stabilization in other regions for environmental purposes. The use of binary binders based on calcium and aluminum should be avoided because of their potential contribution to the formation of ettringite in sulfate soils.