Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Natural-Origin Sorbent
3.2. Removal of Biogenic Raw Materials from Aquatic Solutions with the Use of Natural-Origin Sorbent
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kondili, E.; Kaldellis, J.K.; Papapostolou, C. A novel systemic approach to water resources optimisation in areas with limited water resources. Desalination 2010, 250, 297–301. [Google Scholar] [CrossRef]
- Ramm, K. Considerations Related to the Application of the EU Water Reuse Regulation to the Production of Snow from Reclaimed Water. Circ. Econ. Sustain. 2021. [Google Scholar] [CrossRef]
- Tang, W.; Chen, B.D.; Wang, Z.L. Recent Progress in Power Generation from Water/Liquid Droplet Interaction with Solid Surfaces. Adv. Funct. Mater. 2019, 29, 1901069. [Google Scholar] [CrossRef]
- Kandeal, A.W.; Joseph, A.; Elsharkawy, M.; Elkadeem, M.R.; Hamada, M.A.; Khalil, A.; Moustapha, M.E.; Sharshir, S.W. Research progress on recent technologies of water harvesting from atmospheric air: A detailed review. Sustain. Energy Technol. Assess. 2022, 52, 102000. [Google Scholar] [CrossRef]
- National Snow and Ice Data Center. Facts about Glaciers. 2022. Available online: https://nsidc.org/cryosphere/glaciers (accessed on 16 March 2022).
- Kuhlman, T.; Farrington, J. What is sustainability? Sustainability 2010, 2, 3436–3448. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Yu, Y.; Yang, S.; Lv, Y.; Sarker, M.N.I. Urban Resilience for Urban Sustainability: Concepts, Dimensions, and Perspectives. Sustainability 2022, 14, 2481. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Donnenfeld, Z.; Crookes, C.; Hedden, S. A Delicate Balance: Water scarcity in South Africa; Southern Africa Report 13; Institute for Security Studies: Pretoria, South Africa, 2018; pp. 1–24. [Google Scholar]
- Abdelhaleem, F.S.; Basiouny, M.; Ashour, E.; Mahmoud, A. Application of remote sensing and geographic information systems in irrigation water management under water scarcity conditions in Fayoum, Egypt. J. Environ. Manage. 2021, 299, 113683. [Google Scholar] [CrossRef]
- Gleick, P.H. Roadmap for sustainable water resources in southwestern North America. Proc. Natl. Acad. Sci. USA 2010, 107, 21300–21305. [Google Scholar] [CrossRef] [Green Version]
- Morote, Á.F.; Olcina, J.; Hernández, M. The use of non-conventional water resources as a means of adaptation to drought and climate change in semi-arid regions: South-eastern Spain. Water 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- European Environment Agency (EEA). Use of Freshwater Resources in Europe. 2022. Available online: https://www.eea.europa.eu/data-and-maps/indicators/use-of-freshwater-resources-3/assessment-4 (accessed on 16 March 2022).
- DeNicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Climate change and water scarcity: The case of Saudi Arabia. Ann. Glob. Health 2015, 81, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Luo, W.; Al-Ansari, N.; Elbeltagi, A.; El-Rawy, M.; Farres, H.N.; Gabr, M.E.S. Farmers’ awareness in the context of climate change: An underutilized way for ensuring sustainable farmland adaptation and surface water quality. Sustainability 2021, 13, 11802. [Google Scholar] [CrossRef]
- Nisar, M.B.; Shah, S.A.R.; Tariq, M.O.; Waseem, M. Sustainable wastewater treatment and utilization: A conceptual innovative recycling solution system for water resource recovery. Sustainability 2020, 12, 10350. [Google Scholar] [CrossRef]
- Preisner, M.; Smol, M.; Szołdrowska, D. Trends, insights and effects of the Urban Wastewater Treatment Directive (91/271/EEC) implementation in the light of the Polish coastal zone eutrophication. Environ. Manag. 2021, 67, 342–354. [Google Scholar] [CrossRef]
- Preisner, M.; Smol, M.; Szołdrowska, D. A toxic-free environment ambition in the light of the polish baltic sea coastal zone pollution by heavy metals. Desalin. Water Treat. 2021, 232, 225–235. [Google Scholar] [CrossRef]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying barriers and opportunities for a circular phosphorus economy in the Baltic Sea region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef]
- Smol, M. The importance of sustainable phosphorus management in the circular economy (CE) model: The Polish case study. J. Mater. Cycles Waste Manag. 2019, 21, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. Mitigation of eutrophication caused by wastewater discharge: A simulation-based approach. Ambio 2021, 50, 413–424. [Google Scholar] [CrossRef]
- Dvarioniene, J.; Kruopiene, J.; Stankevičiene, J. Application of cleaner technologies in milk processing industry to improve the environmental efficiency. Clean Technol. Environ. Policy 2012, 14, 1037–1045. [Google Scholar] [CrossRef]
- Wagner, T.; Erickson, L.E. Sustainable Management of Eutrophic Lakes and Reservoirs. J. Environ. Prot. Irvine. Calif. 2017, 8, 436–463. [Google Scholar] [CrossRef] [Green Version]
- Stępniewski, K.; Łaszewski, M. Spatial and seasonal dynamics of inorganic nitrogen and phosphorous compounds in an orchard-dominated catchment with anthropogenic impacts. Sustainability 2021, 13, 11337. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Lahav, O.; Asher, R.B.; Gendel, Y. Potential applications of indirect electrochemical ammonia oxidation within the operation of freshwater and saline-water recirculating aquaculture systems. Aquac. Eng. 2015, 65, 55–64. [Google Scholar] [CrossRef]
- Smol, M. The use of membrane processes for the removal of phosphorus from wastewater. Desalin. Water Treat. 2018, 128, 397–406. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. Biotreatment of raisin and winery wastewaters and simultaneous biodiesel production using a Leptolyngbya-based microbial consortium. J. Clean. Prod. 2017, 148, 185–193. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Ayele, L.; Pérez, E.; Mayoral, Á.; Chebude, Y.; Díaz, I. Synthesis of zeolite A using raw kaolin from Ethiopia and its application in removal of Cr(III) from tannery wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Rasalingam, S.; Peng, R.; Koodali, R.T. Removal of hazardous pollutants from wastewaters: Applications of TiO2-SiO2 mixed oxide materials. J. Nanomater. 2014, 10, 17405. [Google Scholar]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Hak, Y.K.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Kamiński, W.; Tomczak, E. Low-cost applications for water treatment. Proc. ECOpole 2014, 8, 189–194. [Google Scholar] [CrossRef]
- Zabochnicka-Światek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014, 23, 551–561. [Google Scholar]
- Smol, M.; Marcinek, P.; Duda, J.; Szołdrowska, D. Importance of sustainable mineral resource management in implementing the circular economy (CE) model and the European green deal strategy. Resources 2020, 9, 55. [Google Scholar] [CrossRef]
- Smol, M. Inventory and Comparison of Performance Indicators in Circular Economy Roadmaps of the European Countries. Circ. Econ. Sustain. 2021. [Google Scholar] [CrossRef]
- Szymczyk, S.; Glinska-Lewczuk, K. Causes of loading the water of the eutrophic Lake Jagielek with nitrogen and phosphorus compounds under the meteorological conditions of Olsztyn Lakeland. J. Elementol. 2007, 12, 347–361. [Google Scholar]
- European Commission. The European Green Deal Sets out How to Make Europe the First Climate-Neutral Continent by 2050, Boosting the Economy, Improving People’s Health and Quality of Life, Caring for Nature, and Leaving No One Behind. Press. Available online: https://ec.europa.eu/commission/pre (accessed on 10 March 2022).
- European Commission. Communication from Commission. Chemicals Strategy for Sustainability—Towards a Toxic-Free Environment. (COM No. 667, 2020). 2020. Available online: https://ec.europa.eu/environment/strategy/chemicals-strategy_en (accessed on 30 March 2022).
- Gubernat, S.; Masłoń, A.; Czarnota, J.; Koszelnik, P. Reactive materials in the removal of phosphorus compounds from wastewater-A review. Materials 2020, 13, 3377. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryła, A. Choosing of reactive material for phosphorous removal from water and wastewater on the example of lightweight aggregate Pollytag®. Inżynieria Ekol. 2014, 39, 33–41. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A. Properties of lime-siliceous rock opoka as reactive material to remove phosphorous from water and wastewater. Infrastrukt. Ekol. Teren. Wiej. 2014, 1, 227–238. [Google Scholar]
- Chlipalski, J. The use of opoka rock sorbent for wastewater treatment. Rynek Instal. 2006, 6, 83–84. [Google Scholar]
- Kartikasari, D.D.; Mada, U.G.; Mada, U.G. Testing of Polonite, Gazobeton, Parepare Black Volcanic Sand Beach, and Kadidiri Coral Beach Sand for phosphorus removal from water and wastewater in constructed wetland. ASEAN J. Syst. Eng. 2013, 1, 44–49. [Google Scholar]
- Jóźwiakowski, K. Experiment of increasing effectiveness of phosphorus removal in a model of wastewater treatment plant. Inżynieria Rol. 2006, 5, 249–256. [Google Scholar]
- Belkanova, M.Y.; Buntov, A.S. Opoka in coagulation of slightly polluted surface water. IOP Conf. Ser. Mater. Sci. Eng. 2018, 451, 012216. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Renman, A.; Renman, G.; Poll, K. Phosphate removal by mineral-based sorbents used in filters for small-scale wastewater treatment. Water Res. 2008, 42, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karczmarczyk, A.; Bus, A. Testing of reactive materials for phosphorus removal from water and wastewater—Comparative study. Ann. Warsaw Univ. Life Sci.-SGGW. L. Reclam. 2014, 46, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Johansson, L.; Gustafsson, J.P. Phosphate removal using blast furnace slags and opoka-mechanisms. Water Res. 2000, 34, 259–265. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Bus, A.; Baryła, A. The use of reactive materials in rainwater management in urban residentials. Infrastruct. Ecol. Rural Areas 2015, 4, 1089–1096. [Google Scholar]
- Szendera, M.; Turek-Szytow, J. The use of sorption properties of selected sorbents for the removal of organic compounds on the example of catechol. In Contemporary Problems of Environmental Protection and Energy; Silesian University of Technology: Gliwice, Poland, 2019; pp. 225–266. ISBN 9788395008771. [Google Scholar]
- Valentukeviciene, M.; Zurauskiene, R.; Boussouga, Y.A. Fluoride Removal from Groundwater by Technological Process Optimization. Ecol. Chem. Eng. S 2019, 26, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Jakubowska, P.; Borkowski, G.; Brząkalski, D.; Sztorch, B.; Kloziński, A.; Przekop, R.E. The Accelerated Aging Impact on Mechanical and Thermal Properties of Polypropylene Composites with Sedimentary Rock Opoka-Hybrid Natural Filler. Materials 2022, 15, 338. [Google Scholar] [CrossRef]
- Shachneva, E.; Archibasova, D. Adsorption of cadmium ions from aqueous solutions on modified sorbents. Chem. Chem. Technol. 2018, 12, 182–187. [Google Scholar] [CrossRef]
- Kavaliauskaite, I.; Denafas, G.; Uibu, M.; Kuusik, R. Natural minerals opoka and glaukonite as sorbents for acidic gases. Environ. Res. Eng. Manag. 2006, 3, 36–42. [Google Scholar]
- Wdowin, M.; Franus, W.; Panek, R. Preliminary results of usage possibilities of carbonate and zeolitic sorbents in CO2 capture. Fresenius Environ. Bull. 2012, 21, 3726–3734. [Google Scholar]
- Othman, A.; Dumitrescu, E.; Andreescu, D.; Andreescu, S. Nanoporous sorbents for the removal and recovery of phosphorus from eutrophic waters: Sustainability challenges and solutions. ACS Sustain. Chem. Eng. 2018, 6, 12542–12561. [Google Scholar] [CrossRef]
- Brogowski, Z.; Renman, G. Characterization of Opoka as a basis for its use in wastewater treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]

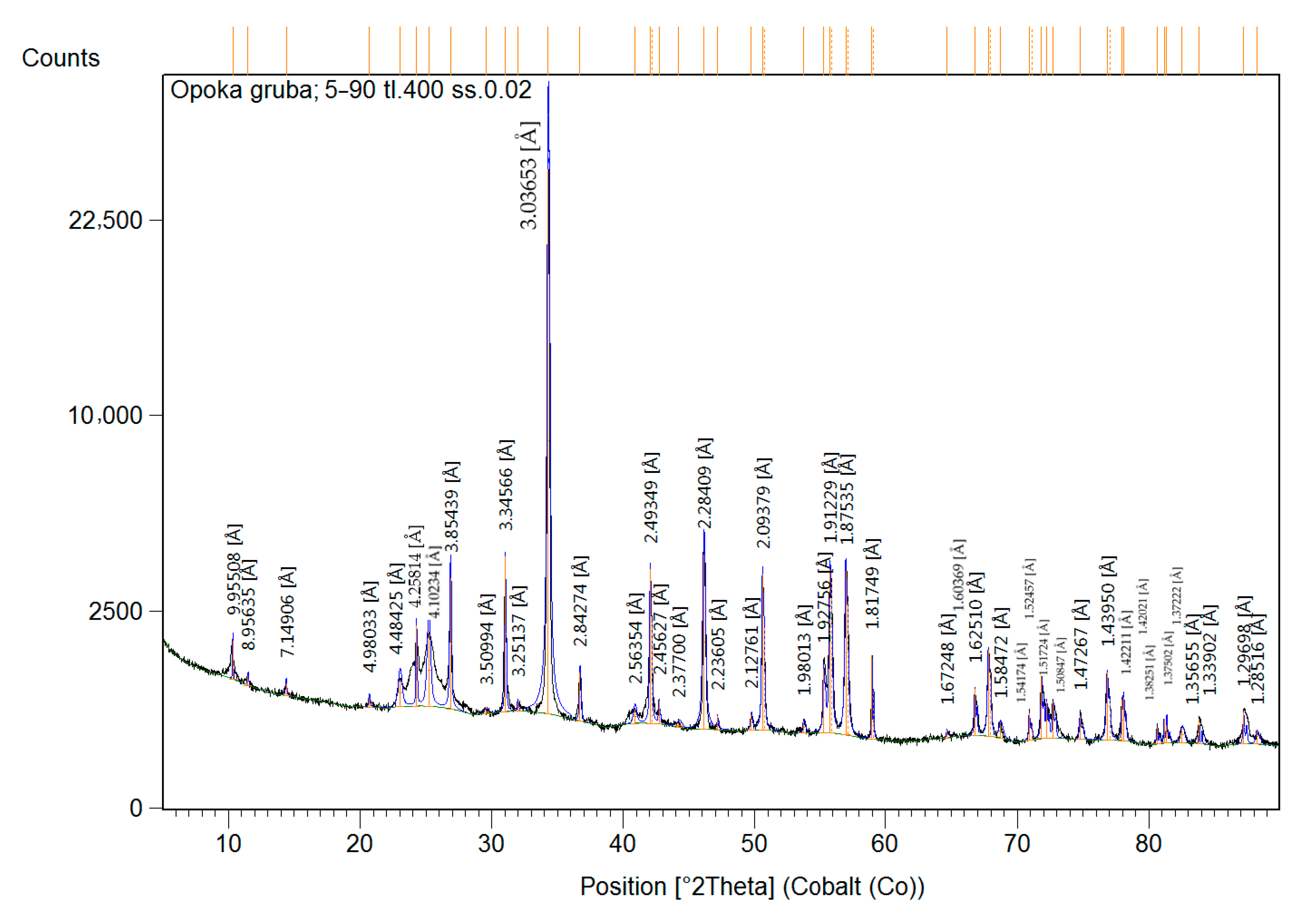
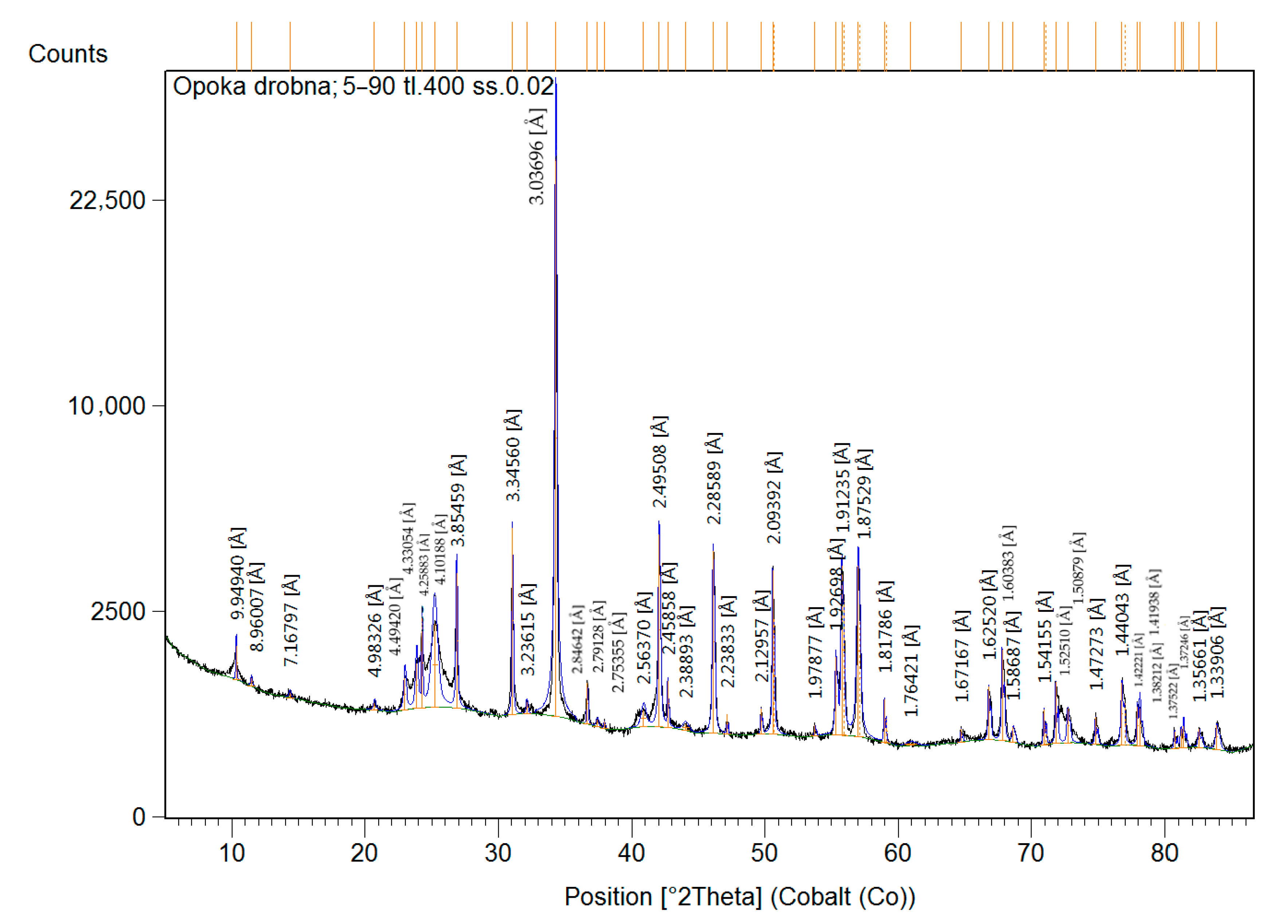
| Mineral Name | Chemical Formula | SemiQuant (%) | |
|---|---|---|---|
| Coarse-Grained | Fine-Grained | ||
| Calcite | CaCO3 | 69 | 71 |
| Quartz | SiO2 | 9 | 7 |
| Cristobalite low | SiO2 | 10 | 11 |
| Tridymite | SiO2 | 8 | 8 |
| Illite 2M1 | K (Al4Si2O9 (OH)3) | 3 | 2 |
| Kaolinite 1A | Al4 (OH)8 (Si4O10) | 0.2 | 0.5 |
| Clinoptilolite | Ca3.16Si36O72 (H2O)21.80 | 0.5 | 0.8 |
| Indicator | Type of Material | |
|---|---|---|
| Fine-Grained | Coarse-Grained | |
| pH | 8.6 | 8.5 |
| reactivity | 0.7495 | 0.7022 |
| Neutralization value | 32.5 (for CaO) | 30.5 (for CaO) |
| Water Sample | pH | NH4+ (mg/dm3) | NO3− (mg/dm3) | PO43− (mg/dm3) | TOC (mg/dm3) |
|---|---|---|---|---|---|
| W1 | 7.5 | <0.1 | <3.0 | 0.02 | 1.1 |
| W2 | 8.1 | 0.9 | 4.4 | 33.3 | 41.7 |
| W3 | 8.1 | 0.5 | 2.1 | 16.7 | 27.5 |
| Water Sample | Sorbent Type | Removal Efficiency (%) | |||
|---|---|---|---|---|---|
| NH4+ | NH4+ | NH4+ | TOC | ||
| W1 | S1 | - | - | 100 | 66.4 |
| S2 | - | - | 100 | 27.3 | |
| W2 | S1 | 47.7 | 33.0 | 53.8 | 52.6 |
| S2 | 26.3 | 27.0 | 38.0 | 46.5 | |
| W3 | S1 | 55.4 | 49.2 | 67.8 | 62.9 |
| S2 | 18.9 | 30.3 | 54.0 | 28.2 | |
| Water Sample | Sorbent Type | Removal Efficiency (%) | |||
|---|---|---|---|---|---|
| NH4+ | NH4+ | NH4+ | TOC | ||
| W1 | S1 | - | - | 100 | 69.7 |
| S2 | - | - | 100 | 63.4 | |
| W2 | S1 | 84.3 | 49.0 | 96.5 | 79.1 |
| S2 | 86.1 | 42.8 | 90.8 | 65.4 | |
| W3 | S1 | 39.2 | 51.7 | 96.6 | 69.3 |
| S2 | 66.9 | 33.7 | 83.0 | 55.0 | |
| Treated Medium | Removal Efficiency | Source | |
|---|---|---|---|
| P | N | ||
| Domestic sewage | 90% | - | [43] |
| Domestic sewage | 91% | [44] | |
| Domestic sewage | 90.9% | - | [45] |
| Septic tank effluent | 80% | 11% | [46] |
| Synthetic solution | >95% | - | [47] |
| Synthetic solution | 99.3% | [48] | |
| Synthetic sewage | <50% | - | [49] |
| Swim pond | 95% | - | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smol, M.; Włóka, D. Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment. Sustainability 2022, 14, 6432. https://doi.org/10.3390/su14116432
Smol M, Włóka D. Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment. Sustainability. 2022; 14(11):6432. https://doi.org/10.3390/su14116432
Chicago/Turabian StyleSmol, Marzena, and Dariusz Włóka. 2022. "Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment" Sustainability 14, no. 11: 6432. https://doi.org/10.3390/su14116432
APA StyleSmol, M., & Włóka, D. (2022). Use of Natural Sorbents in the Processes of Removing Biogenic Compounds from the Aquatic Environment. Sustainability, 14(11), 6432. https://doi.org/10.3390/su14116432







