Factors Influencing Earthworm Fauna in Parks in Megacity Beijing, China: An Application of a Synthetic and Simple Index (ESI)
Abstract
:1. Introduction
2. Materials and Methods
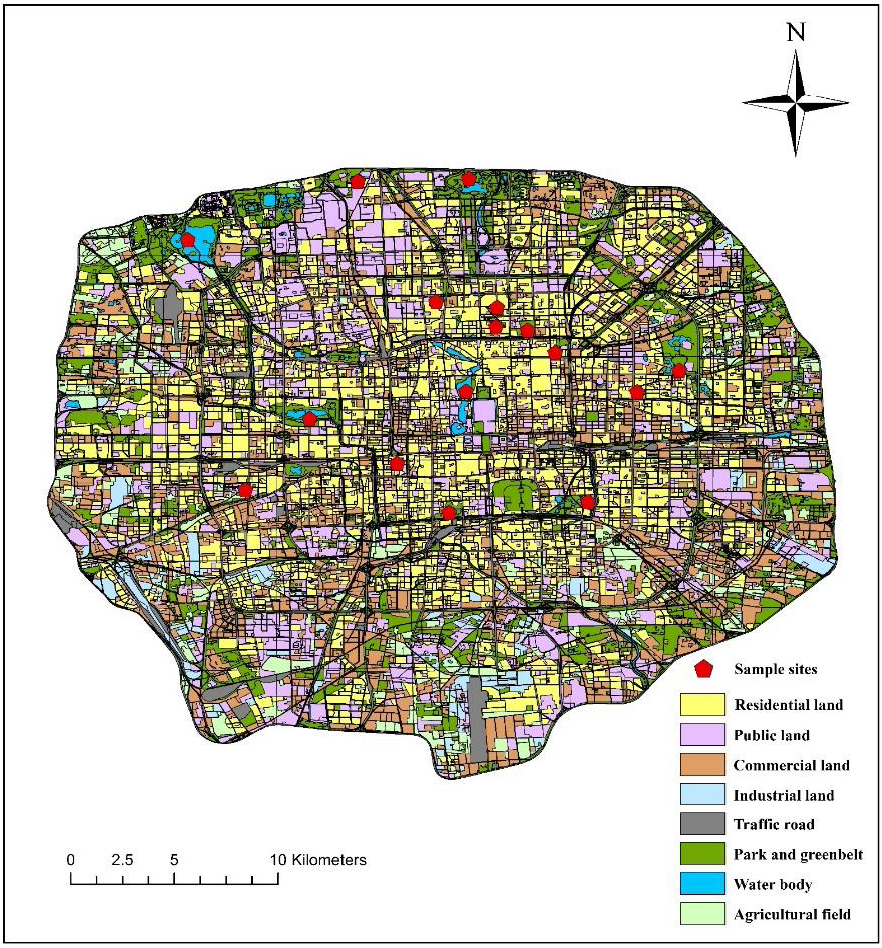
2.1. Study Sites
2.2. Earthworm Sampling
2.3. Soil and Litter Samplings and Chemical Analyses
2.4. Earthworm Ecological Characteristics
2.5. Calculation of ESI
2.6. Data Collection and Statistical Analyses
3. Results
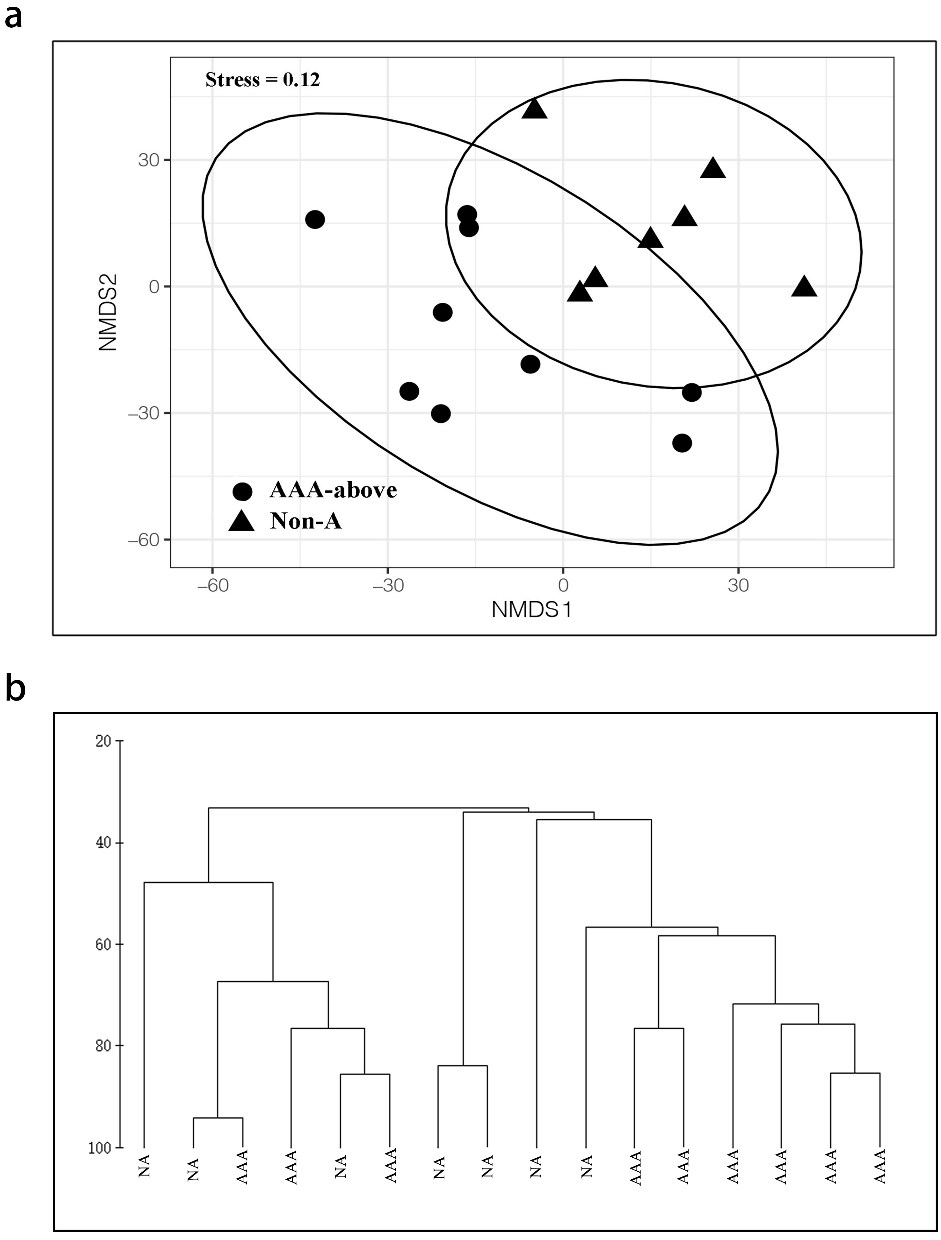
3.1. The Characteristics of Earthworm Population, Community Composition and Distribution in Urban Parks in Beijing
3.2. Park Traits That Affected the Earthworm Fauna
3.3. Relationships between Earthworm Fauna and Soil Properties
4. Discussion
5. Conclusions
- The ecological characteristics of earthworms in parks were comparable to those in other land use types in Beijing. Based on the dominance index, M. guillelmi, B. parvus, M. schmardae, M. californica, and O. occidentalis were the main species. Endogeic and anecic species were the dominant ecological groups.
- Urbanization factors, including construction age, geographical location, and visitor volume, significantly affected the earthworm population characteristics. Meanwhile, park intrinsic features, including greenspace area, park area, and tourist attraction rating, had significant effects on earthworm community composition, thereby influencing the species biodiversity and ESI.
- Earthworm community composition was significantly influenced by soil properties. Soil moisture contents and MBC were the crucial factors. The soil bulk density affected the biomass and average individual biomass, while the pH influenced the density and diversity of earthworms. Potential impacts from park traits on earthworm ecological characteristics were probably related to soil properties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ooms, A.; Dias, A.; van Oosten, A.; Cornelissen, J.; Ellers, J.; Berg, M. Species richness and functional diversity of isopod communities vary across an urbanisation gradient, but the direction and strength depend on soil type. Soil Biol. Biochem. 2020, 148, 107851. [Google Scholar] [CrossRef]
- Caruso, T.; Migliorini, M.; Rota, E.; Bargagli, R. Highly diverse urban soil communities: Does stochasticity play a major role? Appl. Soil Ecol. 2016, 110, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zhang, X.; Zhou, M.; He, S.; Gan, M.; Yang, L.; Wang, K. Impacts of urbanization and landscape pattern on habitat quality using OLS and GWR models in Hangzhou, China. Ecol. Indic. 2020, 117, 106654. [Google Scholar] [CrossRef]
- Zhou, D.; Tian, Y.; Jiang, G. Spatio-temporal investigation of the interactive relationship between urbanization and ecosystem services: Case study of the Jingjinji urban agglomeration, China. Ecol. Indic. 2018, 95, 152–164. [Google Scholar] [CrossRef]
- Ungaro, F.; Maienza, A.; Ugolini, F.; Lanini, G.; Baronti, S.; Calzolari, C. Assessment of joint soil ecosystem services supply in urban green spaces: A case study in Northern Italy. Urban For. Urban Green. 2021, 67, 127455. [Google Scholar] [CrossRef]
- Xie, T.; Wang, M.; Chen, W.; Uwizeyimana, H. Impacts of urbanization and landscape patterns on the earthworm communities in residential areas in Beijing. Sci. Total Environ. 2018, 626, 1261–1269. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J.; Khreis, H.; Triguero-Mas, M.; Gascon, M.; Dadvand, P. Fifty shades of green. Epidemiology 2017, 28, 63–71. [Google Scholar] [CrossRef]
- Tóth, Z.; Szlavecz, K.; Schmidt, D.J.E.; Hornung, E.; Setälä, H.; Yesilonis, I.D.; Kotze, D.J.; Dombos, M.; Pouyat, R.; Mishra, S.; et al. Earthworm assemblages in urban habitats across biogeographical regions. Appl. Soil Ecol. 2020, 151, 103530. [Google Scholar] [CrossRef]
- Glasstetter, M. Earthworm diversity in urban habitats of Basel (Northwestern Switzerland). Zool. Middle East 2012, 58 (Suppl. S4), 95–102. [Google Scholar] [CrossRef]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban For. Urban Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Paszkowski, C.A. The effects of urbanization on North American amphibian species: Identifying new directions for urban conservation. Urban Ecosyst. 2012, 15, 133–147. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Shu, X.; Pei, E.; Yuan, X.; Wang, T.; Wang, Z. Influence of breeding habitat characteristics and landscape heterogeneity on anuran species richness and abundance in urban parks of Shanghai, China. Urban For. Urban Green. 2018, 32, 56–63. [Google Scholar] [CrossRef]
- Dupont, L.; Torres-Leguizamon, M.; René-Corail, P.; Mathieu, J. Landscape features impact connectivity between soil populations: A comparative study of gene flow in earthworms. Mol. Ecol. 2017, 26, 3128–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maréchal, J.; Hoeffner, K.; Marié, X.; Cluzeau, D. Response of earthworm communities to soil engineering and soil isolation in urban landscapes. Ecol. Eng. 2021, 169, 106307. [Google Scholar] [CrossRef]
- Römbke, J.; Jänsch, S.; Didden, W. The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 249–265. [Google Scholar] [CrossRef]
- Syers, J.K.; Springett, J.A. Earthworms and soil fertility. Plant Soil 1984, 76, 93–104. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm Ecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Paoletti, M.G. The role of earthworms for assessment of sustainability and as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 137–155. [Google Scholar] [CrossRef]
- Santorufo, L.; van Gestel, K.; Rocco, A.; Maisto, G. Soil invertebrates as bioindicators of urban soil quality. Environ. Pollut. 2012, 161, 57–63. [Google Scholar] [CrossRef]
- Milutinović, T.; Milanović, J.; Stojanović, M. Application of species-richness estimators for the assessment of earthworm diversity. J. Nat. Hist. 2015, 49, 273–283. [Google Scholar] [CrossRef]
- Bouché, M. PU 43 Stratégies lombriciennes. Ecol. Bull. 1977, 25, 122–132. [Google Scholar]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Gong, Z. Chinese Soil Taxomomy; Science Press: Beijing, China, 2001. [Google Scholar]
- Di, Z.; Dai, W. Soil Science; Science Press: Beijing, China, 2011. [Google Scholar]
- Smetak, K.M.; Johnson-Maynard, J.L.; Lloyd, J.E. Earthworm population density and diversity in different-aged urban systems. Appl. Soil Ecol. 2007, 37, 161–168. [Google Scholar] [CrossRef]
- Dedeke, G.A.; Owagboriaye, F.; Adebambo, A.; Ademolu, K. Earthworm metallothionein production as biomarker of heavy metal pollution in abattoir soil. Appl. Soil Ecol. 2016, 104, 42–47. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Harris, J.; James, I.; Ritz, K. Inefficiency of mustard extraction technique for assessing size and structure of earthworm communities in UK pasture. Soil Biol. Biochem. 2006, 38, 2990–2992. [Google Scholar] [CrossRef]
- Xu, Q. Terrestrial Earthworm (Opisthopora: Olipchaeta) of China, Beijing; China Agriculture Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Mccallum, H.M.; Wilson, J.D.; Beaumont, D.; Sheldon, R.; O’Brien, M.G.; Park, K.J. A role for liming as a conservation intervention? Earthworm abundance is associated with higher soil pH and foraging activity of a threatened shorebird in upland grasslands. Agric. Ecosyst. Environ. 2016, 223, 182–189. [Google Scholar] [CrossRef]
- Wang, M.; Markert, B.; Shen, W.; Chen, W.; Peng, C.; Ouyang, Z. Microbial biomass carbon and enzyme activities of urban soils in Beijing. Environ. Sci. Pollut. Res. 2011, 18, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, T.C.; Haynes, R.J. Influence of agricultural land use on the size and composition of earthworm communities in northern KwaZulu-Natal, South Africa. Appl. Soil Ecol. 2004, 27, 77–88. [Google Scholar] [CrossRef]
- McNaughton, S.J. Relationships among Functional Properties of Californian Grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Doran, J.W.; Coleman, D.C.; Bezdicek, D.F. Defining Soil Quality for a Sustainable Environment; Soil Science Society of America, Inc.: Madison, WI, USA, 1994. [Google Scholar]
- Diack, M.; Stott, D.E. Development of a Soil Quality Index for the Chalmers Silty Clay Loam from the Midwest USA. Available online: https://topsoil.nserl.purdue.edu/nserlweb-old/isco99/pdf/ISCOdisc/SustainingTheGlobalFarm/P024-Diack.pdf (accessed on 28 March 2022).
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indices for evaluating the effect of intensive cropping, fertilisation and manuring for 31. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Zhang, R. Earthworm in meadow brown soil of Beijng. Chin. J. Soil Sci. 1964, 1, 1–33. (In Chinese) [Google Scholar]
- Zhang, Y. Relationship between earthworm diversity and soil environment in Hebei area. J. China Agric. Univ. 2017, 22, 60–68. (In Chinese) [Google Scholar]
- Fang, P.; Wu, W.L.; Xu, Q.; Jiahai, H.; Han, C.; Paoletti, M.G. Assessing Bioindication with Earthworms in An Intensively Farmed Rural Landscape (Yuanqiao and Daqiao Villages in Qianjiang Municipality, Located in Hubei Province, Subtropical China). Crit. Rev. Plant Sci. 1999, 18, 429–455. [Google Scholar] [CrossRef]
- Pižl, V.; Schlaghamerský, J. The impact of pedestrian activity on soil annelids in urban greens. Eur. J. Soil Biol. 2007, 43, S68–S71. [Google Scholar] [CrossRef]
- Saha, A.; Haque, M.; Mm, R. Seasonal abundance of earthworms in different habitats of Rajshahi university and Rajshahi sugar mill areas. Bangladesh J. Zool. 2009, 37, 69–74. [Google Scholar]
- Marcotullio, P.J.; Braimoh, A.K.; Onishi, T. The Impact of Urbanization on Soils. In Land Use and Soil Resources; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Ernst, G.; Emmerling, C. Impact of five different tillage systems on soil organic carbon content and the density, biomass, and community composition of earthworms after a ten year period. Eur. J. Soil Biol. 2009, 45, 247–251. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Pérès, G.; Vandenbulcke, F.; Guernion, M.; Hedde, M.; Beguiristain, T.; Douay, F.; Houot, S.; Piron, D.; Richard, A.; Bispo, A.; et al. Earthworm indicators as tools for soil monitoring, characterization and risk assessment. An example from the national Bioindicator programme (France). Pedobiol. Int. J. Soil Biol. 2011, 54, S77–S87. [Google Scholar] [CrossRef]
- García-Pérez, J.; Alarcón-Gutiérrez, E.; Perroni, Y.; Barois, I. Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl. Soil Ecol. 2014, 83, 230–237. [Google Scholar] [CrossRef]
- Hackenberger, D.K.; Hackenberger, B.K. Earthworm community structure in grassland habitats differentiated by climate type during two consecutive seasons. Eur. J. Soil Biol. 2014, 61, 27–34. [Google Scholar] [CrossRef]
- Margerie, P.; Decaëns, T.; Bureau, F.; Alard, D. Spatial distribution of earthworm species assemblages in a chalky slope of the Seine Valley (Normandy, France). Eur. J. Soil Biol. 2001, 37, 291–296. [Google Scholar] [CrossRef]
- Macarthur, R.H.; Wilson, E.O. An equilibrium theory of insular zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species–area relationship? Glob. Ecol. Biogeogr. 2010, 17, 532–538. [Google Scholar] [CrossRef]
- Heino, J. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 2000, 418, 229–242. [Google Scholar] [CrossRef]
- Pitacco, V.; Mistri, M.; Lipej, L. Species-Area Relationship (SAR) models as tools for estimating faunal biodiversity associated with habitat builder species in sensitive areas: The case of the Mediterranean stony coral (Cladocora caespitosa). Mar. Environ. Res. 2019, 149, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, D.; Baker, G.; Bissett, A.; Thrall, P. Earthworm composition, diversity and biomass under three land use systems in south-eastern Australia. Appl. Soil Ecol. 2015, 88, 32–40. [Google Scholar] [CrossRef]
- Falco, L.B.; Sandler, R.; Momo, F.; Di Ciocco, C.; Saravia, L.; Coviella, C. Earthworm assemblages in different intensity of agricultural uses and their relation to edaphic variables. PeerJ 2015, 3, e979. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Maynard, J.L.; Prather, T.S. Earthworm density and biomass in relation to plant diversity and soil properties in a Palouse prairie remnant. Appl. Soil Ecol. 2013, 72, 119–127. [Google Scholar]
- Berry, E.C.; Jordan, D. Temperature and soil moisture content effects on the growth of Lumbricus terrestris (Oligochaeta: Lumbricidae) under laboratory conditions. Soil Biol. Biochem. 2001, 33, 133–136. [Google Scholar] [CrossRef]
- Wever, L.A.; Lysyk, T.J.; Clapperton, M.J. The influence of soil moisture and temperature on the survival, aestivation, growth and development of juvenile Aporrectodea tuberculata (Eisen) (Lumbricidae). Pedobiologia 2001, 45, 121–133. [Google Scholar] [CrossRef]


| Sample | Density (ind/m2) | Biomass (g/m2) | Individual Biomass (g/ind) | Adult (%) | Species Richness | H | D | J | 1-γ | ESI |
|---|---|---|---|---|---|---|---|---|---|---|
| Urban parks (This study) | 38.6 ± 19.5 | 34.0 ± 23.2 | 0.892 ± 0.514 | 76.2 ± 16.4 | 3.06 ± 1.12 | 0.906 ± 0.375 | 0.556 ± 0.271 | 0.825 ± 0.234 | 0.553± 0.192 | 0.637 ± 0.185 |
| Urban parks (Late August) | 47.3 ± 20.5 | 39.2 ± 26.3 | 0.823 ± 0.614 | 60.0 ± 32.9 | 2.81 ± 1.07 | 0.889 ± 0.360 | 0.488 ± 0.242 | 0.834 ± 0.238 | 0.530 ± 0.186 | 0.585 ± 0.329 |
| Urban parks (Early September) | 28.7 ± 20.1 | 32.0 ± 31.1 | 1.46 ± 20.6 | 78.0 ±28.7 | 2.31 ± 1.21 | 0.669 ± 0.462 | 0.395 ± 0.299 | 0.688 ±0.396 | 0.404 ± 0.259 | 0.780 ± 0.287 |
| Residential areas, Beijing [6] | 44.6 ± 39.1 | 15.6 ± 14.0 | 0.319 ± 0.325 | 75.3 ± 18.9 | 3.08 ± 2.19 | 2.20 ± 0.081 | ||||
| Natural and arable land, Tongzhou, Beijing [37] | 81.6 ± 5.7 | / | / | / | / | / | / | / | / | / |
| Forest, Hebei [38] | 53.1 | 4 | 0.451 | 0.662 | 0.325 | 0.398 | ||||
| Arable land, Hubei, China [39] | 23 ± 32 | 23 ± 27.4 | 0.8 ± 0.75 | 31 ± 29 | 16 (total) | / | / | / | / | / |
| Urban parks, Moscow, USA (lawn, >75 years) [25] | 437 | 94.12 | / | / | 2.33 ± 1.15 | / | / | / | / | / |
| Urban parks, Bron, Czech [40] | 121 ± 32 (path) | 111–288 (path) | / | / | 6 (total) | / | / | / | / | / |
| 256 ± 68 (lawn) | 51.1 ± 11.5 (lawn) |
| Earthworm Species | Family | Genus | Ecological Groups | Y |
|---|---|---|---|---|
| Amynthas hupeiensis (Michaelsen, 1895) | Megascolecidae | Amynthas | Anecic | 0.01 |
| Amynthas robustus (Perrier, 1872) | Megascolecidae | Amynthas | Endogeic | <0.001 |
| Bimastus parvus (Eisen, 1874) | Lumbricidae | Bimastus | Epigeic | 0.10 |
| Drawida japonica (Michaelsen, 1931) | Moniligastridae | Drawida | Epigeic | <0.001 |
| Metaphire californica (Kinberg, 1867) | Megascolecidae | Metaphire | Endogeic | 0.05 |
| Metaphire guillelmi (Michaelsen, 1895) | Megascolecidae | Metaphire | Anecic | 0.26 |
| Metaphire schmardae (Horst, 1883) | Megascolecidae | Metaphire | Endogeic | 0.03 |
| Ocnerodrilus occidentalis (Eisen, 1878) | Megascolecidae | Ocnerodrilus | Endogeic | 0.05 |
| Age | Distance | Visitor Volume | Greenspace Area | Park Area | Greenspace Ratio | Tourist Attraction Rating | |
|---|---|---|---|---|---|---|---|
| Density | −0.017 | −0.098 | 0.154 | −0.122 | −0.137 | −0.377 | 0.036 |
| Biomass | 0.693 ** | −0.594 * | 0.236 | −0.053 | −0.118 | −0.221 | 0.314 |
| Individual biomass | 0.505 * | −0.621 * | 0.208 | −0.353 | −0.355 | −0.309 | −0.015 |
| Adults | −0.006 | −0.425 | 0.083 | −0.236 | −0.239 | 0.019 | −0.335 |
| Species richness | 0.163 | 0.050 | 0.487 | 0.598 * | 0.645 ** | −0.141 | 0.752 ** |
| A. hupeiensis | 0.261 | −0.306 | −0.202 | −0.025 | −0.122 | 0.052 | −0.128 |
| A. robustus | −0.192 | −0.005 | 0.005 | 0.051 | 0.210 | −0.483 | −0.065 |
| B. parvus | 0.152 | −0.089 | 0.374 | 0.452 | 0.384 | 0.083 | 0.619 * |
| D. japonica | −0.079 | 0.322 | 0.187 | 0.378 | 0.544 * | −0.079 | 0.507 * |
| M. californica | 0.205 | 0.071 | 0.546 * | 0.453 | 0.502 * | 0.504 * | 0.467 |
| M. guillelmi | 0.242 | −0.315 | −0.263 | −0.289 | −0.435 | −0.372 | −0.258 |
| M. schmardae | 0.127 | 0.055 | 0.300 | −0.191 | −0.063 | −0.310 | 0.222 |
| O. occidentalis | 0.240 | −0.230 | −0.084 | 0.236 | 0.152 | −0.134 | 0.266 |
| H | −0.004 | 0.239 | 0.469 | 0.585 * | 0.656 ** | −0.138 | 0.690 ** |
| D | −0.062 | 0.168 | 0.498 * | 0.598 * | 0.680** | −0.168 | 0.675 ** |
| J | −0.295 | 0.424 | 0.047 | −0.016 | 0.091 | −0.417 | 0.031 |
| 1-γ | −0.006 | 0.244 | 0.452 | 0.587 * | 0.653 ** | −0.131 | 0.691 ** |
| Epigeics | 0.203 | −0.293 | −0.133 | −0.375 | −0.400 | −0.364 | −0.407 |
| Endogeics | −0.095 | 0.367 | 0.473 | 0.753 ** | 0.742 ** | 0.273 | 0.769 ** |
| Anecis | −0.159 | 0.142 | −0.075 | 0.006 | 0.072 | 0.211 | −0.023 |
| ESI | 0.436 | −0.248 | 0.442 | 0.385 | 0.394 | −0.241 | 0.690 ** |
| Characteristics | Construction Age | Location | Tourist Attraction Rating | ||||
|---|---|---|---|---|---|---|---|
| <30 Years | 30–100 Years | >100 Years | Outer City | Inner City | Non-A | AAA-Above | |
| Density (ind/m2) | 28.2 ± 4.89 a | 35.6 ± 8.42 a | 51.4 ± 7.46 a | 37.1 ± 4.85 a | 42.1 ± 12.3 a | 25.5 ± 5.87 a | 46.5 ± 6.41 a |
| Biomass (g/m2) | 14.7 ± 4.85 b | 31.9 ± 8.62 ab | 52.4 ± 8.61 a | 27.2 ± 5.43 a | 48.8 ± 12.8 a | 25.2 ± 9.40 a | 40.8 ± 6.70 a |
| Individual biomass (g/m2) | 0.612 ± 0.229 a | 0.873 ± 0.152 a | 1.15 ± 0.292 a | 0.781 ± 0.129 a | 1.14 ± 0.290 a | 0.867 ± 0.270 a | 0.913 ± 0.111 a |
| Adult (%) | 0.783 ± 0.0652 a | 0.751 ± 0.0738 a | 0.760 ± 0.0777 a | 0.728 ± 0.0468 a | 0.836 ± 0.0780 a | 0.800 ± 0.0489 a | 0.733 ± 0.0630 a |
| Species richness | 3.00 ± 0.578 a | 2.57 ± 0.369 a | 3.60 ± 0.400 a | 3.00 ± 0.270 a | 3.00 ± 0.632 a | 2.29 ± 0.360 b | 3.56 ± 0.242 a |
| H | 0.962 ± 0.220 a | 0.749 ± 0.141 a | 1.08 ± 0.132 a | 0.946 ± 0.0921 a | 0.818 ± 0.237 a | 0.671 ± 0.155 b | 1.09 ± 0.0761 a |
| D | 0.633 ± 0.178 a | 0.431 ± 0.0883 a | 0.669 ± 0.988 a | 0.571 ± 0.0714 a | 0.523 ± 0.163 a | 0.387 ± 0.110 b | 0.687 ± 0.0570 a |
| J | 0.906 ± 0.0612 a | 0.752 ± 0.129 a | 0.863 ± 0.0211 a | 0.895 ± 0.0276 a | 0.671 ± 0.168 a | 0.759 ± 0.876 a | 0.876 ± 0.0231 a |
| 1-γ | 0.588 ± 0.103 a | 0.481 ± 0.0826 a | 0.625 ± 0.0604 a | 0.587 ± 0.0394 a | 0.476 ± 0.131 a | 0.438 ± 0.0893 b | 0.642 ± 0.0278 a |
| Epigeics (%) | 0.183 ± 0.119 a | 0.168 ± 0.0627 a | 0.151 ± 0.0782 a | 0.208 ± 0.0549 a | 0.0746 ± 0.0573 a | 0.0615 ± 0.0399 b | 0.248 ± 0.0596 a |
| Endogeics (%) | 0.292 ± 0.128 a | 0.505 ± 0.113 a | 0.282 ± 0.0656 a | 0.384 ± 0.0624 a | 0.387 ± 0.174 a | 0.402 ± 0.137 a | 0.372 ± 0.0581 a |
| Anecics (%) | 0.396 ± 0.147 a | 0.276 ± 0.0979 a | 0.518 ± 0.125 a | 0.338 ± 0.0726 a | 0.477 ± 0.159 a | 0.475 ± 0.133 a | 0.309 ± 0.0648 a |
| ESI | 0.605 ± 0.0843 a | 0.564 ± 0.0807 a | 0.764 ± 0.0385 a | 0.631 ± 0.0404 a | 0.649 ± 0.128a | 0.518 ± 0.0732 b | 0.729 ± 0.0391 a |
| Parameters | Density | Biomass | Individual Biomass | Species Richness | H | D | J | 1-γ | ESI |
|---|---|---|---|---|---|---|---|---|---|
| K | 0.500 * | 0.456 | 0.068 | 0.272 | 0.094 | 0.166 | −0.168 | 0.094 | 0.400 |
| P | 0.165 | 0.462 | 0.594 * | −0.141 | −0.282 | −0.241 | −0.038 | −0.282 | 0.085 |
| Available P | −0.246 | 0.315 | 0.559 * | −0.066 | −0.076 | −0.031 | 0.126 | −0.076 | −0.041 |
| NH4-N | 0.577 * | 0.226 | −0.188 | 0.160 | 0.035 | 0.028 | −0.071 | 0.035 | 0.338 |
| Soluble C | −0.475 | 0.126 | 0.509 * | −0.404 | −0.271 | −0.322 | 0.112 | −0.271 | −0.203 |
| Bulk density | −0.371 | −0.791 ** | −0.500 * | −0.281 | −0.258 | −0.139 | −0.305 | −0.258 | −0.535 * |
| pH | 0.616 * | 0.387 | −0.175 | 0.581 * | 0.580 * | 0.421 | 0.138 | 0.580 * | 0.671 ** |
| Moisture | 0.637 ** | 0.691 ** | 0.388 | 0.557 * | 0.447 | 0.412 | 0.112 | 0.447 | 0.524 ** |
| MBC | 0.765 ** | 0.429 | −0.056 | 0.623 ** | 0.476 | 0.477 | −0.153 | 0.476 | 0.691 ** |
| Parameters | Construction Age | Location | Tourist Attraction Rating | ||||
|---|---|---|---|---|---|---|---|
| <30 Years | 30–100 Years | >100 Years | Outer City | Inner City | Non-A | AAA-Above | |
| K (mg/kg) | 1.76 ± 0.0410 a | 1.81 ± 0.0258 a | 1.83 ± 0.0246 a | 1.79 ± 0.0197 a | 1.82 ± 0.0318 a | 1.81 ± 0.0236 a | 1.80 ± 0.255 a |
| P (mg/kg) | 622 ± 97.2 a | 607 ± 49.7 a | 1013 ± 291 a | 613 ± 42.6 a | 1012 ± 257 a | 806 ± 215 a | 684 ± 80.7 a |
| Available K (mg/Kg) | 184 ± 22.0 a | 203 ± 25.5 a | 180 ± 35.5 a | 163± 15.2 b | 254 ± 17.9 a | 185 ± 20.5 a | 199 ± 26.3 a |
| Available P (mg/Kg) | 21.2 ± 8.83 a | 30.3 ± 15.8 a | 21.7 ± 6.81 a | 15.5 ± 3.72 b | 47 ± 19.6 a | 18.7 ± 5.76 a | 30.5 ± 12.2 a |
| NH4-N (mg/kg) | 14.4 ± 0.872 a | 16.1 ± 0.883 a | 15.5 ± 0.727 a | 15.7 ± 0.619 a | 15.1 ± 0.927 a | 15.3 ± 0.971 a | 15.7 ± 0.530 a |
| Soluble C (mg/kg) | 97.5 ± 29.8 a | 99.6 ± 19.1 a | 95.7 ± 32.0 a | 90.4 ± 15.4 a | 114 ± 30.5 a | 113 ± 23.6 a | 86.1 ± 16.8 a |
| Bulk density | 1.27 ± 0.0749 a | 1.17 ± 0.0496 a | 1.13 ± 0.0287 a | 1.21 ± 0.0377 a | 1.12 ± 0.0485 a | 1.18 ± 0.0614 a | 1.18 ± 0.0319 a |
| pH | 8.49 ± 0.105 a | 8.41 ± 0.0395 a | 8.50 ± 0.0612 a | 8.49 ± 0.0451 a | 8.40 ± 0.0501 a | 8.44 ± 0.0380 a | 8.48 ± 0.0571 a |
| Moisture | 13.3 ± 1.22 a | 16.7 ± 1.13 a | 18.9 ± 1.87 a | 15.7 ± 0.867 a | 18.4 ± 2.29 a | 14.2 ± 1.24 b | 18.4 ± 1.06 a |
| MBC (mg/kg) | 238 ± 26.8 a | 292 ± 35.6 a | 401 ± 79.4 a | 282 ± 23.0 a | 380 ± 89.9 a | 241 ± 20.3 b | 368 ± 49.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Li, X.; Wang, M.; Chen, W.; Faber, J.H. Factors Influencing Earthworm Fauna in Parks in Megacity Beijing, China: An Application of a Synthetic and Simple Index (ESI). Sustainability 2022, 14, 6054. https://doi.org/10.3390/su14106054
Xie T, Li X, Wang M, Chen W, Faber JH. Factors Influencing Earthworm Fauna in Parks in Megacity Beijing, China: An Application of a Synthetic and Simple Index (ESI). Sustainability. 2022; 14(10):6054. https://doi.org/10.3390/su14106054
Chicago/Turabian StyleXie, Tian, Xuzhi Li, Meie Wang, Weiping Chen, and Jack H. Faber. 2022. "Factors Influencing Earthworm Fauna in Parks in Megacity Beijing, China: An Application of a Synthetic and Simple Index (ESI)" Sustainability 14, no. 10: 6054. https://doi.org/10.3390/su14106054
APA StyleXie, T., Li, X., Wang, M., Chen, W., & Faber, J. H. (2022). Factors Influencing Earthworm Fauna in Parks in Megacity Beijing, China: An Application of a Synthetic and Simple Index (ESI). Sustainability, 14(10), 6054. https://doi.org/10.3390/su14106054






