Positive Effects and Optimal Ranges of Tea Saponins on Phytoremediation of Cadmium-Contaminated Soil
Abstract
:1. Introduction
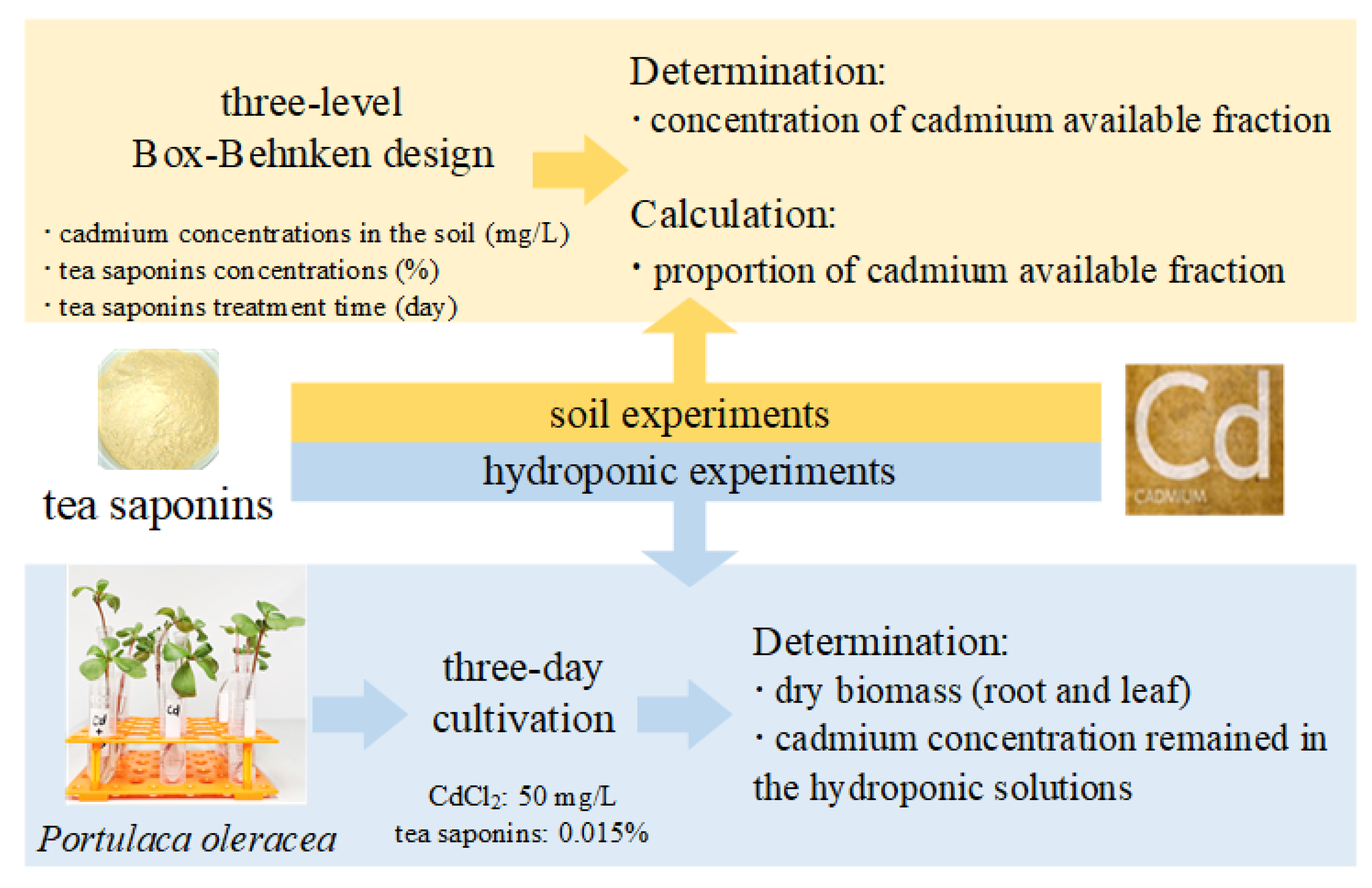
2. Materials and Methods
2.1. Soil Experiments
2.1.1. Soil Preparations
2.1.2. Quantitative Effects of TS
2.2. Plant Cultivation and Experiment
2.3. Cadmium Concentration Determination
2.4. Statistical Analysis
3. Results and Discussion
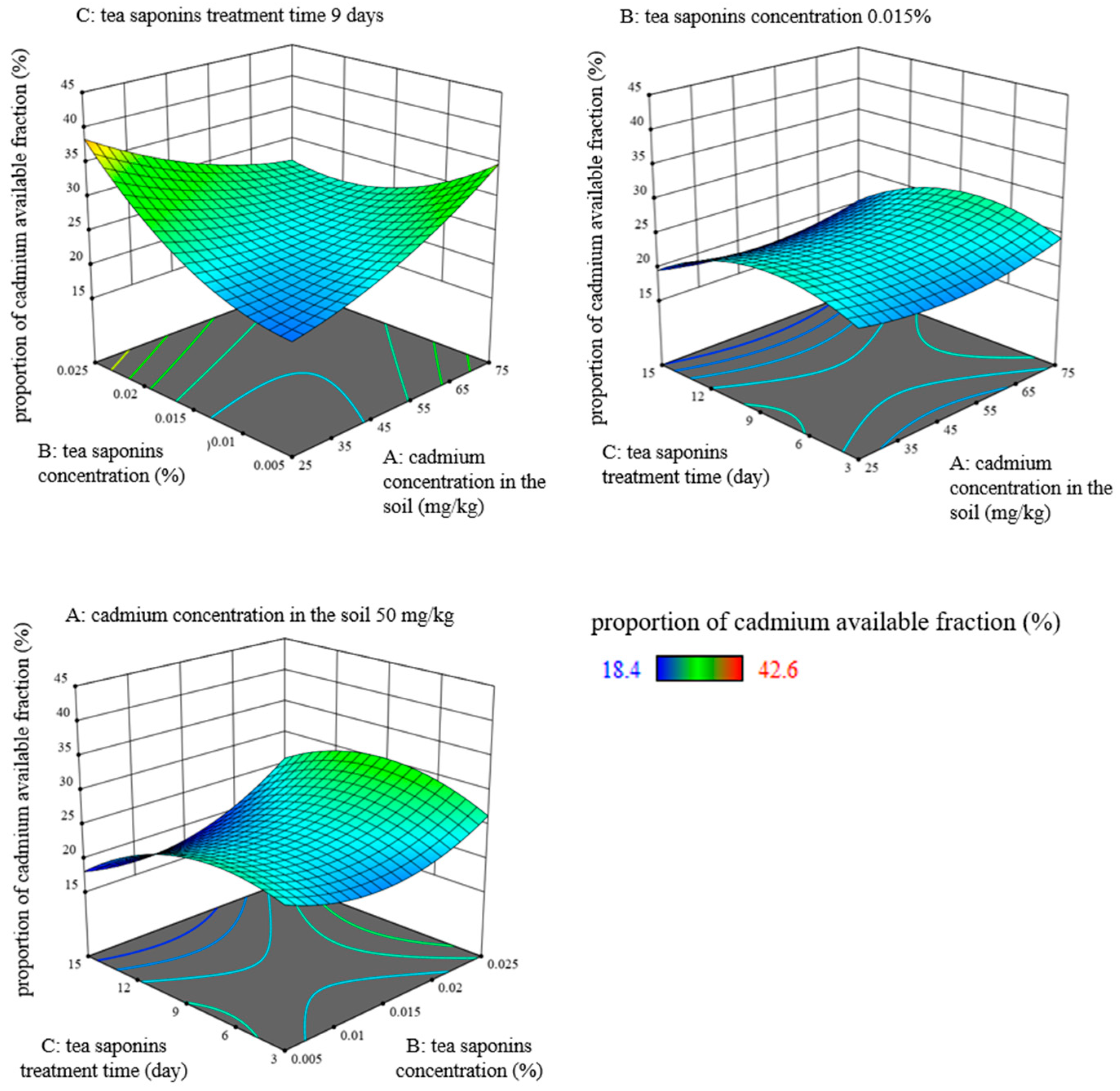
3.1. Effects of Cadmium Concentration in the Soil, TS Concentration, and TS Treatment Time
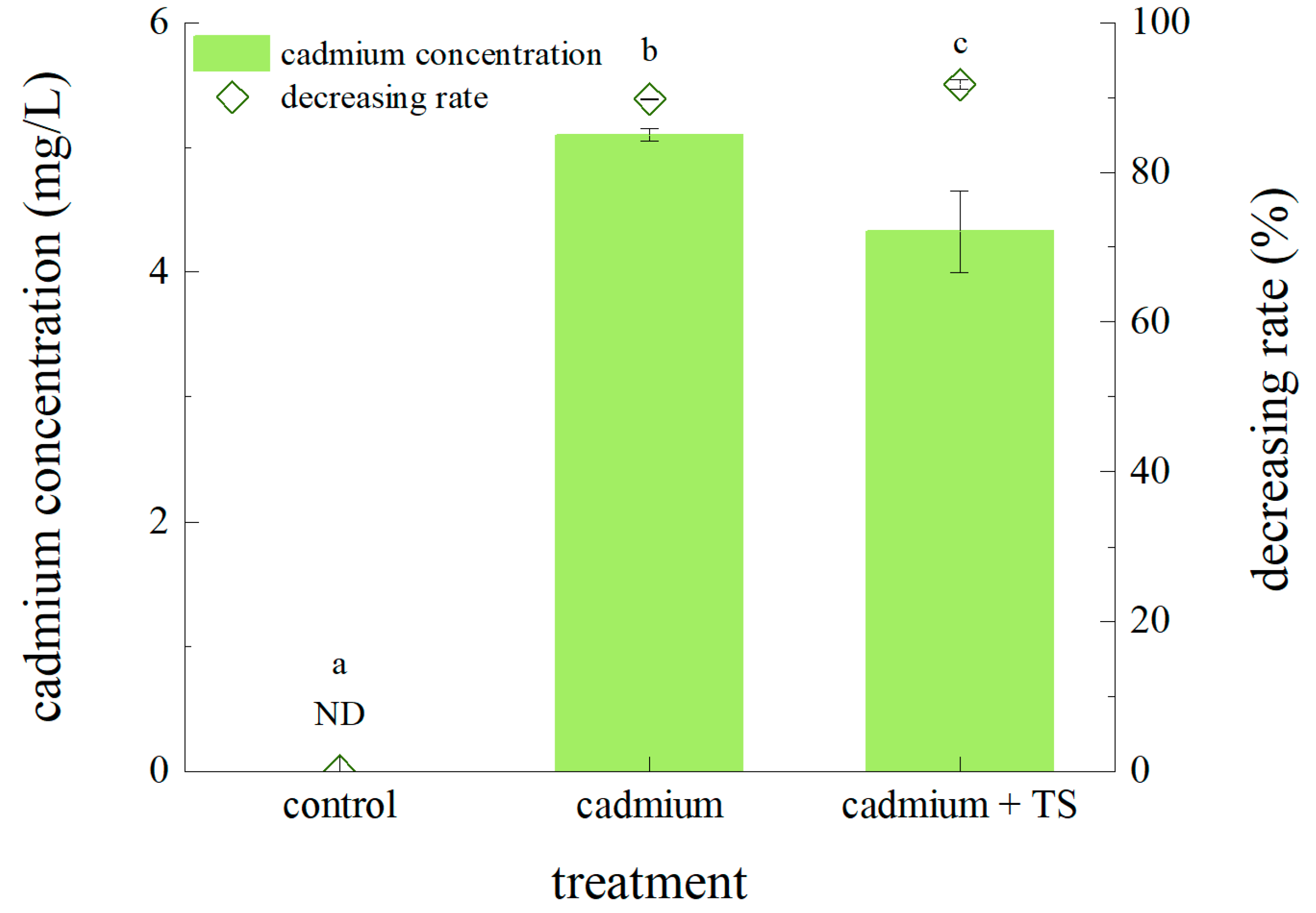
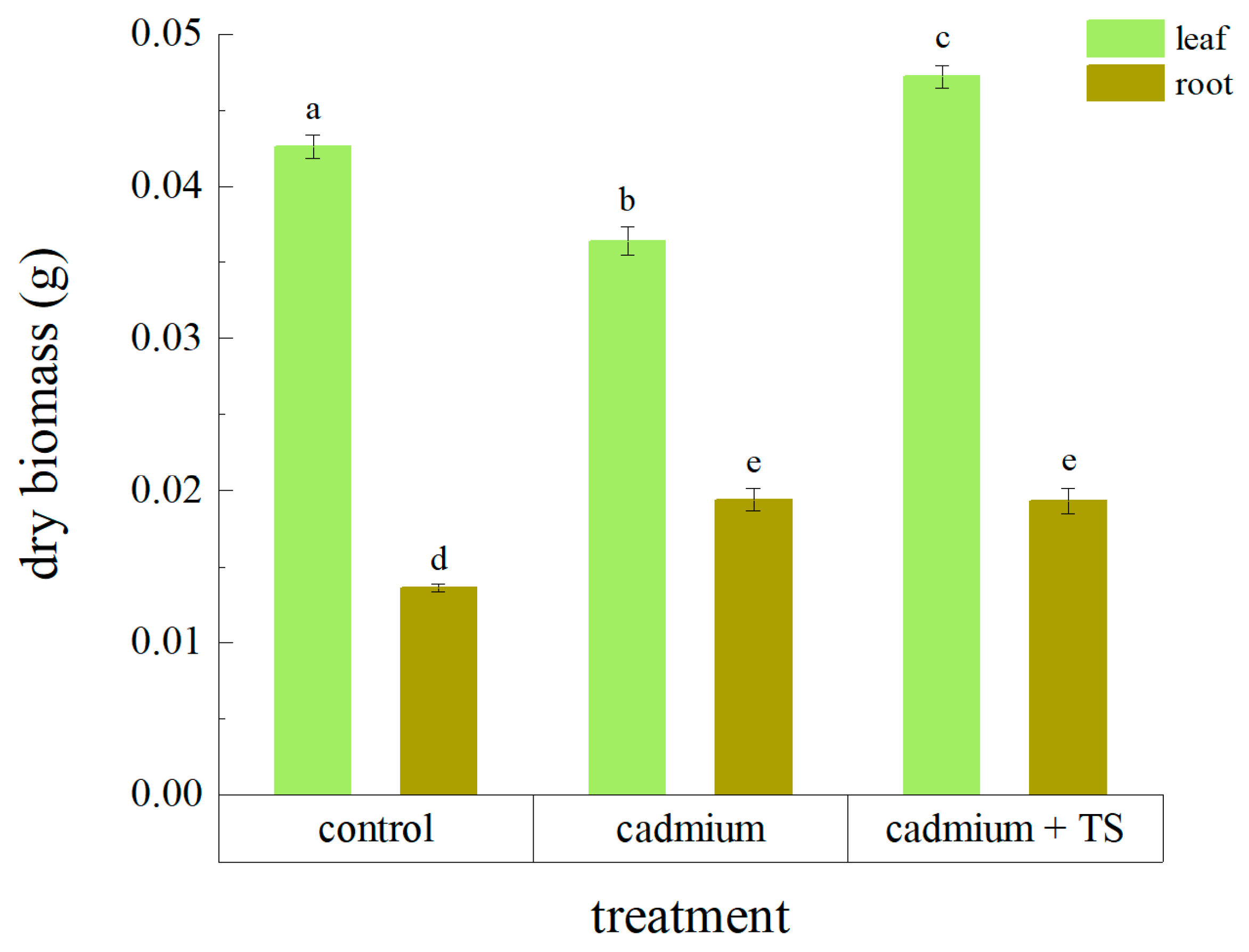
3.2. Effects of TS on Accumulator Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectrometry |
| ANOVA | Analysis of variance |
| BBD | Box-Behnken design |
| EDD | Ethylene diamine disuccinate |
| EDTA | Ethylene diamine tetraacetic acid |
| HS | Humic substances |
| NLMWOAs | Natural low molecular weight organic acids |
| PCBs | Polychlorinated biphenyls |
| SDS | Sodium dodecyl sulfate |
| SE | Standard error |
| TS | Tea saponins |
References
- Ministry of Environmental Protection of the People’s Republic of China; Ministry of Land Resources of the People’s Republic of China. Bulletin of the National Survey on Soil Pollution; Ministry of Environmental Protection of the People’s Republic of China/Ministry of Land Resources of the People’s Republic of China: Beijing, China, 2014.
- Subašić, M.; Šamec, D.; Selović, A.; Karalija, E. Phytoremediation of Cadmium Polluted Soils: Current Status and Approaches for Enhancing. Soil Syst. 2022, 6, 3. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Luo, H.; Wu, H.; Zhang, Y.; Chen, J.; Wu, M.; Xu, G.; Gao, H. Soil contamination with cadmium and potential risk around various mines in China during 2000–2020. J. Environ. Manag. 2022, 310, 114509. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten Chemicals of Major Public Health Concern; WHO: Geneva, Switzerland, 2010; pp. 1–4. [Google Scholar]
- McLaughlin, M.J.; Smolders, E.; Zhao, F.J.; Grant, C.; Montalvo, D. Managing cadmium in agricultural systems. Adv. Agron. 2021, 166, 1–129. [Google Scholar]
- Nordberg, G.F.; Bernard, A.; Diamond, G.L.; Duffus, J.H.; Illing, P.; Nordberg, M.; Bergdahl, I.A.; Jin, T.; Skerfving, S. Risk assessment of effects of cadmium on human health (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 755–808. [Google Scholar] [CrossRef] [Green Version]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J.M. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Lee, J.H. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess Eng. 2013, 18, 431–439. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- Agnello, A.C.; Huguenot, D.; Van Hullebusch, E.D.; Esposito, G. Enhanced phytoremediation: A review of low molecular weight organic acids and surfactants used as amendments. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2531–2576. [Google Scholar] [CrossRef] [Green Version]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water. Air. Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol. 2000, 145, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Cay, S. Enhancement of cadmium uptake by Amaranthus caudatus, an ornamental plant, using tea saponin. Environ. Monit. Assess. 2016, 188, 320. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; He, Y. Tea saponins: Effective natural surfactants beneficial for soil remediation, from preparation to application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Chi, X.; Yan, Z.; Cheng, W. Enhancing plant uptake of polychlorinated biphenyls and cadmium using tea saponin. Bioresour. Technol. 2009, 100, 4649–4653. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Zhang, X.; Hou, Y.; Hu, X.; Liang, X.; Chen, X. Influence of tea saponin on enhancing accessibility of pyrene and cadmium phytoremediated with Lolium multiflorum in co-contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 5705–5711. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Wang, C.; Zhang, X.; Li, H.; Chen, T.; Hou, Y.; Chen, X.; Liang, X. Solubilization Effect of Surfactants on Morphological Transformation of Cadmium and Pyrene in Co-Contaminated Soils. Water Air. Soil Pollut. 2015, 226, 147. [Google Scholar] [CrossRef]
- Xue, Z.; Wu, M.; Hu, H.; Kianpoor Kalkhajeh, Y. Cadmium uptake and transfer by Sedum plumbizincicola using EDTA, tea saponin, and citric acid as activators. Int. J. Phytoremediation 2021, 23, 1052–1060. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Dwivedi, S.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; Singh, N.K.; Chakraborty, S. Phytoremediation efficiency of Portulaca tuberosa rox and Portulaca oleracea L. naturally growing in an industrial effluent irrigated area in Vadodra, Gujrat, India. Environ. Monit Assess 2008, 147, 15–22. [Google Scholar] [CrossRef]
- Yuan, Q.; Chai, F.; Li, J. The Fixed Extraction Performance of Portulaca oleracea for the Heavy Metal Ions in Contaminated Soil. Hubei Agric. Sci. 2018, 57, 30–32. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chimca Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Becker, K. Methods in Molecular Biology; Humana Press: Towota, NJ, USA, 2007. [Google Scholar]
- Midhat, L.; Ouazzani, N.; Hejjaj, A.; Ouhammou, A.; Mandi, L. Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotoxicol. Environ. Saf. 2019, 169, 150–160. [Google Scholar] [CrossRef]
- Caldas, L.F.S.; De Paula, C.E.R.; Brum, D.M.; Cassella, R.J. Application of a four-variables Doehlert design for the multivariate optimization of copper determination in petroleum-derived insulating oils by GFAAS employing the dilute-and-shot approach. Fuel 2013, 105, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Stahle, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar] [CrossRef]
- Gill, G.S.; Kumar, A.; Agarwal, R. Nondestructive grading of black tea based on physical parameters by texture analysis. Biosyst. Eng. 2013, 116, 198–204. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-derived biochar: From production to application in removing heavy metal-contaminated water. Process Saf. Environ. Prot. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
| Run | Cadmium Concentration in the Soil (mg/kg) | Tea Saponins Concentration (%) | Tea Saponins Treatment Time (Day) |
|---|---|---|---|
| 1 | 50 | 0.025 | 3 |
| 2 | 50 | 0.005 | 3 |
| 3 | 25 | 0.025 | 9 |
| 4 | 50 | 0.025 | 15 |
| 5 | 50 | 0.015 | 9 |
| 6 | 75 | 0.015 | 3 |
| 7 | 75 | 0.005 | 9 |
| 8 | 75 | 0.015 | 15 |
| 9 | 50 | 0.015 | 9 |
| 10 | 75 | 0.025 | 9 |
| 11 | 50 | 0.005 | 15 |
| 12 | 25 | 0.015 | 3 |
| 13 | 25 | 0.015 | 15 |
| 14 | 25 | 0.005 | 9 |
| 15 | 50 | 0.015 | 9 |
| Fraction | Cadmium Concentration (mg/L) |
|---|---|
| Available | 0.131 ± 0.009 |
| Bound to carbonates | 0.310 ± 0.042 |
| Bound to Fe-Mn oxides | 0.210 ± 0.021 |
| Bound to organic matter | 0.147 ± 0.018 |
| Residual | 0.0065 ± 0.0007 |
| Parameters | Values | |
|---|---|---|
| Lamp current (mA) | 4.0 | |
| Wavelength (nm) | 228.8 | |
| Slit width (nm) | 0.5 | |
| Range of the standard curve (mg/L) | 0–2.0 | |
| Volume flow rate (L/min) | Air | 13.5 |
| Acetylene | 2.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-L.; He, Y. Positive Effects and Optimal Ranges of Tea Saponins on Phytoremediation of Cadmium-Contaminated Soil. Sustainability 2022, 14, 5941. https://doi.org/10.3390/su14105941
Yu X-L, He Y. Positive Effects and Optimal Ranges of Tea Saponins on Phytoremediation of Cadmium-Contaminated Soil. Sustainability. 2022; 14(10):5941. https://doi.org/10.3390/su14105941
Chicago/Turabian StyleYu, Xiao-Lan, and Yong He. 2022. "Positive Effects and Optimal Ranges of Tea Saponins on Phytoremediation of Cadmium-Contaminated Soil" Sustainability 14, no. 10: 5941. https://doi.org/10.3390/su14105941
APA StyleYu, X.-L., & He, Y. (2022). Positive Effects and Optimal Ranges of Tea Saponins on Phytoremediation of Cadmium-Contaminated Soil. Sustainability, 14(10), 5941. https://doi.org/10.3390/su14105941







