Distribution Patterns of Soil Fauna in Different Forest Habitat Types of North Hebei Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Processing of Samples
2.3. Statistical Analysis
3. Results
3.1. Soil Fauna Communities Composition
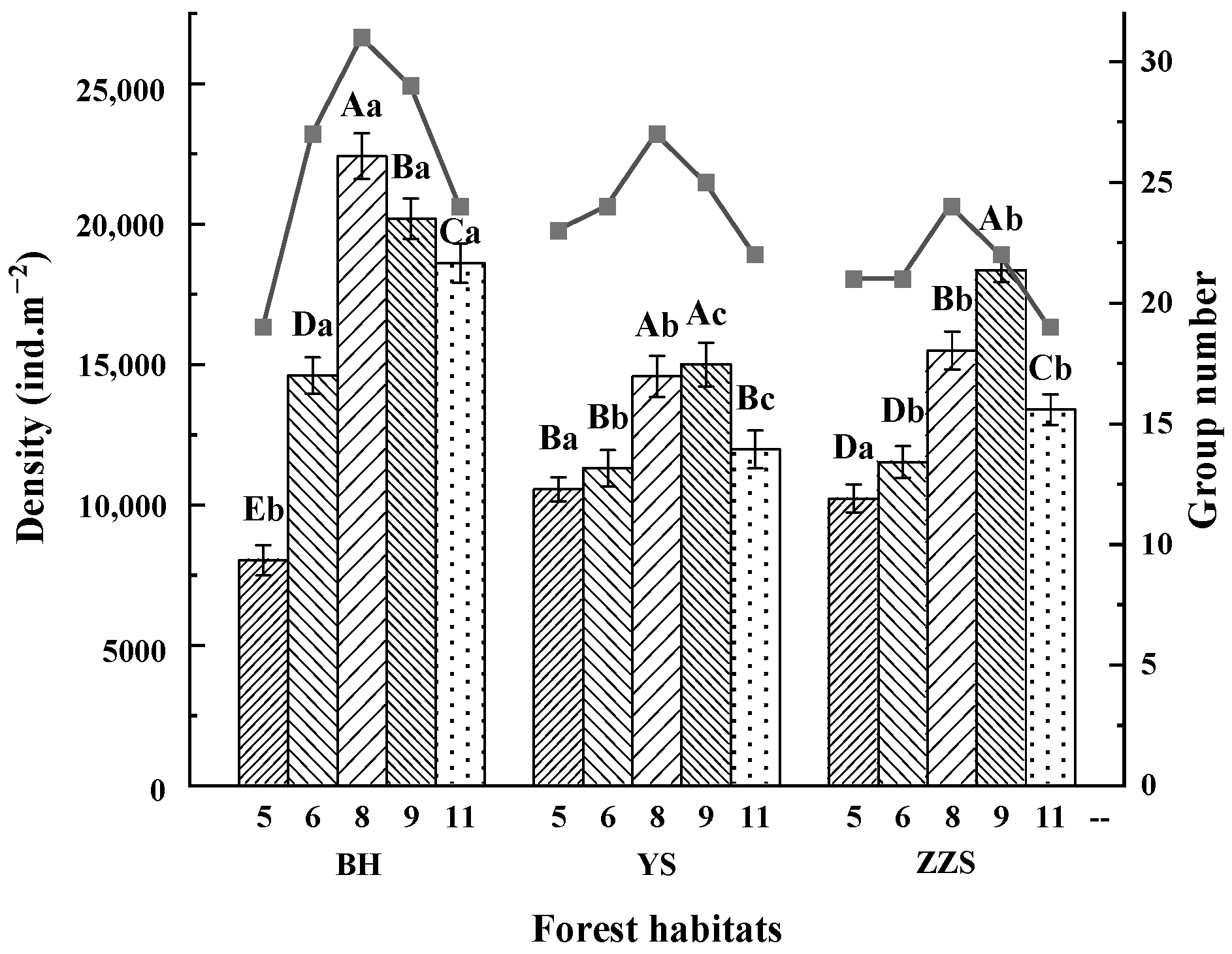
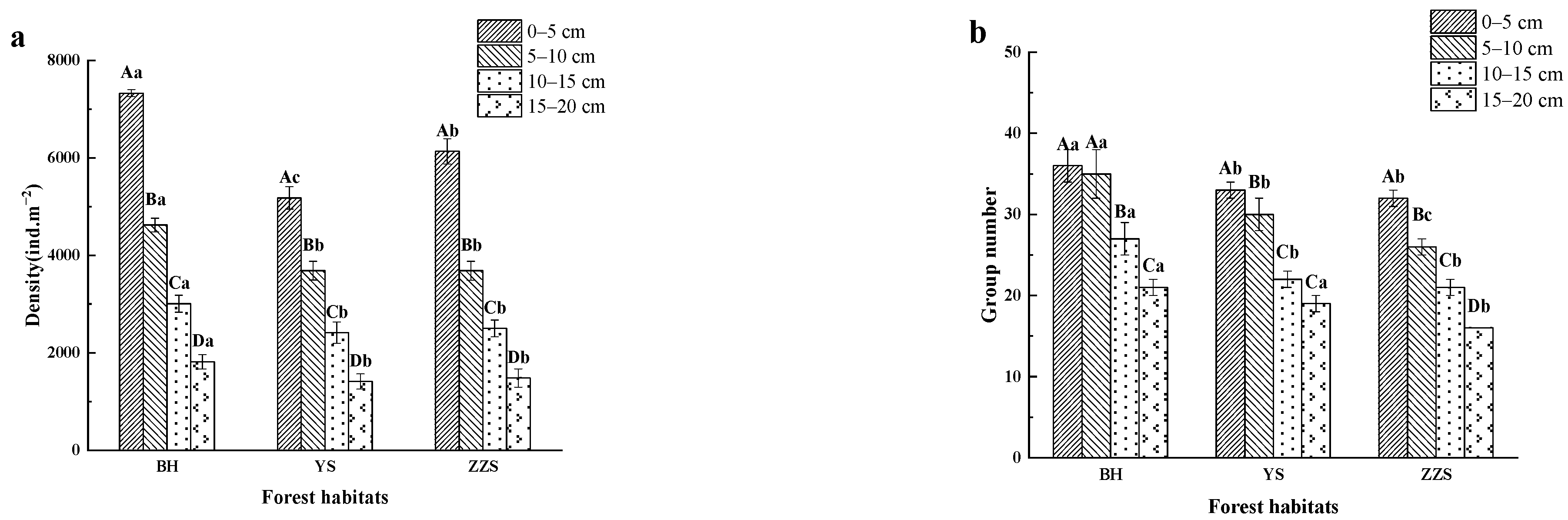
3.2. Distribution of Soil Fauna Communities
3.3. Diversity Characteristics
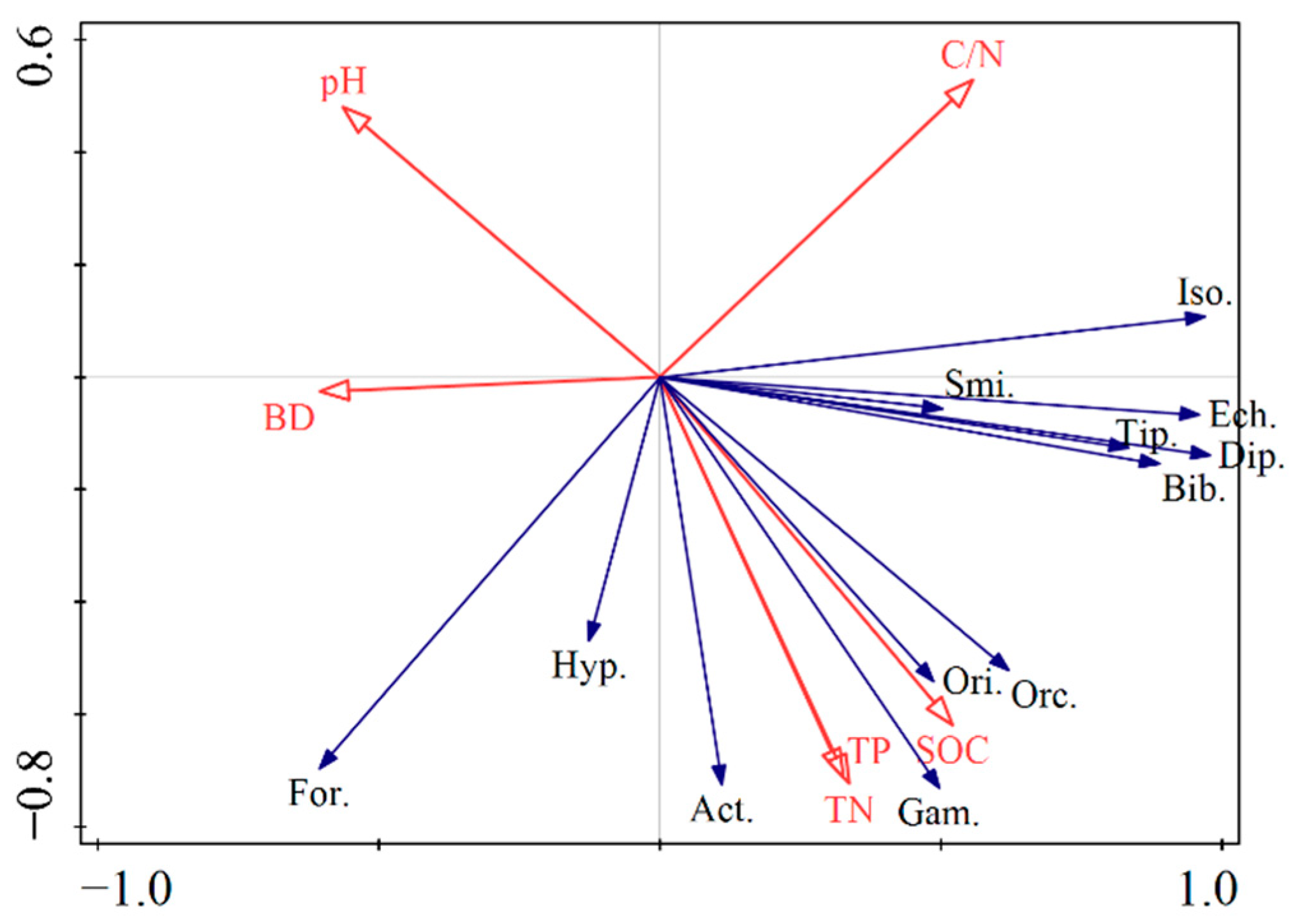
3.4. Effects of Soil Physicochemical Factors on Soil Fauna Communities
4. Discussion
4.1. Spatial Distribution of Soil Fauna in Different Forest Habitats
4.2. Temporal Distribution of Soil Fauna in Different Forest Habitats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Eekeren, N.; Bommele, L.; Bloem, J.; Schouten, T.; Rutgers, M.; de Goede, R.; Reheul, D.; Brussaard, L. Soil biological quality after 36 years of ley-arable cropping, permanent grassland and permanent arable cropping. Appl. Soil Ecol. 2008, 40, 432–446. [Google Scholar] [CrossRef]
- Barajas-Guzmán, G.; Alvarez-Sánchez, J. The relationships between litter fauna and rates of litter decomposition in a tropical rain forest. Appl. Soil Ecol. 2003, 24, 91–100. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; Zoomer, H.R.; Berg, M.P.; de Ruiter, P.C.; Verhoef, H.A.; Martijn Bezemer, T.; van der Putten, W.H. Soil invertebrate fauna enhances grassland succession and diversity. Nature 2003, 422, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, C.M.; Zaborski, E.R.; Wander, M.M. Nematode indicators as integrative measures of soil condition in organic cropping systems. Soil Biol. Biochem. 2013, 64, 103–113. [Google Scholar] [CrossRef]
- Niemeyer, J.C.; Lolata, G.B.; de Carvalho, G.M.; Da Silva, E.M.; Sousa, J.P.; Nogueira, M.A. Microbial indicators of soil health as tools for ecological risk assessment of a metal contaminated site in Brazil. Appl. Soil Ecol. 2012, 59, 96–105. [Google Scholar] [CrossRef]
- Buckerfield, J.C.; Lee, K.E.; Davoren, C.W.; Hannay, J.N. Earthworms as indicators of sustainable production in dryland cropping in Southern Australia. Soil Biol. Biochem. 1997, 29, 547–554. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Hassall, M. Woodlice (Isopoda: Oniscidea): Their potential for assessing sustainability and use as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 157–165. [Google Scholar] [CrossRef]
- Suthar, S. Earthworm communities a bioindicator of arable land management practices: A case study in semiarid region of India. Ecol. Ind. 2009, 9, 588–594. [Google Scholar] [CrossRef]
- Yang, X.; Shao, M.A.; Li, T.C.; Gan, M.; Chen, M.Y. Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indic. 2021, 122, 107236. [Google Scholar] [CrossRef]
- Franklin, E.; Magnusson, W.E.; Luizao, F.J. Relative effects of biotic and abiotic factors on the composition of soil invertebrate communities in an Amazonian savanna. Appl. Soil Ecol. 2004, 29, 259–273. [Google Scholar] [CrossRef]
- Wall, D.H.; Bradford, M.A.; St John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef]
- Hanel, L.; Cerevkova, A. Species and genera of soil nematodes in forest ecosystems of the Vihorlat Protected Landscape Area, Slovakia. Helminthologia 2010, 47, 123–135. [Google Scholar] [CrossRef]
- Joo, S.J.; Yim, M.H.; Nakane, K. Contribution of microarthropods to the decomposition of needle litter in a Japanese cedar (Cryptomeria japonica D. Don) plantation. For. Ecol. Manag. 2006, 234, 192–198. [Google Scholar] [CrossRef]
- Tian, G.; Brussaard, L.; Kang, B.T.; Swift, M.J. Soil Fauna-Mediated Decomposition of Plant Residues under Constrained Environmental Conditions; CAB International: Wallingford, CT, USA, 1997; pp. 125–134. [Google Scholar]
- Farska, J.; Prejzkova, K.; Rusek, J. Management intensity affects traits of soil microarthropod community in montane spruce forest. Appl. Soil Ecol. 2014, 75, 71–79. [Google Scholar] [CrossRef]
- Mulder, C. Driving forces from soil invertebrates to ecosystem functioning: The allometric perspective. Naturwissenschaften 2006, 93, 467–479. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.J.; Gou, Y.B.; Yang, C.L. Soil animal community diversity in the hilly areas around east Dongting lake. J. Hunan Inst. Sci. Technol. (Nat. Sci.) 2008, 21, 73–77. [Google Scholar]
- Korboulewsky, N.; Pereza, G.; Chauvat, M. How tree diversity affects soil fauna diversity: A review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Cavard, X.; Macdonald, S.E.; Bergeron, Y.; Chen, H.Y.H. Importance of mixedwoods for biodiversity conservation: Evidence for understory plants, songbirds, soil fauna, and ectomycorrhizae in northern forests. Environ. Rev. 2011, 19, 142–161. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Ouedraogo, E.; Mando, A.; Brussaard, L. Soil macrofauna affect crop nitrogen and water use efficiencies in semi-arid West Africa. Eur. J. Soil Biol. 2006, 42, 275–277. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2017, 332, 161–172. [Google Scholar] [CrossRef]
- Khemaissia, H.; Jelassi, R.; Souty-Grosset, C.; Nasri-Ammar, K. Amphipoda and Isopoda diversity around Tunisian wetlands (North Africa) in relation to environmental conditions. Afr. J. Ecol. 2018, 56, 455–467. [Google Scholar] [CrossRef]
- Li, J.; Ren, Q.W.; Sun, J.X. Water consumption dominant tree species at Qing shuihe watershed of Chongli district in Zhangjiakou. J. Northwest For. Univ. 2018, 33, 40–46. [Google Scholar]
- Ding, J.; Yang, X.B.; Zhu, C.G.; Xie, J.Z.; Wen, H.D. Spatial pattern and influence factors of soil erosion of Qingshui river basin in Chongli. J. Soil Water Conserv. 2018, 32, 73–80. [Google Scholar]
- Yin, W.Y. Pictorial Keys to Soil Animals of China, 1st ed.; Science Press: Beijing, China, 1998; pp. 1–392. [Google Scholar]
- Zhong, J.M. Insect Classification Atlas, 1st ed.; Jiangsu Science and Technology Press: Nanjing, China, 1985; pp. 1–317. [Google Scholar]
- Ter Braak, C.J.F. The analysis of vegetation environment relationships by canonical correspondence analysis. Vegetatio 1987, 69, 69–77. [Google Scholar] [CrossRef]
- Liu, G.S.; Jiang, N.H.; Zhang, L.D.; Liu, Z.L. Soil Physical and Chemical Analysis and Description of Soil Profiles, 1st ed.; Standards Press of China: Beijing, China, 1996; p. 33. [Google Scholar]
- Page, A.L.; Miller, R.H.; Kenney, D.R. Methods of Soil Analysis, 1st ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 419–422. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sun, J.B.; Liu, H.L.; Zhang, F.D.; Sun, L.; Jin, S. Composition of soil fauna community and its diversity analysis at Maoershan Mountains of Heilongjaing Province. Sci. Silv. Sin. 2006, 42, 71–77. [Google Scholar]
- Zhang, X.P.; Hou, W.L.; Chen, P. Soil animal guilds and their ecological distribution in the northeast of China. Appl. Environ. Biol. 2001, 7, 370–374. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Margalef, R. Perspectives in Ecological Theory; The University of Chicago Press: Chicago, IL, USA, 1970; pp. 111–119. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Lescano, M.N.; Elizalde, L.; Werenkraut, V.; Pirk, G.I.; Flores, G.E. Ant and tenebrionid beetle assemblages in arid lands: Their associations with vegetation types in the Patagonian steppe. J. Arid Environ. 2017, 138, 51–57. [Google Scholar] [CrossRef]
- Zhang, D.; Man, X.L.; Liu, S.Q.; Xu, Z.P. Litter decomposition and nutrient release of typical forest communities in non-growing season in cold temperate zone. J. Beijing For. Univ. 2022, 44, 65–74. [Google Scholar]
- Bohara, M.; Acharya, K.; Perveen, S.; Manevski, K.; Hu, C.S.; Yada, R.K.P.; Shrestha, K.; Li, X.X. In situ litter decomposition and nutrient release from forest trees along an elevation gradient in Central Himalaya. Catena 2020, 194, 104698. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tsukamoto, J. Leaf traits, litter decomposability and forest floor dynamics in an evergreen-and a deciduous-broadleaved forest in warm temperate Japan. Forestry 2013, 86, 441–451. [Google Scholar] [CrossRef]
- Aerts, R.C. Leaf litter chemistry and leaf litter decomposition interrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Huhta, V.; Persson, T.; Setala, H. Functional implications of soil fauna diversity in boreal forests. Appl. Soil Ecol. 1998, 10, 277–288. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, R.; Tan, B.; You, C.M.; Zhang, L.; Zhang, J.; Xu, Z.F.; Schadler, M.R.T.; Scheu, T.F. Nitrogen addition and plant functional type independently modify soil mesofauna effects on litter decomposition. Soil Biol. Biochem. 2021, 160, 108340. [Google Scholar] [CrossRef]
- Szanser, M.; Ilieva-Makulec, K.; Kajak, A.; Gorska, E.; Kusinska, A.; Kisiel, M.; Olejniczak, I.; Russel, S.; Sieminiak, D.; Wojewoda, D. Impact of litter species diversity on decomposition processes and communities of soil organisms. Soil Biol. Biochem. 2011, 43, 9–19. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Bessler, H.; Engels, C.; Temperton, V.M.; Buchmann, N.; Roscher, C.; Kreutziger, Y.; Baade, J.; Habekost, M.; Gleixner, G. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob. Chang. Biol. 2008, 14, 2937–2949. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Wu, X.; Niu, Y.B.; Chen, Y.M.; Dong, C.G.; Qiao, Y.N. Carbon, nitrogen and phosphorus stoichiometry characteristics in leaf, litter and soil at mixed forests of Hippophae rhamnoides in the loess hilly region of China. J. Soil Water Conserv. 2021, 35, 369–376. [Google Scholar]
- Zhao, Y.B.; Ji, T.W.; Zhang, D.J.; Zhang, J. Soil physi-chemical properties, biomass and nutrient contents of forest litter in mixed plantations of Eucalyptus grandis and three tree species. Chin. J. Appl. Environ. Biol. 2015, 21, 948–953. [Google Scholar]
- Liu, C.H.; Shao, Y.; Cao, S.P. Soil fauna community diversity and response to wetland degradation in Nanniwan wetland, Shaanxi, China. Sains Malays. 2021, 50, 1511–1520. [Google Scholar] [CrossRef]
- de Araujo, A.S.F.; Eisenhauer, N.; Nunes, L.A.P.L.; Leite, L.F.C.; Cesarz, S. Soil surface-active fauna in degraded and restored lands of northeast Brazil. Land Degrad. Dev. 2015, 26, 1–8. [Google Scholar] [CrossRef][Green Version]
- Cole, L.; Buckland, S.M.; Bardgett, R.D. Relating microarthropod community structure and diversity to soil fertility manipulations in temperate grassland. Soil Biol. Biochem. 2005, 37, 1707–1717. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Ian Woodward, F.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef]
- van der Wal, A.; Geerts, R.H.E.M.; Korevaar, H.; Schouten, A.J.; Akkerhuis, G.A.J.M.J.O.; Rutgers, M.; Mulder, C. Dissimilar response of plant and soil biota communities to long-term nutrient addition in grasslands. Biol. Fertil. Soils 2009, 45, 663–667. [Google Scholar] [CrossRef]
- Loranger-Merciris, G.; Imbert, D.; Bernhard-Reversat, F.; Ponge, J.F.; Lavelle, P. Soil fauna abundance and diversity in a secondary semi-evergreen forest in Guadeloupe (Lesser Antilles): Influence of soil type and dominant tree species. Biol. Fertil. Soils 2007, 44, 269–276. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Gama-Rodrigues, E.F.; Moco, M.K.D.; Gama-Rodrigues, A.C. Influence of mineral fertilization on edaphic fauna in Acacia auriculiformis (A.CUNN) plantations. Rev. Bras. Cienc. Solo 2014, 38, 39–49. [Google Scholar] [CrossRef]
- Yin, X.Q.; Ma, C.; He, H.S.; Wang, Z.H.; Li, X.Q.; Fu, G.Q.; Liu, J.; Zheng, Y.M. Distribution and diversity patterns of soil fauna in different salinization habitats of Songnen Grasslands, China. Appl. Soil Ecol. 2018, 123, 375–383. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.E.; Shao, F.L.; Yu, X.X. Soil ecological stoichiometry and its influencing factors in natural secondary forest, North Mountain of Hebei Province. Acta Ecol. Sin. 2021, 41, 6267–6279. [Google Scholar]
- Begum, F.; Bajracharya, R.M.; Sitaula, B.K.; Sharma, S. Seasonal dynamics, slope aspect and land use effects on soil mesofauna density in the mid-hills of Nepal. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 290–297. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Ostle, N.J.; McNamara, N.R.; Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 2009, 41, 315–322. [Google Scholar] [CrossRef]


| Forest Type | Altitude (m) | Latitude and Longitude | Aspect | Dominant Species | Tree Layer | Bush Layer |
|---|---|---|---|---|---|---|
| Betula platyphylla (BH) | 1948 | 115°26′36″ E 40°58′48″ N | Northeast direction | Betula platyphylla | Betula platyphylla Malus baccata | Rosa bella Rubus swinhoei Abelia biflora Crataegus pinnatifida Salix cheilophila |
| Picea asperata (YS) | 1890 | 115°27′40″ E 40°57′43″ N | Southwest direction | Picea asperata | Picea asperata Armeniaca sibirica Malus baccata | Potentilla fruticose Rosa bella Spiraea salicifolia |
| Pinus sylvestris (ZZS) | 1900 | 115°27′40″ E 40°57′36″ N | Southwest direction | Pinus sylvestris | Pinus sylvestris Betula platyphylla Larix gmelinii Malus baccata | Potentilla fruticose Rosa bella Rubus swinhoei Abelia biflora Salix cheilophila Spiraea salicifolia |
| Diversity Indexes | Formulas |
|---|---|
| Shannon–Wiener index [35] | |
| Margalef richness index [36] | |
| Simpson dominance index [37] | |
| Pielou evenness index [38] |
| BH | YS | ZZS | Fg. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ind/m2 | % | Deg | Ind/m2 | % | Deg | Ind/m2 | % | Deg | ||
| Monoptera | 33.33 | 0.20% | + | 50.00 | 0.39% | + | 22.22 | 0.16% | + | S |
| Carabidae | 44.44 | 0.26% | + | 16.67 | 0.13% | + | 5.56 | 0.04% | + | Pr |
| Oniscidae | 22.22 | 0.13% | + | 11.11 | 0.09% | + | 5.56 | 0.04% | + | O |
| Tipulidae | 211.11 | 1.26% | ++ | 155.56 | 1.20% | ++ | 111.11 | 0.78% | + | S |
| Geophilidae | 116.67 | 0.70% | + | 38.89 | 0.30% | + | 33.33 | 0.23% | + | Pr |
| Isotomidae | 1733.33 | 10.33% | +++ | 1133.33 | 8.74% | ++ | 1016.67 | 7.13% | ++ | O |
| Eosentomidae | 33.33 | 0.20% | + | 66.67 | 0.51% | + | 33.33 | 0.23% | + | S |
| Phlaeothripidae | 150.00 | 0.89% | + | 33.33 | 0.26% | + | 38.89 | 0.27% | + | O |
| Echinococcidae | 3161.11 | 18.85% | +++ | 2444.44 | 18.84% | +++ | 2500.00 | 17.54% | +++ | O |
| Oribatida | 3088.89 | 18.42% | +++ | 2155.56 | 16.62% | +++ | 3144.44 | 22.07% | +++ | S |
| Elateridae | 16.67 | 0.10% | + | 0.00 | 0.00% | 0.00 | 0.00% | Ph | ||
| Juliformia | 38.89 | 0.23% | + | 5.56 | 0.04% | + | 5.56 | 0.04% | + | S |
| Schizopteridae | 11.11 | 0.07% | + | 11.11 | 0.09% | + | 0.00 | 0.00% | Ph | |
| Bibionidae | 250.00 | 1.49% | ++ | 166.67 | 1.28% | ++ | 127.78 | 0.90% | + | Pr |
| Actinedida | 1794.44 | 10.70% | +++ | 1538.89 | 11.86% | +++ | 2072.22 | 14.54% | +++ | S |
| Diplatyidae | 0.00 | 0.00% | 5.56 | 0.04% | + | 0.00 | 0.00% | O | ||
| Coleoptera larvae | 144.44 | 0.86% | + | 66.67 | 0.51% | + | 44.44 | 0.31% | + | Ph |
| Hypogastruridae | 961.11 | 5.73% | ++ | 1066.67 | 8.22% | ++ | 966.67 | 6.78% | ++ | O |
| Diptera larvae | 322.22 | 1.92% | ++ | 161.11 | 1.24% | ++ | 166.67 | 1.17% | ++ | Ph |
| Enchytraeidae | 27.78 | 0.17% | + | 22.22 | 0.17% | + | 22.22 | 0.16% | + | S |
| Lithobilidae | 0.00 | 0.00% | 0.00 | 0.00% | 11.11 | 0.08% | + | Pr | ||
| Cydnidae | 27.78 | 0.17% | + | 11.11 | 0.09% | + | 0.00 | 0.00% | Ph | |
| Stylommatophora | 22.22 | 0.13% | + | 0.00 | 0.00% | 5.56 | 0.04% | + | S | |
| Curculionidae | 100.00 | 0.60% | + | 16.67 | 0.13% | + | 27.78 | 0.19% | + | Ph |
| Araneae | 61.11 | 0.36% | + | 33.33 | 0.26% | + | 38.89 | 0.27% | + | Ph |
| Mycetophilidae | 166.67 | 0.99% | + | 83.33 | 0.64% | + | 44.44 | 0.31% | + | S |
| Scorpionida | 44.44 | 0.26% | + | 77.78 | 0.60% | + | 33.33 | 0.23% | + | S |
| Elateridae | 22.22 | 0.13% | + | 11.11 | 0.09% | + | 5.56 | 0.04% | + | Ph |
| Formicidae | 83.33 | 0.50% | + | 166.67 | 1.28% | ++ | 194.44 | 1.36% | ++ | O |
| Staphylinidae | 61.11 | 0.36% | + | 44.44 | 0.34% | + | 5.56 | 0.04% | + | Pr |
| Muscidae | 138.89 | 0.83% | + | 116.67 | 0.90% | + | 61.11 | 0.43% | + | S |
| Sminthuridae | 922.22 | 5.50% | ++ | 927.78 | 7.15% | ++ | 766.67 | 5.38% | ++ | O |
| Orchesellidae | 711.11 | 4.24% | ++ | 327.78 | 2.53% | ++ | 627.78 | 4.41% | ++ | O |
| Lumbricidae | 22.22 | 0.13% | + | 16.67 | 0.13% | + | 5.56 | 0.04% | + | S |
| Gamasida | 1994.44 | 11.89% | +++ | 1777.78 | 13.70% | +++ | 1983.33 | 13.92% | +++ | S |
| Katydidae | 27.78 | 0.17% | + | 27.78 | 0.21% | + | 5.56 | 0.04% | + | Ph |
| Rotifera | 100.00 | 0.60% | + | 66.67 | 0.51% | + | 77.78 | 0.55% | + | O |
| Nematoda | 100.00 | 0.60% | + | 105.56 | 0.81% | + | 38.89 | 0.27% | + | O |
| Corydiidae | 5.56 | 0.03% | + | 11.11 | 0.09% | + | 0.00 | 0.00% | O | |
| Density | 16,772 | 12,972 | 14,250 | |||||||
| Group number | 37 | 36 | 34 | |||||||
| Sample | Soil Layer (cm) | Diversity (H) | Abundance (D) | Evenness (J) | Dominance (C) |
|---|---|---|---|---|---|
| BH | 0–5 | 2.497 | 4.872 | 0.697 | 0.115 |
| 5–10 | 2.542 | 5.057 | 0.715 | 0.114 | |
| 10–15 | 2.453 | 4.131 | 0.744 | 0.123 | |
| 15–20 | 2.440 | 3.454 | 0.801 | 0.115 | |
| YS | 0–5 | 2.490 | 4.523 | 0.930 | 0.343 |
| 5–10 | 2.485 | 4.446 | 0.731 | 0.113 | |
| 10–15 | 2.309 | 3.437 | 0.747 | 0.128 | |
| 15–20 | 2.255 | 3.239 | 0.766 | 0.143 | |
| ZZS | 0–5 | 2.305 | 4.407 | 0.665 | 0.132 |
| 5–10 | 2.301 | 3.817 | 0.706 | 0.133 | |
| 10–15 | 2.233 | 3.259 | 0.733 | 0.142 | |
| 15–20 | 2.105 | 2.679 | 0.759 | 0.153 |
| pH | SOC | TN | TP | BD | C/N | |
|---|---|---|---|---|---|---|
| Tip | −0.720 * | 0.359 | 0.26 | 0.159 | −0.3 | 0.26 |
| Iso | −0.473 | 0.336 | 0.136 | 0.172 | −0.485 | 0.638 |
| Ech | −0.481 | 0.473 | 0.271 | 0.368 | −0.559 | 0.627 |
| Ori | −0.38 | 0.818 ** | 0.737 * | 0.861 ** | −0.578 | 0.143 |
| Bib | −0.700 * | 0.411 | 0.293 | 0.226 | −0.362 | 0.313 |
| Act | −0.269 | 0.732 * | 0.763 * | 0.832 ** | −0.316 | −0.251 |
| Hyp | −0.356 | −0.069 | 0.056 | −0.096 | 0.461 | −0.477 |
| Dip | −0.571 | 0.545 | 0.38 | 0.335 | −0.506 | 0.478 |
| For | −0.097 | 0.092 | 0.315 | 0.228 | 0.471 | −0.844 ** |
| Smi | −0.433 | 0.009 | −0.051 | −0.145 | 0.08 | 0.197 |
| Orc | −0.465 | 0.774 * | 0.658 | 0.787 * | −0.574 | 0.266 |
| Gam | −0.485 | 0.761 * | 0.694 * | 0.812 ** | −0.395 | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lin, Q.; Huang, T.; Feng, Y.; Zhang, S. Distribution Patterns of Soil Fauna in Different Forest Habitat Types of North Hebei Mountains, China. Sustainability 2022, 14, 5934. https://doi.org/10.3390/su14105934
Zhang H, Lin Q, Huang T, Feng Y, Zhang S. Distribution Patterns of Soil Fauna in Different Forest Habitat Types of North Hebei Mountains, China. Sustainability. 2022; 14(10):5934. https://doi.org/10.3390/su14105934
Chicago/Turabian StyleZhang, Huayong, Qingxia Lin, Tousheng Huang, Yu Feng, and Shijia Zhang. 2022. "Distribution Patterns of Soil Fauna in Different Forest Habitat Types of North Hebei Mountains, China" Sustainability 14, no. 10: 5934. https://doi.org/10.3390/su14105934
APA StyleZhang, H., Lin, Q., Huang, T., Feng, Y., & Zhang, S. (2022). Distribution Patterns of Soil Fauna in Different Forest Habitat Types of North Hebei Mountains, China. Sustainability, 14(10), 5934. https://doi.org/10.3390/su14105934







