Biogenic Nanosilica Synthesis Employing Agro-Waste Rice Straw and Its Application Study in Photocatalytic Degradation of Cationic Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Raw Material and Rice Straw Ash Preparation
2.2. Nano Silica Extraction from Rice Straw Ash
2.3. Characterization of Rice Straw Ash and Nanosilica Powders
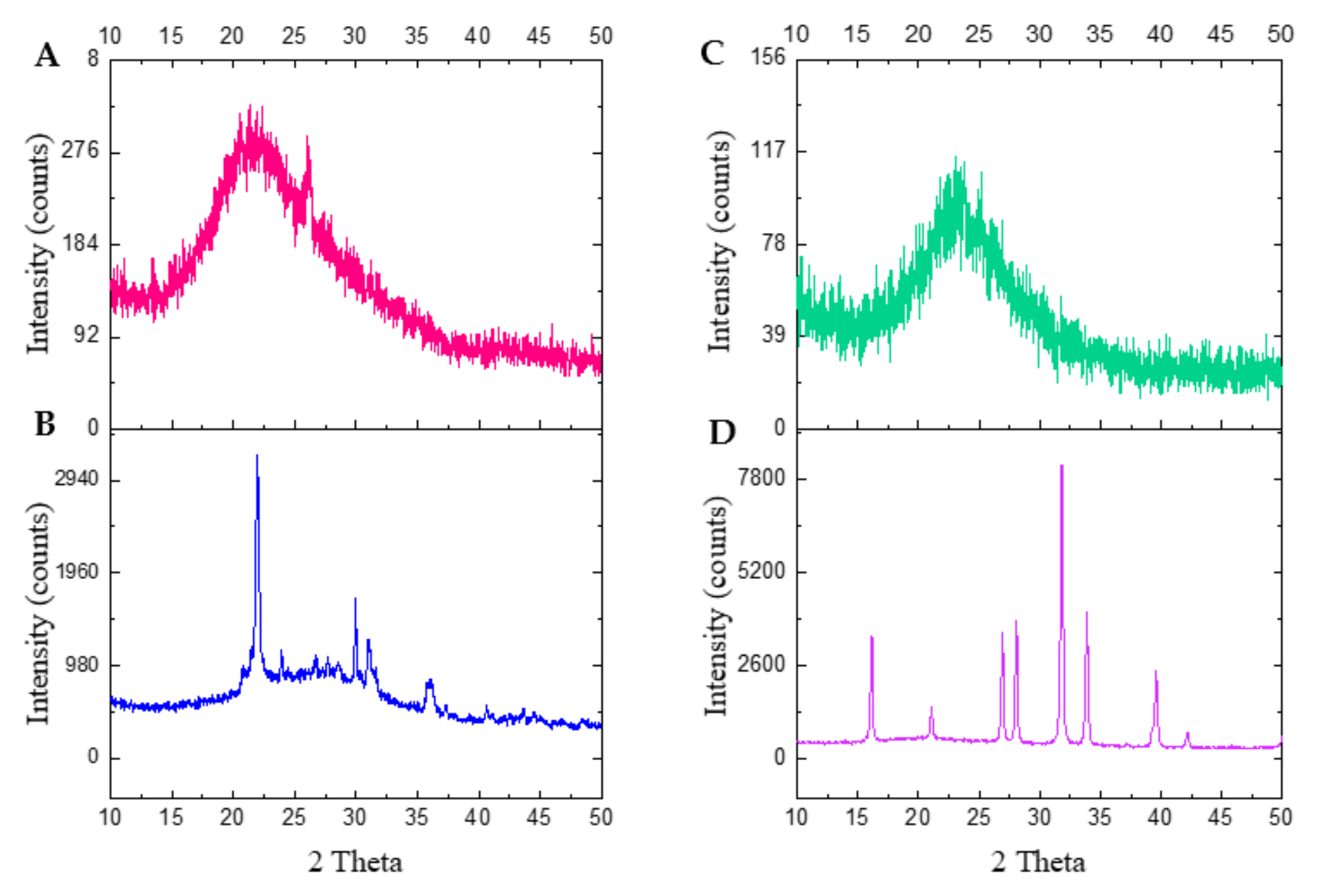
2.3.1. X-ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
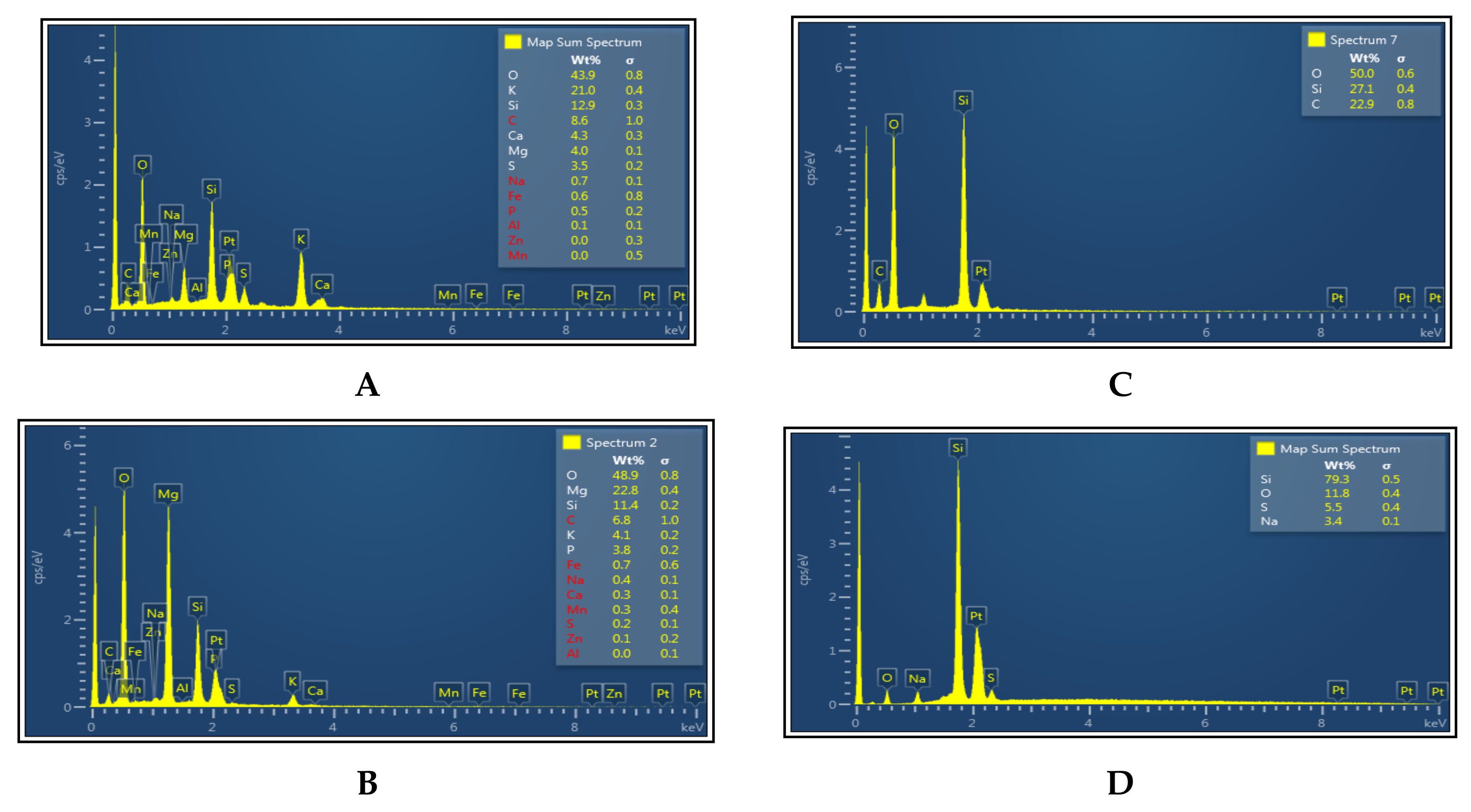
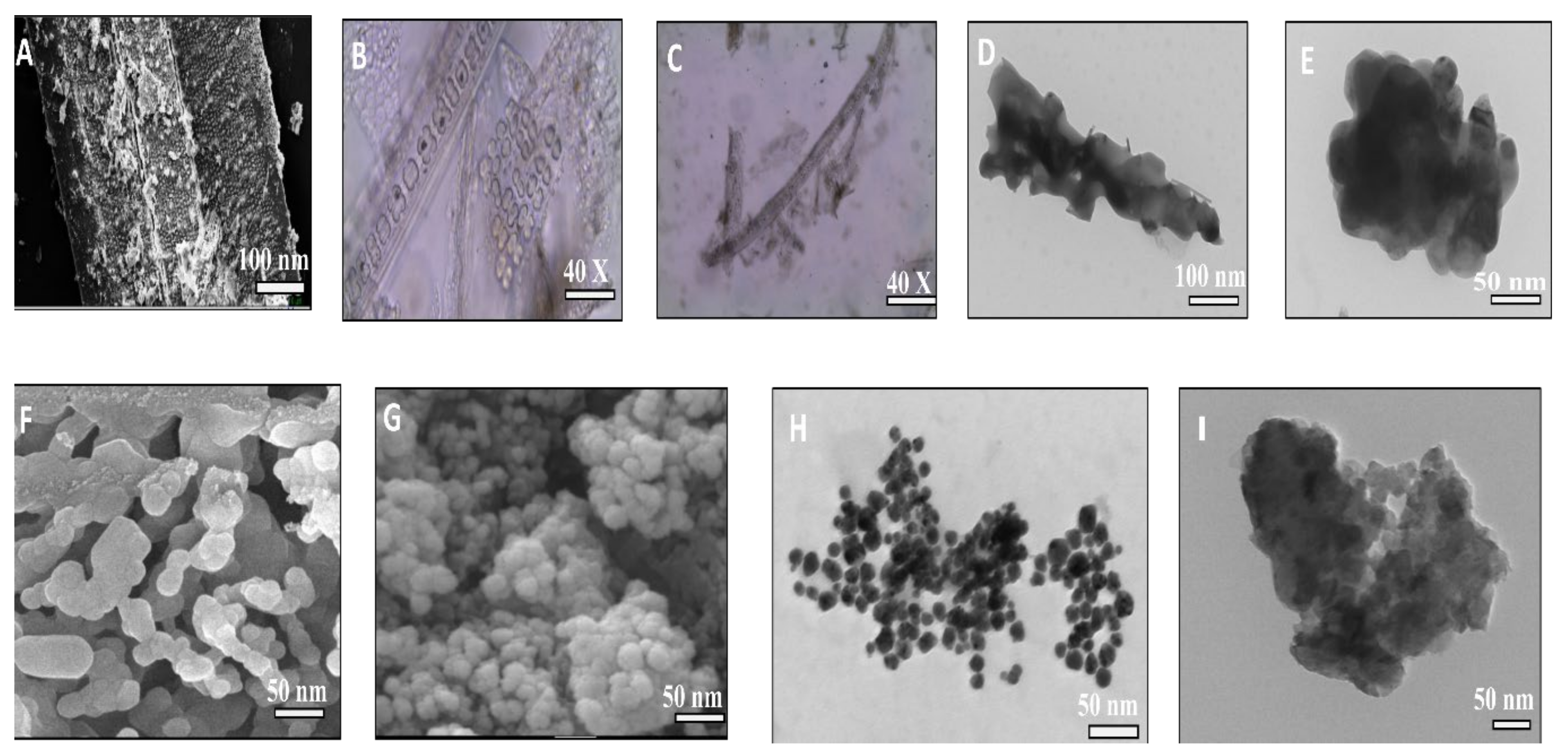
2.3.3. Field Emission Scanning Electron Microscope-Energy Dispersive Spectroscopy (FE-SEM-EDX)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Surface Area and Porosity
2.3.6. Photocatalytic Degradation of Methylene Blue Dye
3. Results
3.1. X-ray Diffraction Analysis
3.2. EDX Analysis
3.3. Fourier Transform Infrared Analysis
3.4. Morphology Studies
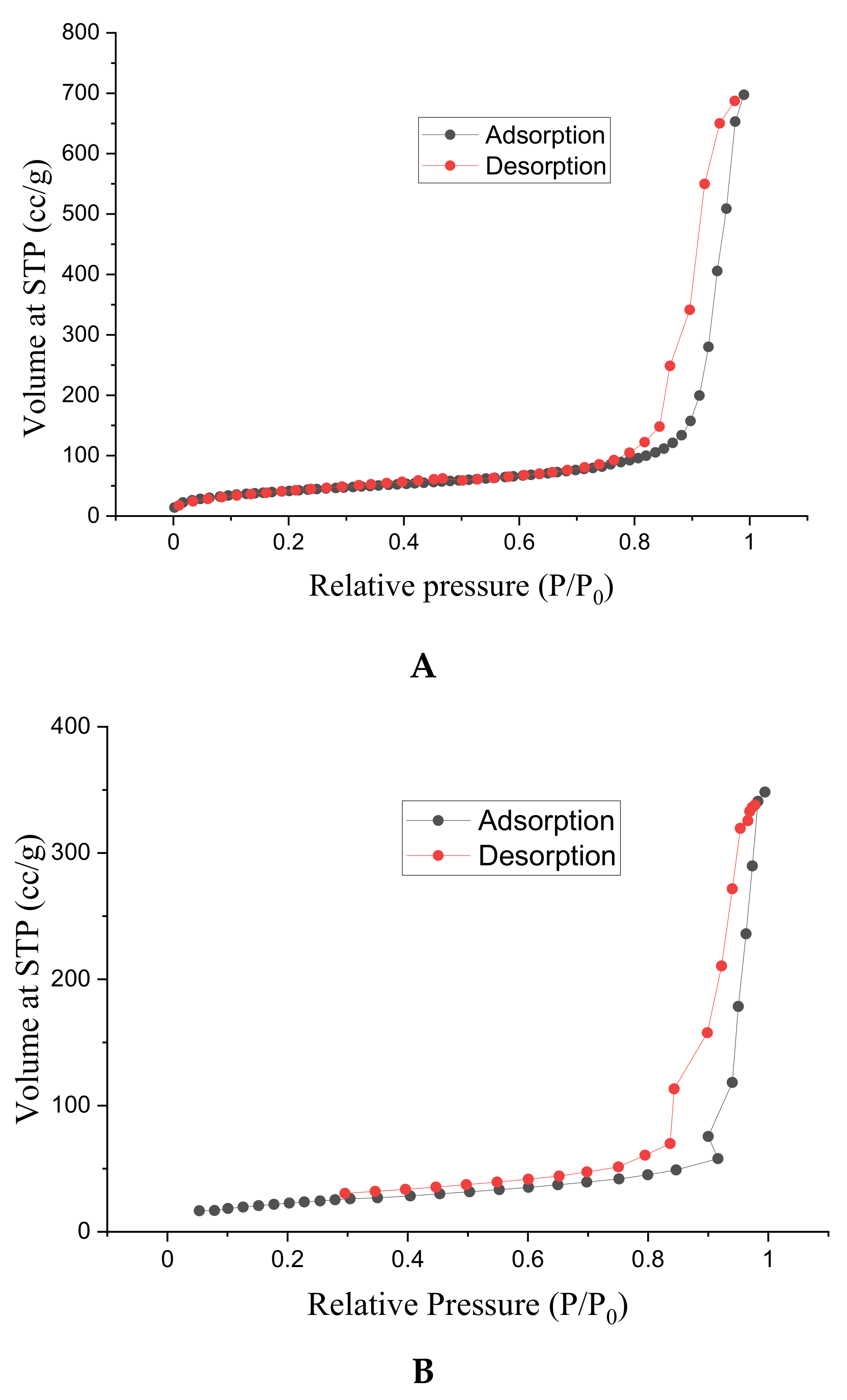
3.5. Surface Area and Porosity Studies
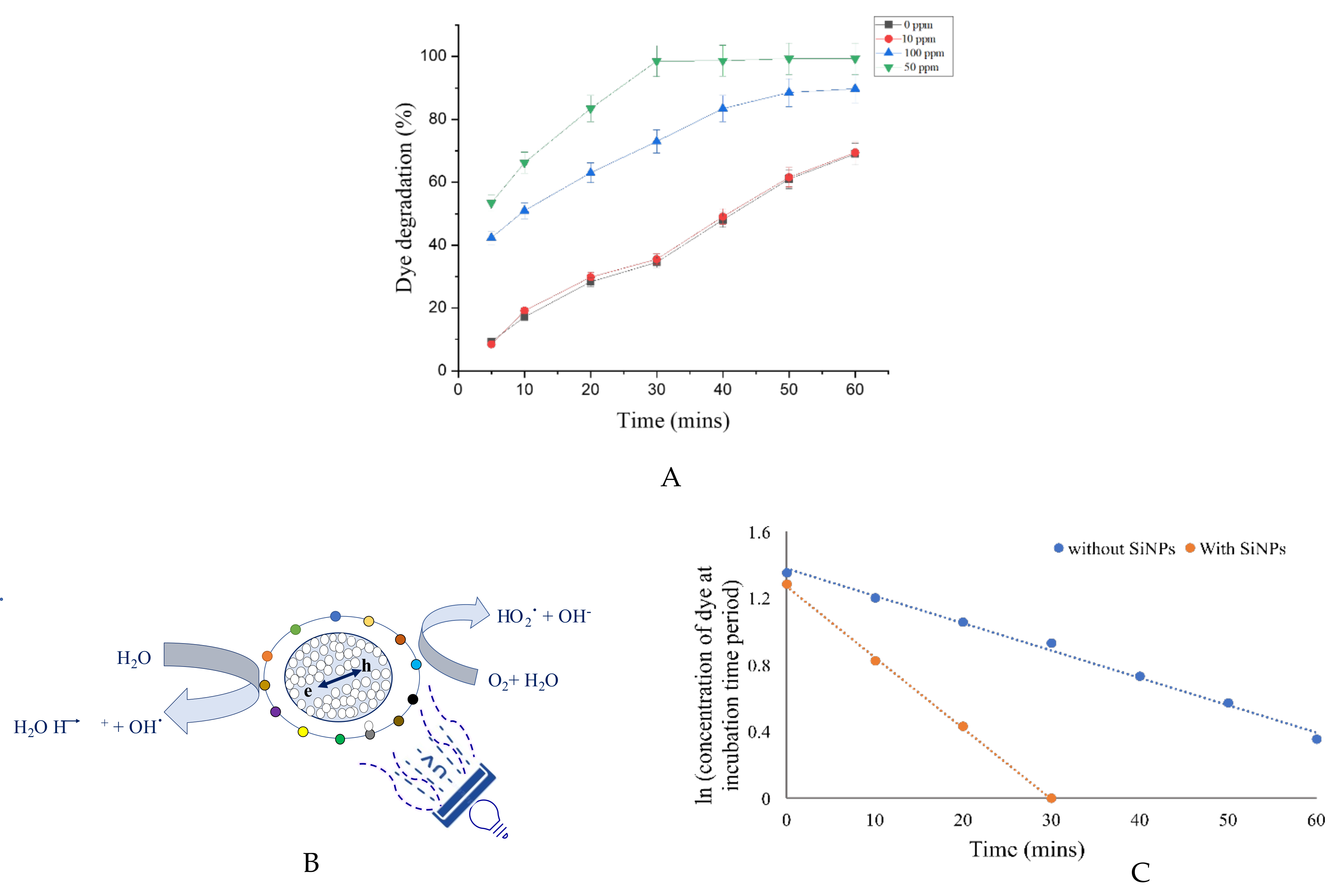
3.6. Decolorization of Cationic Dye Methylene Blue Using Amorphous SiNPs
3.6.1. Mechanism of Photocatalytic Degradation of Methylene Blue Using SiNPs
3.6.2. Kinetic Study of Dye Degradation
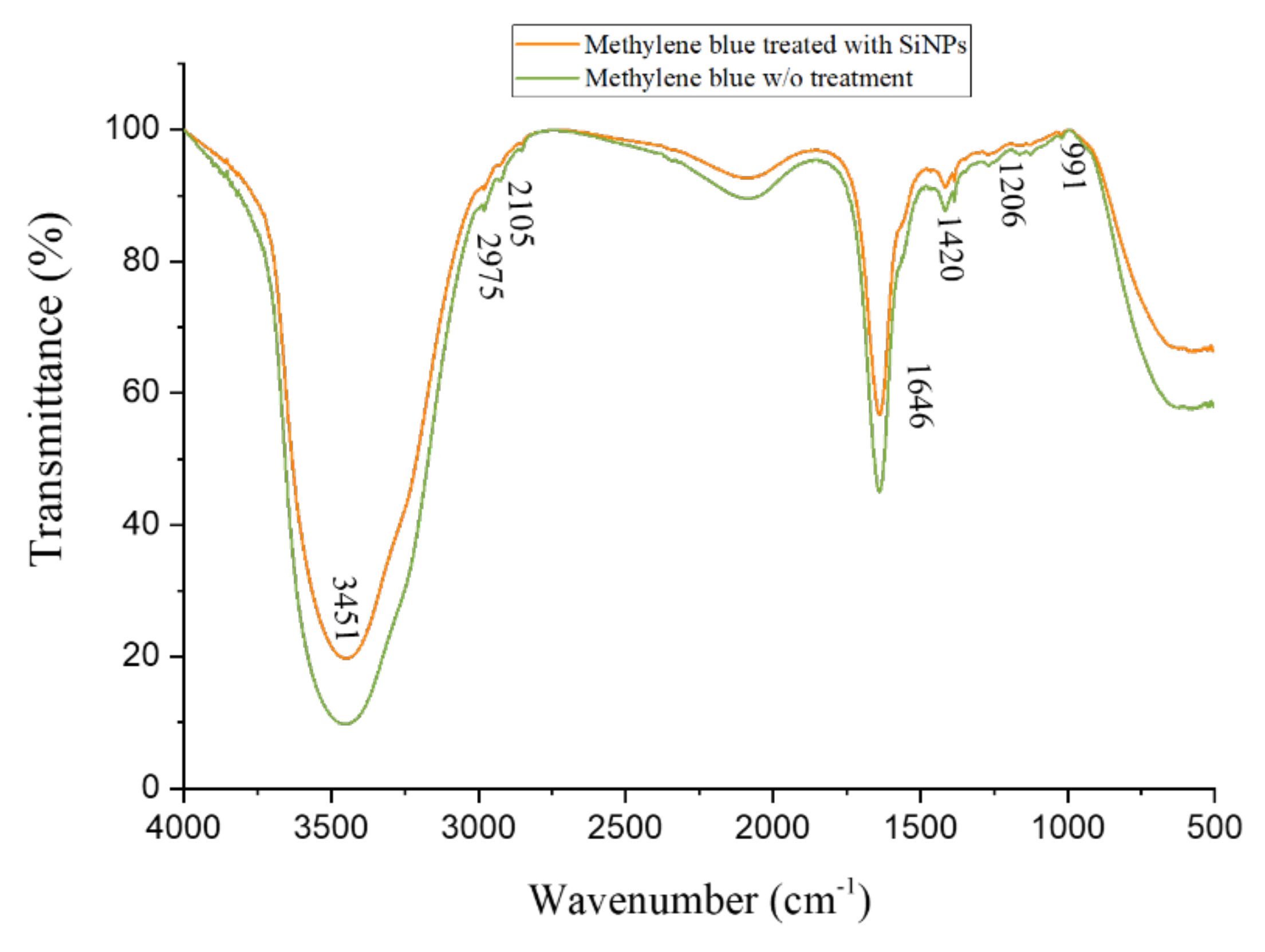
3.6.3. FTIR Studies of Methylene Blue Decolorization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beidaghy Dizaji, H.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high-quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; D’Silva, T.C.; Chandra, R.; Malik, A.; Vijay, V.K.; Misra, A. Strategies for boosting biomethane production from rice straw: A systematic review. Bioresour. Technol. Rep. 2021, 15, 100813. [Google Scholar] [CrossRef]
- Tipayarom, A.; Oanh, N.T.K. Influence of rice straw open burning on levels and profiles of semi-volatile organic compounds in ambient air. Chemosphere 2020, 243, 125379. [Google Scholar] [CrossRef]
- Singh, G.; Arya, S.K. A review on management of rice straw by use of cleaner technologies: Abundant opportunities and expectations for Indian farming. J. Clean. Prod. 2020, 291, 125278. [Google Scholar] [CrossRef]
- Marxen, A.; Klotzbücher, T.; Jahn, R.; Kaiser, K.; Nguyen, V.S.; Schmidt, A.; Schädler, M.; Vetterlein, D. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 2016, 398, 153–163. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Highly pure amorphous silica nano-disks from rice straw. Powder Technol. 2012, 225, 149–155. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, A.; Gupta, A.; Kumar, A.; Hariprasad, P.; Sharma, S. Bioformulation development via valorizing silica-rich spent mushroom substrate with Trichoderma asperellum for plant nutrient and disease management. J. Environ. Manag. 2021, 297, 113278. [Google Scholar] [CrossRef]
- Bahrami, A.; Pech-Canul, M.I.; Gutierrez, C.A.; Soltani, N. Effect of rice-husk ash on properties of laminated and functionally graded Al/SiC composites by one-step pressureless infiltration. J. Alloys Compd. 2015, 644, 256–266. [Google Scholar] [CrossRef]
- Beidaghy Dizaji, H.; Zeng, T.; Hölzig, H.; Bauer, J.; Klöß, G.; Enke, D. Ash transformation mechanism during combustion of rice husk and rice straw. Fuel 2022, 307, 121768. [Google Scholar] [CrossRef]
- Bahrami, A.; Simon, U.; Soltani, N.; Zavareh, S.; Schmidt, J.; Pech-Canul, M.I.; Gurlo, A. Eco-Fabrication of hierarchical porous silica monoliths by ice-templating of rice husk ash. Green Chem. 2017, 19, 188–195. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Mandal, M.K. Synthesis of rice straw extracted nano-silica-composite membrane for CO2 separation. J. Clean. Prod. 2018, 186, 241–252. [Google Scholar] [CrossRef]
- Mor, S.; Manchanda, C.K.; Kansal, S.K.; Ravindra, K. Nanosilica extraction from processed agricultural residue using green technology. J. Clean. Prod. 2017, 143, 1284–1290. [Google Scholar] [CrossRef]
- Kauldhar, B.S.; Yadav, S.K. Turning waste to wealth: A direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J. Clean. Prod. 2018, 194, 158–166. [Google Scholar] [CrossRef]
- Sachan, D.; Ramesh, A.; Das, G. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdelghny, A.M.; Elhadidy, H.; Youssef, A.M. Synthesis and characterization of high surface area nanosilica from rice husk ash by surfactant-free Sol–Gel method. J. Sol-Gel Sci. Technol. 2014, 69, 465–472. [Google Scholar] [CrossRef]
- Handojo, L.; Pramudita, D.; Mangindaan, D.; Indarto, A. Application of nanoparticles in environmental cleanup: Production, potential risks and solutions. Emerg. Eco-Friendly Green Technol. Wastewater Treat. 2020, 18, 45–76. [Google Scholar]
- Liu, X.; Wang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. One-Pot Synthesis of a Magnetic TiO2/PTh/γ-Fe2O3 Heterojunction Nanocomposite for Removing Trace Arsenite via Simultaneous Photocatalytic Oxidation and Adsorption. Ind. Eng. Chem. Res. 2020, 60, 528–540. [Google Scholar] [CrossRef]
- Krawczyk, A.; Domagała-Świątkiewicz, I.; Lis-Krzyścin, A.; Daraż, M. Waste Silica as a Valuable Component of Extensive Green-Roof Substrates. Polish J. Environ. Stud. 2017, 26, 643–653. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Liao, C.; Wang, Y.; He, J.; Fu, C.; Yang, K.; Bai, Z.; Zhang, F. Nano silica diaphragm in-fiber cavity for gas pressure measurement. Sci. Rep. 2017, 7, 787. [Google Scholar] [CrossRef]
- Le, T.M.; Tran, U.P.N.; Duong, Y.H.P.; Nguyen, Q.D.; Tran, V.T.; Mai, P.T.; Le, P.K. Sustainable bioethanol and value-added chemicals production from paddy residues at pilot scale. Clean Technol. Environ. Policy 2021, 1–13. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent progress in synthesis, functionalization, biocompatibility, and biomedical applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef]
- Olivier, G.; Combrinck, R.; Kayondo, M.; Boshoff, W.P. Combined effect of nano-silica, super absorbent polymers, and synthetic fibres on plastic shrinkage cracking in concrete. Constr. Build. Mater. 2018, 192, 85–98. [Google Scholar] [CrossRef]
- Zhang, H.; Goeppert, A.; Olah, G.A.; Prakash, G.K.S. Remarkable effect of moisture on the CO2 adsorption of nano-silica supported linear and branched polyethylenimine. J. CO2 Util. 2017, 19, 91–99. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Naeimirad, M.; Kotek, R.; Ramakrishna, S. A review on aerogel: 3D nanoporous structured fillers in polymer-based nanocomposites. Polym. Compos. 2018, 39, 3383–3408. [Google Scholar] [CrossRef]
- Tolba, G.M.K.; Barakat, N.A.M.; Bastaweesy, A.M.; Ashour, E.A.; Abdelmoez, W.; El-Newehy, M.H.; Al-Deyab, S.S.; Kim, H.Y. Effective and highly recyclable nanosilica produced from the rice husk for effective removal of organic dyes. J. Ind. Eng. Chem. 2015, 29, 134–145. [Google Scholar] [CrossRef]
- Aly, H.F.; Abd-Elhamid, A.I. Photocatalytic degradation of methylene blue dye using silica oxide nanoparticles as a catalyst. Water Environ. Res. 2018, 90, 807–817. [Google Scholar] [CrossRef]
- Stanley, R. Enhanced sunlight photocatalytic degradation of methylene blue by rod-like ZnO-SiO2 nanocomposite. Optik 2019, 180, 134–143. [Google Scholar] [CrossRef]
- Kusdianto, K.; Widiyastuti, W.; Shimada, M.; Qomariyah, L.; Winardi, S. Fabrication of ZnO-SiO2 nanocomposite materials prepared by a spray pyrolysis for the photocatalytic activity under UV and sunlight irradiations. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Borovets, Bulgaria, 26–29 November 2020; IOP Publishing: Bristol, UK, 2020; Volume 778, p. 12105. [Google Scholar] [CrossRef]
- Saleh, R.; Djaja, N.F. UV light photocatalytic degradation of organic dyes with Fe-doped ZnO nanoparticles. Superlattices Microstruct. 2014, 74, 217–233. [Google Scholar] [CrossRef]
- Venkatramanan, V.; Shah, S.; Rai, A.K.; Prasad, R. Nexus between Crop Residue Burning, Bioeconomy and Sustainable Development Goals over North-Western India. Front. Energy Res. 2021, 8, 614212. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Mendes, C.; Adnet, F.; Leite, M.; Furtado, C.R.G.; Sousa, A. Chemical, physical, mechanical, thermal and morphological characterization of corn husk residue. Cellul. Chem. Technol. 2015, 49, 727–735. [Google Scholar]
- Sarkar, P.; Moyez, S.A.; Dey, A.; Roy, S.; Das, S.K. Experimental investigation of photocatalytic and photovoltaic activity of titania/rice husk crystalline nano-silica hybrid composite. Sol. Energy Mater. Sol. Cells 2017, 172, 93–98. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Chen, X.; Tian, S.; Li, K. Synthesis and characterization of silica nanoparticles preparing by low-temperature vapor-phase hydrolysis of SiCl4. Ind. Eng. Chem. Res. 2014, 53, 11884–11890. [Google Scholar] [CrossRef]
- Zamani, A.; Marjani, A.P.; Mousavi, Z. Agricultural waste biomass-assisted nanostructures: Synthesis and application. Green Process. Synth. 2019, 8, 421–429. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. Rice Husk Reuse in the Preparation of SnO2/SiO2 Nanocomposite. Mater. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Beidaghy Dizaji, H.; Zeng, T.; Enke, D. New fuel indexes to predict ash behavior for biogenic silica production. Fuel 2022, 310B, 122345. [Google Scholar] [CrossRef]
- El-Didamony, H.; El-Fadaly, E.; Amer, A.A.; Abazeed, I.H. Synthesis and characterization of low cost nanosilica from sodium silicate solution and their applications in ceramic engobes. Bol. Soc. Esp. Cerám. Vidr. 2020, 59, 31–43. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. High-Purity nano silica powder from rice husk using a simple chemical method. J. Exp. Nanosci. 2014, 9, 272–281. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Li, J.; Yang, Y. Advances in pharmacological research of eugenol. Curr. Opin. Complement. Altern. Med. 2014, 1, 8–11. [Google Scholar]
- Peres, E.C.; Favarin, N.; Slaviero, J.; Almeida, A.R.F.; Enders, M.P.; Muller, E.I.; Dotto, G.L. Bio-nanosilica obtained from rice husk using ultrasound and its potential for dye removal. Mater. Lett. 2018, 231, 72–75. [Google Scholar] [CrossRef]
- Vinoda, B.M.; Vinuth, M.; Bodke, Y.; Manjanna, J. Photocatalytic degradation of toxic methyl red dye using silica nanoparticles synthesized from rice husk ash. J. Env. Anal. Toxicol. 2015, 5, 525–2161. [Google Scholar] [CrossRef]
- Selvaggi, R.; Tarpani, L.; Santuari, A.; Giovagnoli, S.; Latterini, L. Silica nanoparticles assisted photodegradation of acridine orange in aqueous suspensions. Appl. Catal. B Environ. 2015, 168, 363–369. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. Highly pure silica nanoparticles with high adsorption capacity obtained from sugarcane waste ash. ACS Omega 2018, 3, 2618–2627. [Google Scholar] [CrossRef]
- Mohammadi Galangash, M.; Mohaghegh Montazeri, M.; Ghavidast, A.; Shirzad-Siboni, M. Synthesis of carboxyl-functionalized magnetic nanoparticles for adsorption of malachite green from water: Kinetics and thermodynamics studies. J. Chin. Chem. Soc. 2018, 65, 940–950. [Google Scholar] [CrossRef]
- Salimi, F.; Tahmasobi, K.; Karami, C.; Jahangiri, A. Preparation of modified nano-SiO2 by bismuth and iron as a novel remover of methylene blue from water solution. J. Mex. Chem. Soc. 2017, 61, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Badr, Y.; Abd El-Wahed, M.G.; Mahmoud, M.A. Photocatalytic degradation of methyl red dye by silica nanoparticles. J. Hazard. Mater. 2008, 154, 245–253. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloid Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Wang, X.; Han, S.; Zhang, Q.; Zhang, N.; Zhao, D. Photocatalytic oxidation degradation mechanism study of methylene blue dye waste water with GR/iTO2. In Proceedings of the MATEC Web of Conferences, Warsaw, Poland, 5–7 October 2017; EDP Sciences: Les Ulis, France, 2018; Volume 238, p. 3006. [Google Scholar]

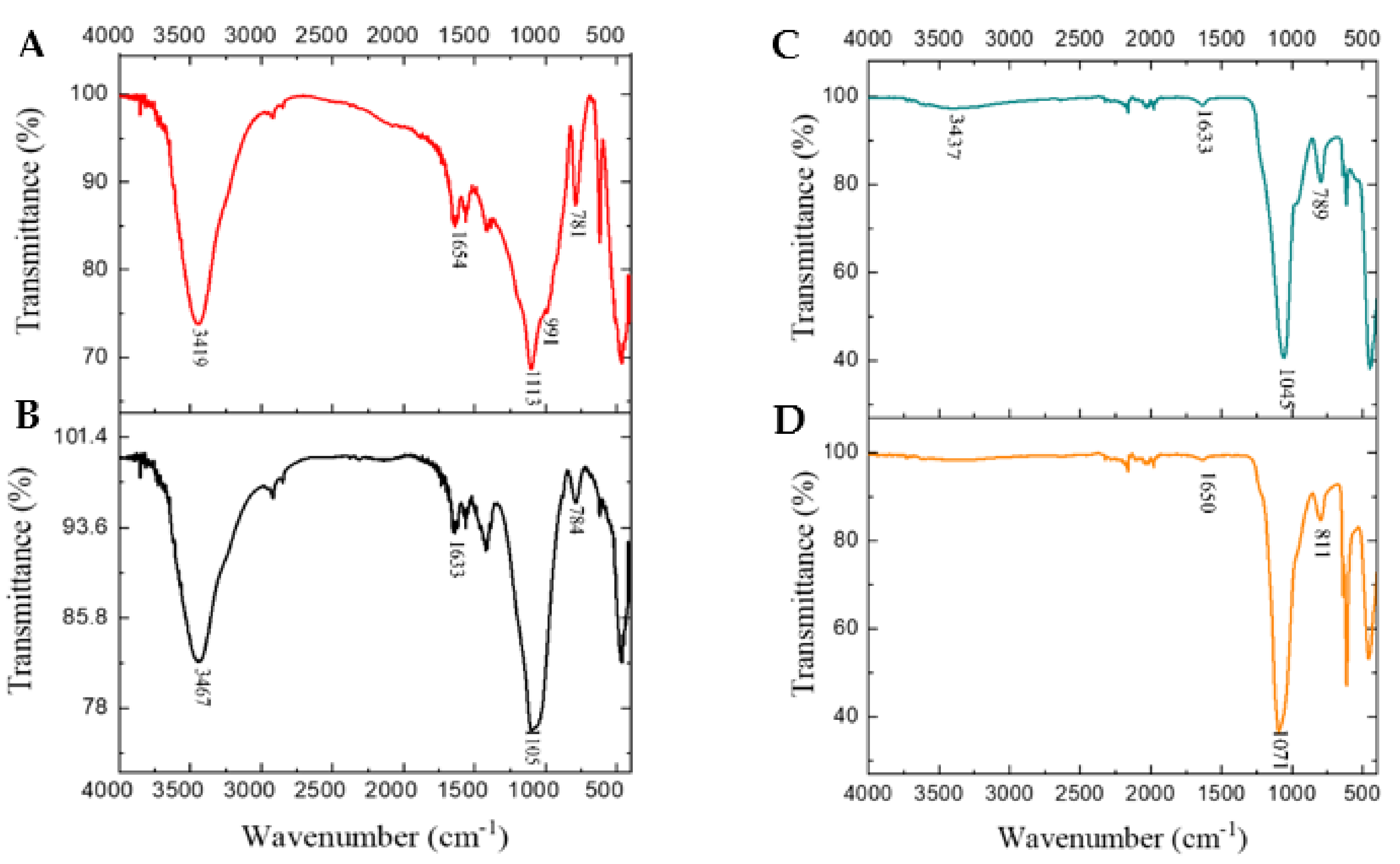
| RSA-600 | RSA-900 | SiNPs (Amorphous) | SiNPs (Crystalline) | Literature | Functional Groups |
|---|---|---|---|---|---|
| 459 | 470 | 462 | 452 | 460 [36] | Si−O |
| 1091 | 11,081 | 1073 | 1101 | 1033 [12] | Si−O−Si |
| 3474 | 3421 | 3423 | NA | 3000–3500 [13] | Si−OH |
| NA | NA | 1663 | 1641 | 1600 [35] | H−OH |
| 795 | 775 | 796 | 817 | 796 [6] | Si−O−Si |
| Dye | Concentration (Dye) and pH | Source of SiNPs | Concentration (Nanosilica) | Source | Degradation (%) | Time | References |
|---|---|---|---|---|---|---|---|
| Crystal Violet | 50 mg/L; pH 7 | Rice husk | 1 g/L | Ultrasound | 80% | 60 min | Peres et al. [41] |
| Methyl red | 0.05 Mm/100 mL; pH 7 | Rice husk | 1 g/100 mL | Sunlight | 95% | 120 min | Vinoda et al. [42] |
| Methylene blue | 50 ppm/50 mL; pH-not reported | Rice husk | 50 mg/100 mL | Ceramic material based on amorphous SiNPs | 80% | 30 min | Tolba et al. [25] |
| Methylene blue | 100 mg/L; pH 11 | Yellow sand | 10 g/L | Ultraviolet light | 100% | 90 s | Aly and Elhamid [26] |
| Methylene blue | 50 ppm; pH 7 | ZnO/SiO2 xerogel | 0.075 g/L | Visible light | 100% | 30 min | Stanley [27] |
| Acridine orange | 1 × 10−5 M | 3-aminopropyl-functionalized silica NPs | 10 mg/3 mL | Ultraviolet light | 58% | 50 min | Selvaggi et al. [43] |
| Acid ornage | 150 mg/L | Sugar cane ash | 1 g/L | Not reported | 80% | 30 min | Rovani et al. [44] |
| Malachite green | 20 mg/L; pH 9 | Fe3O4@SiO2-COOH NPs | 0.5 g/L | Not reported | 97.5% | 120 min | Galangash et al. [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Dizaji, H.B.; Puttuswamy, H.; Sharma, S. Biogenic Nanosilica Synthesis Employing Agro-Waste Rice Straw and Its Application Study in Photocatalytic Degradation of Cationic Dye. Sustainability 2022, 14, 539. https://doi.org/10.3390/su14010539
Singh G, Dizaji HB, Puttuswamy H, Sharma S. Biogenic Nanosilica Synthesis Employing Agro-Waste Rice Straw and Its Application Study in Photocatalytic Degradation of Cationic Dye. Sustainability. 2022; 14(1):539. https://doi.org/10.3390/su14010539
Chicago/Turabian StyleSingh, Garima, Hossein Beidaghy Dizaji, Hariprasad Puttuswamy, and Satyawati Sharma. 2022. "Biogenic Nanosilica Synthesis Employing Agro-Waste Rice Straw and Its Application Study in Photocatalytic Degradation of Cationic Dye" Sustainability 14, no. 1: 539. https://doi.org/10.3390/su14010539
APA StyleSingh, G., Dizaji, H. B., Puttuswamy, H., & Sharma, S. (2022). Biogenic Nanosilica Synthesis Employing Agro-Waste Rice Straw and Its Application Study in Photocatalytic Degradation of Cationic Dye. Sustainability, 14(1), 539. https://doi.org/10.3390/su14010539







